Abstract
Objective
Injury to the alveolocapillary barrier characterizes ALI/ARDS; therefore determining levels of lung epithelium-specific small proteins in serum may help predict clinical outcomes. We examined whether serum Clara cell protein (CC-16) concentration is correlated with the outcome, mechanical ventilation duration, and incidence of nonpulmonary organ failure.
Design
Prospective multicenter observational study conducted by the Quebec Critical Care Network.
Measurements
Seventy-eight adult ARDS patients requiring mechanical ventilation were enrolled and 28-day mortality was the primary outcome. Ventilatory parameters were computed and blood was sampled daily. Clinical information collected included cause of death, duration of mechanical ventilation, number of ventilator-free days, and organ failures.
Results
Median serum levels of CC-16 were significantly higher in nonsurvivors than survivors on days 0–2 (19.93 μg/l, IQR 11.8–44.32, vs. 8.9, 5.66–26.38) and sustained up to day 14. CC-16 levels were correlated positively with the number of failing organs (ρ = 0.3623) and requirement for prolonged mechanical ventilation. Predictors of patient mortality included age, arterial carbon dioxide partial pressure, CC-16, and APACHE II score (odds ratios 1.35, 1.52, 1.37, 1.159, respectively).
Conclusions
Higher initial CC-16 serum level is associated with increased risk of death, fewer ventilator-free days, and increased frequency of nonpulmonary multiple organ failure. CC-16 is a valuable biomarker of ARDS that may help predict outcome among ARDS patients with high-risk mortality.
Similar content being viewed by others
Introduction
The acute respiratory distress syndrome (ARDS) is a serious clinical condition commonly observed in intensive care units, with an incidence of 5–15 cases per 100,000 per year in developed countries [1]. Until recently ARDS has been associated with mortality rates of 40–60% [1, 2, 3]; however, new ventilatory strategies have resulted in substantial improvements. Investigators of the ARDS Network have demonstrated that a low volume-controlled ventilation allows a decreased overall mortality [4, 5]. This protective ventilatory strategy has led to an increased number of ventilator-free days (VFDs) and reduction in ARDS mortality to less than 40% [4, 5]. Hallmarks of ARDS are lung inflammation and edema with protein leakages [6, 7, 8, 9]. However, the nature of this leakage and its potential utility as a prognostic indicator of clinical outcomes has not been extensively investigated. It is now clear that leakage through a porous alveolar-capillary barrier is bidirectional during ARDS [6, 8, 9]. Of the many biomarkers of protein leakage lung epithelium-specific small proteins are the most relevant for the clinical monitoring of lung injury [6]. Included in this class are the surfactant-associated proteins secreted from type II and Clara epithelial cells [e.g., surfactant proteins (SP) A–D), alveolar type I epithelial membrane proteins (e.g., HI-56), secretory products of type II epithelial cells (e.g., KL-6), and a Clara cell specific protein (CC-16 or CC-10) [6, 10, 11, 12, 13]. Several investigations have validated SP-A, SP-B, SP-D, and KL-6 as important biomarkers of lung injury and clinical outcome in patients with ARDS [6, 10, 11, 12, 13] or as predictor of ARDS onset in at-risk patients [11]. In this context, pneumoproteins such as CC-16 (16 kDa) or SP-B (15kDa) which are similar in molecular size to several locally produced inflammatory mediators (tumor necrosis factor α, interleukin 1β) may follow the same pathway across the alveolar-capillary barrier, helped by a loss of size selectivity from injured lungs [6].
It has been postulated that ventilator-induced lung injury plays a role in the onset of multiple organ dysfunction sometimes progressing to severe multiple organ failure. Two underlying, often coexisting, causes have been proposed: (a) locally produced cytokines and mediators and (2) bacteria, bacteria-derived toxins, and/or other factors released in and translocated from the lung systemically to distal organs, with the loss of compartmentalization seen in ARDS [14, 15, 16]. Subsequent to observations which established epithelial biomarker leakage as a tool for monitoring alveolar-capillary barrier permeability in experimental mechanically ventilated lungs [8] we decided to evaluate this method over the course of human ARDS. The primary purpose of the study was to determine whether baseline blood concentrations of CC-16 is predictive of survival in a cohort of patients with ARDS. Secondary objectives were to examine the correlation between CC-16 baseline values and the number of nonpulmonary organ failures and VFDs during the course of ARDS.
Methods
Study design
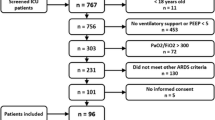
This prospective multicenter cohort study was conducted from 2000 to 2002 at six clinical centers of the Quebec Critical Care Network. The study protocol was approved by the ethics review committees in each of the participating centers, and written consent was obtained from all included patients. All 78 consecutive adult patients 18 years or older requiring assisted mechanical ventilation and meeting the criteria for ARDS according to the North American–European consensus conference (NAECC) definition [17] were eligible. The time window of enrollment for studied patients was within 48 h from the onset of ARDS. An internal control group of ICU ventilated patients at risk of ARDS was constituted for the first 48 h comparison (n = 12: six extrapulmonary sepsis, two hemorrhagic shocks with multiple transfusions, two aspirations of gastric content, and two traumas with multiple fractures). ARDS patients were followed up to 28 days or death. Subset analysis was performed with nonsmokers (never smoking or at least 6-month from cessation) and active smokers.
Study procedures
Blood samples were drawn via an arterial line and sera were stored at –80°C. Serum levels of CC-16 were determined using an automated latex immunoassay [18] whose accuracy was confirmed by comparison with an enzyme-linked immunosorbent assay [19]. For the follow-up CC-16 study the highest value available was taken into account. Because CC-16 half-life is dependent on the glomerular filtration rate, plasma creatinine content was determined by the Jaffe [20] assay.
During the course of the study clinical and prognostic data were collected. Baseline data included demographic information, cause of ARDS, risk factors, Acute Physiology and Chronic Health Evaluation (APACHE) II score at the onset of ARDS, Lung Injury Score (LIS), and causes of death. During follow-up each patient was assessed with regard to ventilatory parameters. Blood samples were taken daily during the first week, every 3 days afterwards, and up to day 28 (when possible) or death. After 28 days of follow-up data were collected on survival, cause of death, duration of mechanical ventilation, number of VFDs, and associated organ failures. In patients who had experienced organ failures data were collected on the day of maximum alterations from the onset of ARDS and maximum alteration of the organ according to the Sequential Organ Failure Assessment (SOFA) score system [21]. Criteria for organ dysfunction were based upon the SOFA score, with level 1 renal failure defined as blood creatinine above 160 μmol/l [21]. All clinical and biological data were analyzed using a computerized informatics application (Epithelial Biomarkers) created especially for this study.
Patients
The mean age of the 78 patients (61.5% men) was 63 ± 16 years (median 55, range 40.5–67). Risk factors of ARDS were: nonpulmonary sepsis (n = 19), pneumonia (n = 40), aspiration (n = 8), trauma (n = 6), acute pancreatitis (n = 3), and other (n = 2). Two-thirds (62.8%) of ARDS cases were from direct causes. Median baseline parameters were: APACHE II score 21 (range 16–26), ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2/FIO2) 97.5 (76.5–150), PaCO2 47.5 mmHg (37–53.25), minute ventilation 10 l/min (8.4–13.6), tidal volume (Vt) 8.4 ml/kg ideal body weight (6.8–10.3), pH 7.34 (7.28–7.42), blood creatinine 98 μmol/l (63–183), LIS 3.25 (2.75–3.5). Baseline characteristics of outcome groups are described in Table 1.
Statistical methods
The relationship between two variables was assessed with the SAS Proc Mixed (SAS version 8.2, SAS Institute, Cary, N.C., USA). Descriptive results are presented as median and interquartile range (IQR). Baseline characteristics of dead and surviving patients were compared using Pearson's χ2 test for proportions and the Mann-Whitney U test for quantitative variables. Each variable with a p value of 0.20 or less on a bivariate analysis was introduced in a backward logistic-regression model. The least significant variable was discarded and the model reconstructed until only significant variables (p ≤ 0.05 with the Wald statistic) were left in the model, and the likelihood ratio test was established. Areas under the receiver operating characteristic (ROC) curves were calculated for the entire model and for each of the predictive variables. Optimal positive and negative likelihood ratios were determined for natural logarithm (Ln) CC-16 because normal distribution was not reached. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) software (version 12, Chicago, Ill., USA). Subsets of patients with different VFDs were compared using the Kruskal-Wallis test with posttest analysis of variance and correlations between parameters were analyzed using Spearman's rank coefficient in a two-tailed test, both for nonparameteric data.
Results
The 28-day survival rate for the cohort was 62% (49/78 patients alive), which was close to the survival rate after release from the intensive care unit (50/78). Causes of death in the 29 nonsurvivors were as follows: 5 terminal respiratory failure, 18 multiple organ failure, 6 other (3 neurological terminal events, 2 cessation of active treatment, 1 refractory shock). Outcome groups' characteristics of organ dysfunction/failure are detailed in Table 2. During the 28-day observation period nine patients were treated with venovenous hemodialysis for renal failure; three had to be treated within the first 2 days. Twenty-one patients received glucocorticoids, nine of whom were treated within the first 2 days for reasons other than ARDS. The underlying conditions for steroid treatment in this latter group included previous obstructive respiratory disease in two, adrenal failure with circulatory shock in five, primary adrenal failure in one, and refractory ARDS in one. Eight patients received nitric oxide (four on days 0–1), ten were turned in prone position (three on days 0–2), and five were given an alveolar recruitment maneuver (40 cmH2O end-inspiratory pressure for 40 s) on days 0–2.
CC-16 and other predictors of survival
Univariate analysis
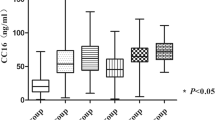
As illustrated in Fig. 1a, serum levels of CC-16 on days 0–2 were significantly higher in patients who died within the observation period (median 8.9 μg/l, IQR 5.66–26.38) than in those who survived (19.93 μg/l, 11.8–44.32; p = 0.01). This difference remained statistically significant when patients with renal failure were excluded from the analysis because of interference between CC-16 half-life and glomerular filtration rate dependency (nonsurvivors 16.32 μg/l, 10.98–43.48 vs. survivors 8.89 μg/l (5.88–19.8; Fig. 1b). Active smokers (n = 34) exhibited initial CC-16 blood contents of 28.1 ± 36.2 μg/l and nonsmokers (n = 40) 29.9 ± 47 μg/l (p = 0.855, 4 missing data). In addition, the active smokers/nonsmokers ratio was 25/20 in survivors and 9/20 in nonsurvivors (p = 0.0388, 4 missing data). While active smoking habit has been reported to decrease serum levels of CC-16, it was neither an independent factor of initial CC-16 blood content nor an indicator of outcome in our study. Serial measurements of CC-16 revealed no statistically significant differences between survivors and nonsurvivors during the two last weeks of observation, but a sustained increased blood content in nonsurvivors during the two first weeks from the onset (p < 0.05; Fig. 1c).
CC-16 blood concentrations on days 0–2 from the onset of ARDS as a function of outcome. a All studied patients. b Patients without renal failure. c Serial changes in CC-16 blood concentrations in survivors vs. nonsurvivors with ARDS. Values per patient-period were the highest observed during the follow-up and arbitrary partitioned in blocks of several-days up to day 28. n Number of patients sampled at the corresponding period. White boxes Survivors; gray boxes nonsurvivors; horizontal line median; box 25th–75th percentile range; error bars 10th–90th percentile range. Mann-Whitney U test, two-tailed
Multivariate analysis
A multivariate analysis determined the following four variables to be predictors of patient mortality: age, PaCO2, Ln (CC-16), and APACHE II score (Table 3). ROC analysis was performed on statistically selected parameters to examine their utility as predictors of death. The ROC is shown in Fig. 2 using the variable Ln (CC-16). Ln CC-16 higher than 2 (7.4 μg/l) yields a sensitivity of 90% and specificity of 60%, and Ln CC-16 higher than 4 (54.6 μg/l) 21% sensitivity and 91% specificity. Other significant independent parameters from the multiple regression analysis are: age (AUC 0.699), APACHE II score (0.704), and PaCO2 (0.634). Data from all four predictors yield an AUC of 0.865. The Hosmer-Lemeshow test indicated that the fit of the model was good (C = 6.97, p = 0.54; H = 2.94, and p = 0.976). The likelihood ratio test indicated that the final model with only four independent variables did not differ significantly from the initial model (χ2 = 7.7, p = 0.320).
Receiver operating characteristic curve relating serum CC-16 concentration (Ln) on days 0–2 of ARDS and survival. Area under the curve (AUC) is 0.684 (0.56–0.803) and represents the fraction of patients who died that would have a positive test: high CC-16 blood concentration, greater than 54.6 μg/l for specificity greater than or equal to 90%. Vertical axis Number of true positive values (sensitivity); horizontal axis number of false positive values (1-specificity); diagonal segments produced by ties
CC-16, mechanical ventilation, and ventilator-free days
The mean ventilation time for the total cohort was 17.6 ± 16 days. The mean number of VFDs was 9.7 ± 1 for the total cohort, comparable to that observed in recent large trials of ARDS [4, 16, 22]. In general, patients experiencing no VFDs died or in rare cases were still receiving assisted ventilation at 28 days. Patients with fewer than 7 VFDs had higher CC-16 values than those with 7–14 days (p = 0.0286) and those with more than 14 days (p = 0.0003; Fig. 3).
Pneumoproteins and nonrespiratory organ failures
There was a direct correlation between CC-16 levels and number of failing organs (Spearman's ρ = 0.3623, 95% confidence interval 0.14–0.55, p = 0.0014, n = 78; Fig. 4). This relationship was maintained when patients with renal failure were excluded from the analysis (Spearman's ρ = 0.24, 95% confidence interval 0.007–0.44, p = 0.0381; Fig. 4).
Relationship between pneumoprotein blood concentrations on days 0–2 and the number of failing organs associated with ARDS during the course of the disease. Failing organs as defined by SOFA score and excluding central nervous system (see Table 2). Including renal failure (gray bars) and excluding renal failure (black bars)
Discussion
This observational cohort study of ARDS patients demonstrates a prognostic value for the pneumoprotein CC-16. Specifically, the higher the initial CC-16 serum levels are after the onset of ARDS, the worse are the associated clinical outcomes. These include increased risk of death, fewer VFDs and increased frequency of nonpulmonary multiple organ failure.
ARDS survival and CC-16
Although no standardized recommendations regarding mechanical ventilation or weaning procedures were provided to participating centers, overall mortality rate in this study was similar to those of other multicenter studies, i.e., 30–40% [4, 16, 23]. A principal observation in this investigation is that early measurement of serum CC-16 is correlated with outcome of patients at the onset of ARDS. This observation is unlikely related to the ventilator setting because baseline selected Vt-normalized to the ideal body weight and minute ventilation were similar in the two outcome groups. CC-16 is a more specific independent predictor of death than age, even when renal failure is discarded from analyses. Lung injury in ARDS induces loss of size selectivity of the alveolar capillary barrier which physiologically restricts 10- to 20-kDa macromolecule transport [6, 8]. Higher serum levels of lung-specific proteins can issue from nonrestrictive pulmonary leakage or increased epithelial cell damage. These pneumoproteins are considered to be sensitive and specific lung biomarkers of alveolar-capillary barrier alteration, particularly in ARDS [6, 10, 11, 12]. CC-16 is secreted by bronchiolar Clara cells in a region commonly affected by ARDS-related diffuse alveolar damage, and leaks across the alveolar-capillary barrier into the bloodstream in a number of inflammatory lung diseases, appearing to be one of the best candidates for monitoring lung hyperpermeability [6, 8]. In addition, higher CC-16 bronchoalveolar lavage fluid values have been reported in ARDS patients with lower concentrations in nonsurvivors [24], suggesting a possible enhanced lung permeability associated with poor outcome.
Several general risk factors as well as non-lung-specific biomarkers of ARDS have also been proposed to help physicians in predicting outcomes [1, 14, 25]. Biomarkers more targeted to pulmonary tissues, but different from CC-16, have also been tested in the effort to predict ARDS outcome. Elevated serum SP-D and KL-6 but not SP-A are good predictors of death in ARDS [12, 13]. However, SP-D, widely expressed in the intestine, can easily translocate from hyperpermeable gut in critical conditions, and KL-6 is a large protein whose modulation of diffusion is not well understood [13]. SP-B looks promising as a predictor of ARDS onset in at-risk patients [11], but there is pending issue as to its value for predicting ARDS outcome.
Mechanical ventilation of ARDS and CC-16
Duration of mechanical ventilation is currently considered a sensitive clinical marker of outcome in critically ill patients [26]. Although this study was not designed to provide specific validation because no standardized procedure of ventilation was mandatory, the average duration of mechanical ventilation and the median number of VFDs reported in this study fall within the range of other investigations [4, 11, 16, 22, 27]. A marker that can predict the required time needed for invasive ventilation could become a valuable component of a multiparameter assessment model to score morbidity index, difficulty and duration of rehabilitation, and cost of care. Specifically, the higher the early CC-16 blood concentration was, the fewer were VFDs in this study, which is consistent with the association reported between mechanical ventilation and CC-16 leakage in a rat model of hyperoxia [8]. High plasma levels of SP-D at baseline were also associated with fewer VFDs in a human ARDS study [12]. Ventilation-dependent PaCO2 was identified in this study as an independent indicator of outcomes and one of the four predictors of death. Indeed, the observed higher baseline PaCO2 in nonsurvivors was disease-dependent rather than related to differing ventilatory parameter settings, because minute ventilations were similar in both outcome groups [28].
Ventilator-associated nonpulmonary organ failure and CC-16
The primary cause of death in this cohort was multiple organ failure, as an independent factor of poor prognosis, a finding consistent with previous studies [4, 12, 25, 29]. There is growing evidence suggesting that bacteria, endotoxins, and inflammatory mediators contained in and/or produced by the lung can leak into systemic bloodstream [15, 16, 30, 31]. Clinical observations together with ex vivo and in vivo experimental models of acute lung injury/ARDS demonstrate that lung-derived overproduction of inflammatory mediators, enhanced by inappropriate mechanical ventilation, can be dispersed systemically into the bloodstream through a loss of compartmentalization, triggering distal organ apoptosis and failure, and increasing mortality [16, 31, 32, 33, 34, 35].
The relationship between early CC-16 blood concentration and frequency of nonpulmonary organ failure in this study remained after discarding potentially confounding data from patients with renal failure, as has been recently reported with SP-D blood levels [12]. Since CC-16 is not committed in acute lung injury/ARDS or multiple organ failure induction but rather is an anti-inflammatory molecule [6, 24], this correlation likely reflects an increased passage of aggressive “companion” mediators (e.g., tumor necrosis factor α, interleukin 1β) which accompany CC-16 across the alveolar-capillary barrier.
Limitations of the present study include the relatively small size of the population studied, a long enrollment period due to the limited number of centers, the uncommon incidence of ARDS, absence of consensus on ventilation and weaning strategies, potential impact of the first ARDS Network publication [4], assessment of only one biomarker, and relative dispersion of CC-16 blood levels between survivors and nonsurvivors. On the other hand, baseline CC-16 blood measurement as a predictor of ARDS patient's outcome adds above and beyond other lung epithelial biomarkers for several reasons: it is (a) more lung-specific than SP-D, (b) easier to measure than SP-B, (c) more accurate than SP-A, and (d) smaller and more sensitive than KL-6. Although glomerular filtration rate limited, CC-16 is a valuable marker of ARDS outcome which can be easily measured with commercially available enzyme-linked immunosorbent assay kits.
In conclusion, higher initial serum concentration of CC-16 is associated with (a) worse clinical outcome in ARDS patients with or without kidney failure, (b) prolonged mechanical ventilation, and (c) increased frequency and severity of nonpulmonary organ failure. CC-16 measurement in addition to other relevant parameters may assist critical care physicians in better predicting mortality among their high-risk ARDS patients. Because CC-16 cutoff predictive values were chosen by a post-hoc analysis and the cohort patient was limited, a larger prospective ARDS patients' study is needed to further recommended CC-16 blood measurement for clinical use.
References
Atabai K, Matthay MA (2002) Acute lung injury and the acute respiratory distress syndrome: definitions and epidemiology. Thorax 57:452–458
Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. N Engl J Med 342:1334–1349
Berthiaume Y, Lesur O, Dagenais A (1999) Treatment of the adult respiratory syndrome: plea for rescue therapy of the alveolar epithelium. Thorax 54:150–160
The ARDS Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Dos Santos CC, Slutsky AS (2000) Mechanisms of ventilator-induced lung injury: a perspective. J Appl Physiol 89:1645–1655
Hermans C, Bernard A (1999) Lung epithelium specific proteins: characteristics and potential applications as markers. Am J Respir Crit Care Med 159:646–678
Dreyfuss D, Saumon G (1998) Ventilator-induced lung injury. Am J Respir Crit Care Med 157:294–323
Lesur O, Hermans C, Chalifour JF, Picotte J, Levy B, Bernard A, Lane D (2003) Pneumoprotein (CC-16) vascular transfer during mechanical ventilation in rats: effect of KGF pretreatment. Am J Physiol Lung Cell Mol Physiol 284:L410–L419
Piantadosi CA, Schwartz DA (2004) The acute respiratory distress syndrome. Ann Intern Med 141:460–470
Doyle IR, Bersten AD, Nicholas TE (1997) Surfactant proteins-A and -B are elevated in plasma of patients with acute respiratory failure. Am J Respir Crit Care Med 156:1217–1229
Bersten AD, Hunt T, Nicholas TE, Doyle IR (2001) Elevated plasma surfactant protein-B predicts development of ARDS in patients with acute respiratory failure. Am J Respir Crit Care Med 164:648–652
Eisner MD, Parsons P, Matthay MA, and the Acute Respiratory Distress Network (2003) Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax 58:983–988
Ishizaka A, Matsuda T, Albertine KH, Koh H, Tasaka S, Hasegawa N, Kohno N, Kotani T, Morisaki H, Takeda J, Nakamura M, Fang X, Martin TR, Matthay MA, Hashimoto S (2004) Elevation of KL-6, a lung epithelial cell marker, in plasma and epithelial lining fluid in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 286:L1088–L1094
Pittet JF, McKersie RC, Martin TR, Matthay MA (1997) Biological markers of ALI: prognostic and pathogenetic significance. Am J Respir Crit Care Med 155:1187–1205
Dreyfuss D, Saumon G (1998) From ventilator-induced lung injury to multiple organ dysfunction? Intensive Care Med 24:102–104
Ranieri MV, Suter PM, Tortorella C, de Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS (1999) Effect of mechanical ventilation on inflammatory mediators in patients with ARDS. a randomized controlled trial. JAMA 282:54–61
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R (1994) The American-European Consensus conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149:818–824
Bernard A, Lauwerys R, Noel A, Vandeleene B, Lambert A (1991) Determination by latex immunoassay of protein 1 in normal and pathological urine. Clin Chim Acta 201:231–245
Hermans C, Osman A, Nyberg BI, Peterson C, Bernard A (1998) Determinants of Clara cell protein (CC-16) concentration in serum: a reassessment with two different immunoassays. Clin Chim Acta 272:101–110
Jaffe M (1886) Über den Niederschlag welchen Pikrinsäure in normalen Harn erzeugt und über eine neue Reaction des Kreatinins. Physiol Chem 10:391–400
Ferreira FL, Bota D, Bross A, Melot C, Vincent JL (2001) Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 286:1754–1758
Zeiher BG, Artigas A, Vincent JL, Dmietrienko A, Jackson K, Thompson BT, Bernard G (2004) Neutrophil elastase inhibition in acute lung injury: result of the STRIVE study. Crit Care Med 32:1695–1702
Jorens PG, Sibille Y, Gulding NJ, van Overveld FJ, Herman AG, Bossaert L, De Backer WA, Lauwerys R, Flower RJ, Bernard A (2005) Potential role of Clara cell protein, an endogenous phospholipase A2 inhibitor, in acute lung injury. Eur Respir J 8:1647–1653
Cheng IW, Ware LB, Greene KE, Nuckton TJ, Eisner MD, Matthay MA (2003) Prognostic value of surfactant proteins A and D in patients with acute lung injury. Crit Care Med 31:20–27
Moran JL, Solomon P, Fox V, Salagaras M, Williams PJ, Quinlan K, Bersten AD (2004) Modelling thirty-day mortality in the ARDS in a adult ICU. Anaesth Intensive Care 32:317–329
Combes A, Costa M-A, Trouillet J-L, Baudot J, Mokhtari M, Gibert C, Chastre J (2003) Morbidity, mortality, and quality-of-life outcomes of patients requiring ≥ 14 days of mechanical ventilation. Crit Care Med 31:1373–1381
Vincent JL, Brase R, Santman F, Suter PM, McLuckie A, Dhainaut JF, Park Y, Karmel J (2001) A multi-centre, double-blind, placebo-controlled study of liposomal prostaglandin E1 (TLC C-53) in patients with ARDS. Intensive Care Med 27:1578–1583
Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, Matthay MA (2002) Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 346:1281–1286
Ferring M, Vincent JL (1997) Is outcome from ARDS related to the severity of respiratory failure? Eur Respir J 10:1297–1300
Slutsky AS, Tremblay LN (1998) Multiple system organ failure. is mechanical ventilation a contributing factor? Am J Respir Crit Care Med 157:1721–1725
Plötz FB, Slutsky AS, van Vught AJ, Heijnen CJ (2004) Ventilator-induced lung injury and multiple system organ failure: a critical review of facts and hypotheses. Intensive Care Med 30:1865–1872
Nahum A, Hoyt J, Schmitz L, Moody J, Shapiro R, Marini JJ (1997) Effect of mechanical ventilation strategy on dissemination of intratracheally instilled Escherichia coli in dogs. Crit Care Med 25:1733–1743
Chiumello D, Pristine G, Slutsky AS (1999) Mechanical ventilation affects local and systemic cytokines in an animal model of ARDS. Am J Respir Crit Care Med 160:109–116
Lin CY, Zhang H, Cheng KC, Slutsky AS (2003) Mechanical ventilation may increase susceptibility to the development of bacteremia. Crit Care Med 31:1429–1434
Imai Y, Parodo J, Kajikawa O, de Perrot M, Fischer S, Edwards V, Cutz E, Liu M, Keshavjee S, Martin TR, Marshall JC, Ranieri VM, Slutsky AS (2003) Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of ARDS. JAMA 289:2104–2112
Acknowledgements
We thank Christine Gambier-Lesur for her assistance in data collection and entry, Deborah Cook for critical reviewing of and advise on the manuscript, and Bruce Wilson and Marilyn Krelenbaum for helping in manuscript edition.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Financial support was provided by the Réseau en Santé Respiratoire du FRSQ (O.L.). O.L. and G.B. are research scholars, and E.R. is a national scholar of the FRSQ.
This article is discussed in the editorial available at: http://dx.doi.org/10.1007/s00134-006-0236-0
Rights and permissions
About this article
Cite this article
Lesur, O., Langevin, S., Berthiaume, Y. et al. Outcome value of Clara cell protein in serum of patients with acute respiratory distress syndrome. Intensive Care Med 32, 1167–1174 (2006). https://doi.org/10.1007/s00134-006-0235-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-006-0235-1