Abstract
The aim of this study was to assess the serum concentrations of Clara cell secretory protein (CC16) in association with acute and chronic lung injury in mechanically ventilated preterm neonates. Thirty-five preterm neonates (gestational age [GA] ≤31 weeks) with acute respiratory failure were enrolled. Of these, 23 neonates requiring ventilatory support within 2 h after birth comprised the mechanically ventilated group (MV group), and 12 neonates who were not ventilated made up the nonventilated group (NV group). Serum CC16 was measured (using enzyme-linked immunosorbent assay [ELISA]) within 2 h (T0) and at 72 h (T1) after birth, at day 14 of life (T2) and at 36 weeks postmenstrual age (T3). The median CC16 concentrations were significantly higher in the MV group compared to the NV group at all times. Analysis with respect to differences observed in the group characteristics showed that GA, Apgar score at 5 min and mechanical ventilation were significant covariates of serum CC16 at T0. All neonates in the NV group and 18 cases in the MV group, respectively, survived discharge. Ventilated survivors with later bronchopulmonary dysplasia (BPD; oxygen requirement at T3, n = 7) had significantly higher CC16 at all times compared to nonventilated neonates. Elevated serum CC16 levels at T2 were predictive of BPD development. In conclusion, our results show that serum CC16 increases significantly in preterm neonates ventilated early after birth and remains high in those with later BPD. Further research is needed to validate the usefulness of CC16 as a peripheral blood biomarker of acute and chronic lung injury.
Similar content being viewed by others
Introduction
Clara cell secretory protein (CC16, CC10 or CCSP) is a 15.8-kD protein secreted all along the tracheobronchial epithelium, especially in the terminal bronchioles, where nonciliated Clara cells are localised. Although the precise role of CC16 remains to be elucidated, several properties and functions in vitro suggest an important role of this protein for the protection of the lung against inflammation. These include the inhibition of enzymes such as cytosolic phospholipase A2, immune cells (neutrophils, monocytes, fibroblasts) and cytokines (interferon-gamma) involved in the inflammatory process. Furthermore, CC16 attenuates surfactant degradation and oxidative stress [7, 10].
Under normal conditions, CC16, which is almost exclusively expressed in the lumen of the respiratory system, is also present in the bloodstream of healthy subjects, albeit in much lower concentrations, following its passive transfer across the air–blood barrier [10]. However, clinical studies have shown that circulating CC16 rises in various pulmonary diseases characterised by increased epithelial permeability, such as acute respiratory distress syndrome [18], idiopathic interstitial pneumonia [26] and sarcoidosis [12], as well as after exposure to environmental air pollutants [6] or fire smoke [5]. On the basis of this evidence, CC16 has been proposed as a potential, non-invasive, peripheral, blood biomarker specific to the lung injury.
Mechanical ventilation is known to disrupt alveolar integrity [9], constituting a critical component of lung injury in preterm neonates and progression to bronchopulmonary dysplasia (BPD) [24]. Some inflammatory markers in tracheal aspirates and peripheral blood heretofore used for the early detection of lung injury in preterm neonates receiving mechanical ventilation, such as proinflammatory cytokines interleukin-1 beta and 6 [16, 25], are, rather, products of a systemic inflammatory response and not specific to lung disease [13]. In addition, the tracheal aspirate technique is invasive, and, apparently, it can be performed only in intubated neonates. Increased permeability of the alveoli leading to a large bidirectional protein flux between airways and circulation is considered to be a hallmark feature of respiratory distress syndrome (RDS) [14, 15], thus, theoretically permitting the diffusion of CC16 into the vascular compartment. In this context, we formulated the hypothesis that CC16 would increase acutely in the blood of ventilated preterm neonates during the initial phase of mechanical respiratory support proportionally to respiratory disease severity, and that this increase would persist in those who later develop BPD. To date, there is only one study regarding circulating CC16 concentrations in ventilated preterm and term neonates [19]. Nevertheless, in the aforementioned study, serum CC16 was not critically assessed in association with parameters related to mechanical ventilation or the severity of the respiratory failure, and, most importantly, measurements of serum CC16 were confined within the first week of life.
Based on these considerations, we designed this study with the aim of evaluating the profile of serum CC16 concentrations in preterm neonates requiring mechanical ventilation in association with acute and chronic lung injury, as documented clinically by BPD development.
Patients and methods
Study population
The study was approved by the human research review board of our institution and an informed written consent was obtained from all parents.
In this prospective observational study, inborn, preterm neonates (gestational age [GA]≤31 weeks) with acute respiratory failure were included. The enrolled neonates were categorised into: (a) the nonventilated group (NV group), in which supplemental oxygen or nasal continuous positive airway pressure (NCPAP) were applied and (b) the mechanically ventilated group (MV group), in which synchronised intermittent mandatory ventilation was established within the first 2 h of life.
In order to preclude the confounding effect of several perinatal conditions on alveolar permeability and surfactant metabolism and, consequently, on CC16 serum levels, neonates born to mothers with diabetes or clinical chorioamnionitis and those with severe perinatal asphyxia, severe bleeding disorders (platelets count <50,000/mm3), possible (clinical and laboratory evidence) or confirmed (positive blood culture) early onset sepsis, as well as congenital infections and anomalies, were excluded. Refusal of parental consent was also an exclusion criterion. Furthermore, the plan of the study was that each eligible neonate continued in the assigned group with respect to respiratory support until successful extubation, death or the development of BPD. Thus, neonates initially assigned to the NV group who required mechanical ventilation after day 3 of life were excluded from the final analysis. BPD was defined as the need for oxygen at 36 weeks postmenstrual age.
In all neonates, the demographic and perinatal characteristics (sex, birth weight [BW], GA, maternal smoking, preeclampsia/eclampsia, intrauterine growth retardation, premature rupture of membranes >24 h, prenatal steroid administration, mode of delivery, Apgar score), as well as pulmonary data (severity and cause of acute respiratory failure and surfactant administration) were recorded. The severity of acute respiratory failure was assessed using the alveolar-arterial difference of oxygen [AaDO2=(FiO2*713)−PaCO2−PaO2, FiO2; fraction of inspired oxygen, PaO2 and PaCO2; partial pressure of arterial oxygen and carbon dioxide, respectively]. In ventilated neonates, the oxygenation index [(mean airway pressure*FiO2*100)/PaO2] was also calculated. Diagnosis of the cause of respiratory failure was based on the X-ray findings. Serum creatinine was used to evaluate renal functions possibly affecting serum CC16 concentrations. The mean arterial pressure was also recorded. The outcome parameters collected included survival, the development of BPD, as well as the duration of mechanical ventilation, NCPAP and supplemental oxygen.
Pulmonary and supportive care
Pressure-limited synchronised intermittent mandatory ventilation was the ventilation mode applied. However, neonates in the MV group were allowed NCPAP before the initiation of mechanical ventilation, as was crossover from MV to high-frequency oscillatory ventilation for refractory respiratory failure. Exogenous surfactant (Beractant, 100 mg/Kg/dose) was given within the first 2 h after birth and for two further doses. Prophylactic surfactant administration in the delivery room was not performed. Following extubation, infants were put on NCPAP. General supportive treatment was at the discretion of the attending neonatologist, in line with the protocols applied in our department.
Blood sampling and serum CC16 assay
Arterial blood was drawn from all enrolled neonates for CC16 measurement after their stabilisation in the neonatal intensive care unit within the first 2 h of life (T0), and before the first dose of surfactant in those treated, as well as at 72 h (T1) after birth, at day 14 of life (T2) and at 36 weeks postmenstrual age in those still in hospital, or before discharge (T3). Sera were stored at −80°C until pneumoprotein measurements. Neonates who succumbed before the second blood sampling were excluded from the statistical analysis.
The serum human CC16 concentrations were measured using quantitative colorimetric sandwich enzyme-linked immunosorbent assay (ELISA) kits (BioVendor GmbH, Germany) following the manufacturer’s instructions. Each sample was run in duplicate and the mean concentration was calculated. The lower limit of detection and assay sensitivity were 0.02 ng/mL and 0.5 ng/mL, respectively.
Statistical analysis
The numerical data are expressed as medians with ranges, as not all of the parameters studied followed a normal distribution (Kolmogorov-Smirnov test). Differences in numeric variables were assessed using Mann-Whitney, Friedman’s or Kruskal-Wallis nonparametric two-tailed tests, with post hoc analysis performed (Dunn’s multiple comparison tests) when indicated. Fisher’s exact test was used for the categorical variables. Spearman’s nonparametric correlation was used to describe the relation between two variables. Multiple regression was used to assess the influence of demographic and perinatal characteristics on CC16 levels at T0. To validate the usefulness of CC16 in predicting BPD, receiver operating characteristic (ROC) curves were calculated at the different time points and cut-off levels determined when a significant result was obtained. Values of p < 0.05 were considered to be significant. Statistical analysis was performed by using the Statistical Package for the Social Sciences (SPSS) software for Windows (version 15.0, SPSS, Inc.) and GraphPad InStat.
Results
Forty-one neonates were initially enrolled in the study. Two of them assigned to the NV group were excluded from the final analysis because mechanical ventilation was required after the first 3 days of life, and four from the MV group were excluded due to death prior to the second blood sampling (n = 3) and the diagnosis of congenital heart disease (n = 1). Thus, our study population consisted of 35 neonates assigned to the NV (n = 12) and MV (n = 23) groups, respectively. The surviving neonates had blood sampling at all time points, whereas all of those cases who died had sampling at T0 and T1. The power of the study to detect the observed differences in serum CC16 levels between ventilated and nonventilated preterm neonates was calculated to be 95.4% (at level a = 5% using a two-sided independent samples t-test).
Demographic and perinatal and neonatal characteristics
As shown in Table 1, the two groups differed significantly with respect to GA, BW, intubation in the delivery room and Apgar scores at 1 and 5 min, whereas the remaining demographic and perinatal characteristics were comparable. The cause of acute respiratory failure in the nonventilated neonates was mild RDS (n = 5) and transient tachypnea of the newborn (n = 7). Among those requiring mechanical ventilation, 18 had RDS, three had lung immaturity and two transient tachypnea of the newborn. Five and seven neonates in the NV group needed respiratory support by means of NCPAP and oxygen hood, respectively. Compared to NV, the MV group had significantly higher AaDO2 (249.9 mmHg [40.4–552.6] vs. 102.5 mmHg [61.9–208.2], P = 0.005) at T0. All neonates with RDS within the MV group were treated with exogenous surfactant. Most neonates improved over time, resulting in less mechanical respiratory support; 12 and six neonates assigned to the MV group were on a ventilator at T1 and T2, respectively, whereas none received mechanical ventilation at T3. Three neonates in the MV group required high-frequency oscillatory ventilation as a rescue therapy. All neonates in the NV group survived discharge. However, five (21.7%) neonates in the MV group died of pulmonary causes (n = 2), severe intraventricular haemorrhage (n = 2) and sepsis-necrotising enterocolitis (n = 1) before day 14 of life. In one neonate dying of refractory arterial hypotension, hydrocortisone was given on day 3 of life. No other neonate received inhaled or systematic steroids to facilitate extubation. Of the surviving ventilated neonates, seven (38.9%) developed BPD. No neonate developed BPD in the NV group. Survivors in the MV group with BPD had a significantly higher duration of mechanical ventilation (29 days [9–73] vs. 2 days [0.5–11], P < 0.001), NCPAP (22 days [4–32] vs. 1 day [0.5–16], P = 0.02) and supplemental oxygen (85 days [70–187] vs. 5 days [1.5–48.5], P < 0.001) as compared to those without BPD.
Pneumoprotein serum concentrations
Simple bivariate correlations showed that serum CC16 values at birth (T0) did not correlate significantly with GA, BW and Apgar score at 1 min, but they did have a significant inverse correlation with Apgar score at 5 min (Table 2). As a second step, multiple regression analysis was carried out using all previous variables, including mode of respiratory support, as possible confounders. It was found that GA, Apgar score at 5 min and mechanical ventilation were significant covariates (P < 0.001) of initial CC16 levels (Table 2).
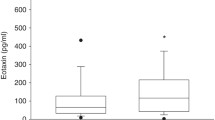
At T0, the CC16 levels were significantly higher in the MV group as compared to the NV group (Fig. 1). On follow-up, the CC16 levels decreased in both groups (Friedman’s test; NV group: P = 0.006; MV group: P < 0.001), although these levels remained significantly higher in the MV group (Fig. 1). Within the MV group, neonates with RDS had higher CC16 concentrations at T0 and at T1, albeit not significantly, as compared to those without RDS (data not shown). Serum CC16 was not correlated with AaDO2, the oxygenation index (MV group) or the mean arterial pressure, as well as creatinine at T0 and T1.
Boxplots of serum CC16 concentrations at the different time points in nonventilated (NV group, n = 12) and mechanically ventilated (MV group, n = 23) preterm neonates. The values at T2 and T3 refer to ventilated survivors (n = 18). The circles and asterisks indicate outliers and extreme values, respectively
In order to investigate the relationship between CC16 levels and BPD development, survivors in the MV group were further divided into two subgroups with or without later BPD. At T0, both subgroups of ventilated neonates had significantly higher serum CC16 as compared to nonventilated cases. However, on follow-up, the differences were significant only between ventilated neonates with later BPD and nonventilated cases at T2 and T3 (Fig. 2). It is worth noting that six out of the seven neonates who developed BPD were still mechanically ventilated at T2.
Boxplots of serum CC16 concentrations at the different time points in nonventilated neonates (n = 12) and the subgroups of surviving ventilated ones who did (n = 7) or did not (n = 11) develop later bronchopulmonary dysplasia (BPD). Kruskal-Wallis with Dunn’s multiple comparison tests; #P < 0.05. The circles and asterisks indicate outliers and extreme values, respectively
Finally, using ROC curve analysis, it was found that only CC16 values of the whole study population on day 14 of life resulted in a significantly large area under the curve (AUC=0.913, P = 0.01) with respect to later BPD. An optimal cut-off level for CC16 at T2 as determined by Youden’s index (0.68) was 10.54 ng/mL, yielding a sensitivity of 85.7% and a specificity of 82.6% in predicting BPD development.
Discussion
The results of this study show that ventilated neonates have significantly increased serum CC16 concentrations early after birth, which remain significantly higher in those subsequently developing BPD.
Studies carried out in animals with acute lung injury [2, 11] and in adults receiving mechanical ventilation for acute respiratory failure have demonstrated increased circulating CC16 attributed to enhanced leakage across the bronchoalveolar–blood barrier [8, 18]. In the only existing relevant study in neonates, however, mechanical ventilation has been associated with a significant rise of serum CC16 in moderately preterm neonates [19]. Furthermore, we demonstrated that the increase of the specific protein in the blood occurred early, even after a short-term exposure of preterm neonates to the injurious effect of mechanical ventilation, thus, confirming its sensitivity in detecting subtle defects in the epithelial permeability [27].
Nevertheless, several other possibilities should be taken into consideration for the differences in serum CC16 levels noted early after birth. For example, these differences could reflect a developmental maturation phenomenon, as ventilated neonates were of smaller gestational age. Previous studies showed that CC16 in rabbit foetal lung [22], human amniotic fluid [4], as well as in tracheal aspirate [17] and serum [19] of neonates, increases as a function of gestational age. In the present study, such a conclusion is verified, as GA is shown to be significantly associated with CC16 levels soon after birth when adjusting for Apgar score at 5 min and ventilatory support. Thus, serum CC16 levels should be lower in more immature neonates. The fact that, however, we found higher serum CC16 in immature ventilated neonates when compared to more mature nonventilated cases, favours the view of a possible contributory role of ventilatory support, including that provided during resuscitation at birth. Nevertheless, it is difficult for the effect of mechanical ventilation on serum CC16 to be distinguished from that of lung immaturity, unless measurements were performed in cord blood, that is, before any respiratory intervention. Such an approach was not selected in the current study due to the possibility of contamination with maternal blood [22]. Considering the causal link between maternal infection/inflammation and premature birth, intrauterine infection could also account for the increased CC16 [23]. In lipopolysaccharide-induced acute lung inflammation, an increased leakage of CC16 in the serum has been documented in animals [2] and humans [20]. Furthermore, neonates with postnatal infections were reported as having elevated CC16 levels in the bronchoalveolar lavage [17]. In the present study, clinical chorioamnionitis and possible or proven neonatal infection were a priori exclusion criteria. An additional confounder is prenatal steroid administration to the mothers. The experimental data showed increased transcriptional up-regulation of CC16 production by pulmonary epithelial cells after glucocorticoid exposure in utero [21]. Moreover, steroids were found to block the release of CC16 in the blood [20], a finding not yet confirmed by others [2]. Interestingly, antenatal and postnatal glucocorticoid administration in neonates was shown not to influence CC16 levels in tracheal aspirates [17] and serum [19]. In this study, the proportion of prenatal steroids was comparably high in both groups, while only one ventilated neonate received steroids postnatally. Lastly, from the analysis of our results, the serum creatinine and mean arterial pressure were found to not correlate with serum CC16, despite data indicating that the blood levels of CC16 depend on its clearance from the circulation [8].
According to our initial hypothesis, circulating CC16 was expected to be higher in ventilated neonates with RDS, simultaneously reflecting derangement in oxygenation. In adults with acute respiratory failure, serum CC16 was found to be related to blood oxygenation [8]. We, indeed, documented higher CC16 levels in ventilated neonates with RDS, although not significantly within the first few hours after birth. This finding should be investigated in larger studies, as the number of ventilated neonates without RDS included in this study was small. However, no association could be demonstrated between serum CC16 and blood oxygenation during the acute phase of RDS. Possibly, the protein, apart from the disrupted alveoli predominately affecting oxygenation, may follow other route(s) for its lung to blood passage, especially when positive pressure ventilation is performed. A possible mechanism may involve destructive injury of the terminal conductive airways at the junction with the gas exchange units.
Further analysis of our results showed that persistently high serum CC16 levels up to day 14 of life were predictive of BPD development. Increased serum CC16 is suggestive of an ongoing injury of the bronchoalveolar–blood barrier. Yet, as the maximal increase of serum CC16 was observed in neonates still receiving ventilation at this time point, this increase reflected ventilator-induced lung damage rather than BPD development per se. From the clinical point of view, the early detection of increased CC16 in serum is important, since neonatologists are given the opportunity to intervene in an attempt to minimise lung injury, e.g. by using more lung-protective ventilatory strategies [1] or certain pharmacological agents [3].
One limitation of this study was the fact that the histological examination of the placenta was not performed and, thus, the possibility of a subclinical infection underlying preterm delivery cannot be ruled out. In addition, the number of enrolled neonates was relatively small, and the use of more sophisticated statistical analyses, such as logistic regression, was, therefore, not possible. Nevertheless, the study had enough power to confidently detect differences in the serum CC16 levels between ventilated and nonventilated preterm neonates.
In conclusion, our data provide evidence that serum CC16 concentrations may be useful as a peripheral blood biomarker in evaluating acute and chronic lung injury in ventilated preterm neonates. The clinical significance of increased serum CC16 for monitoring lung injury in preterm neonates should be validated in larger studies.
Abbreviations
- AaDO2 :
-
Alveolar-arterial difference of oxygen
- BPD:
-
Bronchopulmonary dysplasia
- BW:
-
Birth weight
- CC16:
-
Clara cell 16-kD secretory protein
- FiO2 :
-
Fraction of inspired oxygen
- GA:
-
Gestational age
- MV group:
-
Mechanically ventilated group
- NCPAP:
-
Nasal continuous positive airway pressure
- NV group:
-
Nonventilated group
- PaCO2 :
-
Partial pressure of arterial carbon dioxide
- PaO2 :
-
Partial pressure of arterial oxygen
- RDS:
-
Respiratory distress syndrome
References
Ambalavanan N, Carlo WA (2006) Ventilatory strategies in the prevention and management of bronchopulmonary dysplasia. Semin Perinatol 30:192–199
Arsalane K, Broeckaert F, Knoops B, Wiedig M, Toubeau G, Bernard A (2000) Clara cell specific protein (CC16) expression after acute lung inflammation induced by intratracheal lipopolysaccharide administration. Am J Respir Crit Care Med 161:1624–1630
Baveja R, Christou H (2006) Pharmacological strategies in the prevention and management of bronchopulmonary dysplasia. Semin Perinatol 30:209–218
Bernard A, Thielemans N, Lauwerys R, Langhendries JP, Van Lierde M, Freund MM (1994) Clara cell protein in human amniotic fluid: a potential marker of fetal lung growth. Pediatr Res 36:771–775
Bernard A, Hermans C, Van Houte G (1997) Transient increase of serum Clara cell protein (CC16) after exposure to smoke. Occup Environ Med 54:63–65
Blomberg A, Mudway I, Svensson M, Hagenbjörk-Gustafsson A, Thomasson L, Helleday R, Dumont X, Forsberg B, Nordberg G, Bernard A (2003) Clara cell protein as a biomarker for ozone-induced lung injury in humans. Eur Respir J 22:883–888
Broeckaert F, Bernard A (2000) Clara cell secretory protein (CC16): characteristics and perspectives as lung peripheral biomarker. Clin Exp Allergy 30:469–475
Doyle IR, Hermans C, Bernard A, Nicholas TE, Bersten AD (1998) Clearance of Clara cell secretory protein 16 (CC16) and surfactant proteins A and B from blood in acute respiratory failure. Am J Respir Crit Care Med 158:1528–1535
Dreyfuss D, Saumon G (1998) Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 157:294–323
Hermans C, Bernard A (1999) Lung epithelium-specific proteins: characteristics and potential applications as markers. Am J Respir Crit Care Med 159:646–678
Hermans C, Knoops B, Wiedig M, Arsalane K, Toubeau G, Falmagne P, Bernard A (1999) Clara cell protein as a marker of Clara cell damage and bronchoalveolar blood barrier permeability. Eur Respir J 13:1014–1021
Hermans C, Petrek M, Kolek V, Weynand B, Pieters T, Lambert M, Bernard A (2001) Serum Clara cell protein (CC16), a marker of the integrity of the air–blood barrier in sarcoidosis. Eur Respir J 18:507–514
Hillman NH, Moss TJ, Kallapur SG, Bachurski C, Pillow JJ, Polglase GR, Nitsos I, Kramer BW, Jobe AH (2007) Brief, large tidal volume ventilation initiates lung injury and a systemic response in fetal sheep. Am J Respir Crit Care Med 176:575–581
Jefferies AL, Coates G, O’Brodovich H (1984) Pulmonary epithelial permeability in hyaline-membrane disease. N Engl J Med 311:1075–1080
Jobe A, Jacobs H, Ikegami M, Berry D (1985) Lung protein leaks in ventilated lambs: effects of gestational age. J Appl Physiol 58:1246–1251
Kotecha S, Wilson L, Wangoo A, Silverman M, Shaw RJ (1996) Increase in interleukin (IL)-1 beta and IL-6 in bronchoalveolar lavage fluid obtained from infants with chronic lung disease of prematurity. Pediatr Res 40:250–256
Lassus P, Nevalainen TJ, Eskola JU, Andersson S (2000) Clara-cell secretory protein in preterm infants’ tracheal aspirates correlates with maturity and increases in infection. Pediatr Pulmonol 30:466–469
Lesur O, Langevin S, Berthiaume Y, Légaré M, Skrobik Y, Bellemare JF, Lévy B, Fortier Y, Lauzier F, Bravo G, Nickmilder M, Rousseau E, Bernard A; Critical Care Research Group of the Québec Respiratory Health Network (2006) Outcome value of Clara cell protein in serum of patients with acute respiratory distress syndrome. Intensive Care Med 32:1167–1174
Loughran-Fowlds A, Oei J, Wang H, Xu H, Wimalasundera N, Egan C, Henry R, Lui K (2006) The influence of gestation and mechanical ventilation on serum clara cell secretory protein (CC10) concentrations in ventilated and nonventilated newborn infants. Pediatr Res 60:103–108
Michel O, Murdoch R, Bernard A (2005) Inhaled LPS induces blood release of Clara cell specific protein (CC16) in human beings. J Allergy Clin Immunol 115:1143–1147
Oshika E, Liu S, Ung LP, Singh G, Shinozuka H, Michalopoulos GK, Katyal SL (1998) Glucocorticoid-induced effects on pattern formation and epithelial cell differentiation in early embryonic rat lungs. Pediatr Res 43:305–314
Peri A, Dubin NH, Dhanireddy R, Mukherjee AB (1995) Uteroglobin gene expression in the rabbit uterus throughout gestation and in the fetal lung. Relationship between uteroglobin and eicosanoid levels in the developing fetal lung. J Clin Invest 96:343–353
Romero R, Espinoza J, Chaiworapongsa T, Kalache K (2002) Infection and prematurity and the role of preventive strategies. Semin Neonatol 7:259–274
Speer CP (2003) Inflammation and bronchopulmonary dysplasia. Semin Neonatol 8:29–38
Vento G, Capoluongo E, Matassa PG, Concolino P, Vendettuoli V, Vaccarella C, Frezza S, Zuppi C, Romagnoli C, Ameglio F (2006) Serum levels of seven cytokines in premature ventilated newborns: correlations with old and new forms of bronchopulmonary dysplasia. Intensive Care Med 32:723–730
Ye Q, Fujita M, Ouchi H, Inoshima I, Maeyama T, Kuwano K, Horiuchi Y, Hara N, Nakanishi Y (2004) Serum CC-10 in inflammatory lung diseases. Respiration 71:505–510
Yoshikawa S, King JA, Reynolds SD, Stripp BR, Parker JC (2004) Time and pressure dependence of transvascular Clara cell protein, albumin, and IgG transport during ventilator-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol 286:L604–L612
Acknowledgement
We would like to thank Dr. Christos Nakas for his assistance with the statistical analysis carried out in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarafidis, K., Stathopoulou, T., Diamanti, E. et al. Clara cell secretory protein (CC16) as a peripheral blood biomarker of lung injury in ventilated preterm neonates. Eur J Pediatr 167, 1297–1303 (2008). https://doi.org/10.1007/s00431-008-0712-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-008-0712-3