Abstract
Neonatal hyperglycaemia is frequent and requires insulin therapy. To resolve the difficulties encountered by paediatricians in stabilising glycaemia, the preparation and administration of insulin aspart were assessed and optimised. After high-performance liquid chromatography (HPLC-UV) assessment of insulin aspart preparations made according to the old protocol, a new protocol was drawn up. Dosage reliability of solutions prepared by paediatric nurses was evaluated by HPLC-UV. This new protocol was also tested in a Y-infusion situation and the need to saturate infusion tubes assessed. Wide deviations in insulin aspart concentrations were found between theoretical concentrations and preparations made according to the old protocol. Glycated insulin aspart was found in the majority of these preparations. The new protocol significantly reduced the variability of data and relative deviations around the target value. It also eliminated the formation of glycated insulin even in the case of co-infusion of parenteral nutrition and confirmed the need to saturate infusion tubes.
Conclusion: The revision of the insulin therapy protocol reduced the variability of insulin concentration in preparations and avoided the administration of glycated derivatives potentially toxic for neonates.
What is Known: • Insulin preparation in NICUs is a risky task because it is a two-step preparation • Diluted in dextrose, insulin aspart is unstable, with formation of potentially toxic glycated derivatives What is New: • This work proposes a new insulin therapy protocol validated by HPLC-UV for NICU allowing suppression of the formation of glycated insulin, to significantly reduce deviations from theoretical concentrations and to limit adsorption phenomena • This protocol is validated in case of co-infusion of parenteral nutrition |





Similar content being viewed by others
Data availability
Data and materials are not in a public depository and are available upon request.
Code availability
N/A
Abbreviations
- D5%:
-
Dextrose
- D50%:
-
50% dextrose
- HPLC-UV:
-
High-performance liquid chromatography
- IPN:
-
Individualised parenteral nutrition
- NICUs:
-
Neonatal intensive care units
- Q1:
-
1st quartile
- Q3:
-
3rd quartile
References
Mitanchez-Mokhtari D, Walter-Nicolet E, Farges C, Egrot F, Tréluyer JM, Hubert P (2004) Modalités d’administration de l’insuline par voie intraveineuse chez le nouveau-né prématuré. Arch Pediatr 11:1054–1059. https://doi.org/10.1016/j.arcped.2004.03.027
Préta L-H, Henry H, Masse M, Barthélémy C, Carta N, Foulon C, Goossens JF, Kouach M, Lannoy D, Storme L, Décaudin B, Genay S, Odou P (2020) Instability of insulin aspart diluted in dextrose. Diabetes Care 43:e77–e78. https://doi.org/10.2337/dc19-2462
Hunter SJ, Boyd AC, O’Harte FPM et al (2003) Demonstration of glycated insulin in human diabetic plasma and decreased biological activity assessed by euglycemic-hyperinsulinemic clamp technique in humans. Diabetes 52:492–498. https://doi.org/10.2337/diabetes.52.2.492
McKillop AM, Lindsay JR, Au S et al (2006) Meal-dependent regulation of circulating glycated insulin in type 2 diabetic subjects. Horm Metab Res 38:94–97. https://doi.org/10.1055/s-2006-925125
Shahriyary L, Riazi G, Lornejad MR, Ghezlou M, Bigdeli B, Delavari B, Mamashli F, Abbasi S, Davoodi J, Saboury AA (2018) Effect of glycated insulin on the blood-brain barrier permeability: an in vitro study. Arch Biochem Biophys 647:54–66. https://doi.org/10.1016/j.abb.2018.02.004
Masse M, Maton M, Genay S, Blanchemain N, Barthélémy C, Décaudin B, Odou P (2018) In vitro assessment of the influence of intravenous extension set materials on insulin aspart drug delivery. PLoS One 13:e0201623. https://doi.org/10.1371/journal.pone.0201623
https://www.equator-network.org/reporting-guidelines/squire/ (2015) Revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0). Accessed 9 Mar 2021
Préta L-H (2019) Révision et optimisation des pratiques de préparation et d’administration de l’insuline asparte en réanimation néonatale. Université de Lille. https://pepite-depot.univ-lille2.fr/nuxeo/site/esupversions/af81991f-5604-4cfc-84ed-05307bdec563. Accessed 9 Mar 2021
Goodacre S (2015) Uncontrolled before-after studies: discouraged by Cochrane and the EMJ. Emerg Med J 32:507–508. https://doi.org/10.1136/emermed-2015-204761
Ainscough LP, Ford JL, Morecroft CW, Peak M, Turner MA, Nunn AJ, Roberts M (2018) Accuracy of intravenous and enteral preparations involving small volumes for paediatric use: a review. Eur J Hosp Pharm 25:66–71. https://doi.org/10.1136/ejhpharm-2016-001117
Abdel-Wahab YH, O’Harte FP, Boyd AC et al (1997) Glycation of insulin results in reduced biological activity in mice. Acta Diabetol 34:265–270. https://doi.org/10.1007/s005920050086
Boyd AC, Abdel-Wahab YH, McKillop AM et al (2000) Impaired ability of glycated insulin to regulate plasma glucose and stimulate glucose transport and metabolism in mouse abdominal muscle. Biochim Biophys Acta 1523:128–134. https://doi.org/10.1016/s0304-4165(00)00113-6
Foinard A, Décaudin B, Simon N, Barthélémy C, Storme L, Odou P (2014) Vancomycin syringe study shows significant reduction in dosing variability after introducing a revised protocol. Acta Paediatr 103:e93–e94. https://doi.org/10.1111/apa.12484
Knopp JL, Chase JG (2020) Clinical recommendations for managing the impact of insulin adsorptive loss in hospital and diabetes care. J Diabetes Sci Technol 1932296820915875. https://doi.org/10.1177/1932296820915875
Knopp JL, Bishop K, Lerios T, Chase JG (2019) Capacity of infusion lines for insulin adsorption: effect of flow rate on total adsorption. J Diabetes Sci Technol 1932296819876924:109–120. https://doi.org/10.1177/1932296819876924
Eibensteiner F, Laml-Wallner G, Thanhaeuser M, Ristl R, Ely S, Jilma B, Berger A, Haiden N (2020) ELBW infants receive inadvertent sodium load above the recommended intake. Pediatr Res 88:412–420. https://doi.org/10.1038/s41390-020-0867-9
Acknowledgements
We thank Alexandra Tavernier (M.A. University of Glasgow, Professeur Agrégée, France) for English language and editing assistance.
Author information
Authors and Affiliations
Contributions
All the authors made substantial contributions to conception and design of the study and/or acquisition of data and/or analysis and interpretation of data; all the authors participated in drafting or critically revising the manuscript; all the authors gave final approval of the version submitted.
• Conducted the study and carried out the experiments, acquired and analysed the data and drafted the first version of the manuscript: Laure-Hélène Préta
• Supervised the work by contributing to design of the study, interpreting the data and revising the manuscript: Stéphanie Genay, Pascal Odou, Laurent Storme and Bertrand Décaudin
• Assisted in data collection and contributed to the development and execution of the analyses and revised the manuscript: Cécile Malat and Natacha Carta
• Participated in the development of the protocol and interpretation of the data and revised the manuscript: Mohamed Riadh Boukhris and Julie Verspieren
• Approved the final manuscript version: all the authors
Corresponding author
Ethics declarations
Ethics approval and consent to participate
N/A
Consent for publication
N/A
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Daniele De Luca
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
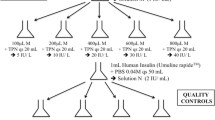
Representation of the infusion set-up performed with the medical devices detailed in Suppl mat 2 (PNG 243 kb)
ESM 2
Medical devices used in infusion set-up (PDF 226 kb)
ESM 3
Regression parameters of the validation method for the range. The given parameters are: a (the slope of the line), b (the intercept at the origin) in mean value ± standard deviation and R2 (the coefficient of determination). The limits of detection (LOD) and quantification (LOQ) are expressed in μg/mL for phenol and metacresol and U/mL for insulin (DOCX 12 kb)
ESM 4
New protocol for the preparation and administration of insulin aspart in NICUs. Changes from the previous protocol are highlighted in red boxes. (PDF 61 kb)
Rights and permissions
About this article
Cite this article
Préta, LH., Genay, S., Malat, C. et al. Optimising insulin aspart practices in a neonatal intensive care unit: a clinical and pharmaco-technical study. Eur J Pediatr 180, 2985–2992 (2021). https://doi.org/10.1007/s00431-021-04041-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-021-04041-y