Abstract
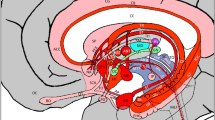
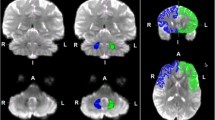
The amygdala is known to have a role in core processes regulated by the limbic system such as motivation, memory, emotion, social behavior, self-awareness as well as certain primitive instincts. Several functional studies have investigated some of these brain tasks of the human limbic system. However, the underlying neuronal fiber connectivity of the amygdalo-diencephalon, as part of the limbic system, has not been delineated separately by prior diffusion-weighted imaging studies. The ability to trace the underlying fiber connections individually will be helpful in understanding the neurophysiology of these tracts in different functions. To date, few diffusion-weighted studies have focused on the amygdala, yet the fine connections of the amygdala, hypothalamus, septum or other adjacent limbic structures have yet to be elucidated by diffusion-weighted tractography studies. We therefore aimed to further investigate these fine neuronal connections using fiber tractography and high spatial resolution diffusion tensor imaging on 3T on 15 healthy right-handed male human subjects (age range 24–37 years). The ventral amygdalofugal pathway, anterior commissure and stria terminalis are the three main efferent pathways of the amygdala. We delineated the detailed trajectories of the ventral amygdalofugal tract, anterior commissure and their connections bilaterally in 15 normal adult human brains. Using a high-resolution diffusion tensor tractography technique, for the first time, we were able to demonstrate the trajectory of amygdalofugal tract and its connections to the hypothalamic and septal nuclei. We further revealed, for the first time, the close relationship of the amygdalofugal tract and anterior commissure with the fornix, stria terminalis and uncinate fasciculus bilaterally in 15 healthy adult human brains.





Similar content being viewed by others
Abbreviations
- AC:
-
Anterior commissure
- AF:
-
Amygdalofugal
- DTI:
-
Diffusion tensor imaging
- DTT:
-
Diffusion tensor tractography
- DWI:
-
Diffusion-weighted imaging
- FACT:
-
Fiber assignment by continuous tracking
- Fx:
-
Fornix
- ROI:
-
Region of interest
- ST:
-
Stria terminalis
- UF:
-
Uncinate fasciculus
References
Allen LS, Gorski RA (1991) Sexual dimorphism of the anterior commissure and massa intermedia of the human brain. J Comp Neurol 312:97–104
Amaral DG (2003) The amygdala, social behavior, and danger detection. Ann N Y Acad Sci 1000:337–347
Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, Blackford JU (2014) BNST neurocircuitry in humans. Neuroimage 2014(91):311–323
Bamiou DE, Sisodiya S, Musiek FE, Luxon LM (2007) The role of the interhemispheric pathway in hearing. Brain Res Rev 56:170–182 [Epub 2007 Jul 17. Review]
Barrick TR, Clark CA (2004) Singularities in diffusion tensor fields and their relevance in white matter fiber tractography. Neuroimage. 22:481–491
Baur V, Hänggi J, Langer N, Jäncke L (2013) Resting-state functional and structural connectivity within an insula-amygdala route specifically index state and trait anxiety. Biol Psychiatry 73:85–92
Bouchard TP, Malykhin N, Martin WR, Hanstock CC, Emery DJ, Fisher NJ, Camicioli RM (2008) Age and dementia-associated atrophy predominates in the hippocampal head and amygdala in Parkinson’s disease. Neurobiol Aging 29:1027–1039
Catani M, Thiebaut de Schotten M (2008) A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex 44:1105–1132
Cattarelli M (1982) The role of the medial olfactory pathways in olfaction: behavioral and electrophysiological data. Behav Brain Res 6:339–364
Coan AC, Morita ME, Campos BM, Bergo FP, Kubota BY, Cendes F (2013) Amygdala enlargement occurs in patients with mesial temporal lobe epilepsy and hippocampal sclerosis with early epilepsy onset. Epilepsy Behav 29:390–394
Cohen MX, Elger CE, Weber B (2008) Amygdala tractography predicts functional connectivity and learning during feedback-guided decision-making. Neuroimage 39:1396–1407
Concha L, Gross DW, Beaulieu C (2005) Diffusion tensor tractography of the limbic system. AJNR Am J Neuroradiol 26:2267–2274
Davis M (1994) The role of the amygdala in emotional learning. Int Rev Neurobiol 1994(36):225–266
Enatsu R, Gonzalez-Martinez J, Bulacio J, Kubota Y, Mosher J, Burgess RC, Najm I, Nair DR (2014) Connections of the limbic network: a corticocortical evoked potentials study. Cortex. doi:10.1016/j.cortex.2014.06.018
Femano PA, Edinger HM, Siegel A (1983) The effects of stimulation of substantia innominata and sensory receiving areas of the forebrain upon the activity of neurons within the amygdala of the anesthetized cat. Brain Res 269:119–132
Fonzo GA, Ramsawh HJ, Flagan TM, Sullivan SG, Simmons AN, Paulus MP, Stein MB (2014) Cognitive-behavioral therapy for generalized anxiety disorder is associated with attenuation of limbic activation to threat-related facial emotions. J Affect Disord 169:76–85
Göttlich M, Krämer UM, Kordon A, Hohagen F, Zurowski B (2014) Decreased limbic and increased fronto-parietal connectivity in unmedicated patients with obsessive-compulsive disorder. Hum Brain Mapp. doi:10.1002/hbm.22574
Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, Orr SP, Kikinis R, Jolesz FA, McCarley RW, Pitman RK (1996) Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry 40:1091–1099
Hasan KM, Kamali A, Kramer LA (2009) Mapping the human brain white matter tracts relative to cortical and deep gray matter using diffusion tensor imaging at high spatial resolution. Magn Reson Imaging 27:631–636
Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, Lozano AM, Mayberg HS (2008) Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex 18:1374–1383
Jones DK (2008) Studying connections in the living human brain with diffusion MRI. Cortex 44:936–952
Josephs KA, Murray ME, Whitwell JL, Parisi JE, Petrucelli L, Jack CR, Petersen RC, Dickson D (2014) Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol 127:441–450
Kamali A, Hasan KM (2014) The importance of using a proper technique and accurate seeding of regions-of-interest in diffusion tensor tractography. J Neurol Sci 339:235–236
Kamali A, Kramer LA, Hasan KM (2010) Feasibility of prefronto-caudate pathway tractography using high resolution diffusion tensor tractography data at 3T. J Neurosci Methods 191:249–254
Kamali A, Flanders AE, Brody J, Hunter JV, Hasan KM (2014a) Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct Funct 219:269–281
Kamali A, Sair HI, Radmanesh A, Hasan KM (2014b) Decoding the superior parietal lobule connections of the superior longitudinal fasciculus/arcuate fasciculus in the human brain. Neuroscience 277:577–583
Kamali A, Yousem DM, Lin DD, Sair HI, Jasti SP, Keser Z, Riascos RF, Hasan KM (2016) Mapping the trajectory of the stria terminalis of the human limbic system using high spatial resolution diffusion tensor tractography. Neurosci Lett. doi:10.1016/j.neulet.2015.09.035
Kooistra CA, Heilman KM (1988) Memory loss from a subcortical white matter infarct. J Neurol Neurosurg Psychiatry 51:866–869
Lanuza E, Font C, Martínez-Marcos A, Martínez-García F (1997) Amygdalo-hypothalamic projections in the lizard Podarcis hispanica: a combined anterograde and retrograde tracing study. J Comp Neurol 384:537–555
McIntosh AM, Maniega SM, Lymer GK, McKirdy J, Hall J, Sussmann JE, Bastin ME, Clayden JD et al (2008) White matter tractography in bipolar disorder and schizophrenia. Biol Psychiatry 64:1088–1092
Miller EJ, Saint Marie LR, Breier MR, Swerdlow NR (2010) Pathways from the ventral hippocampus and caudal amygdala to forebrain regions that regulate sensorimotor gating in the rat. Neuroscience 165:601–611
Mori S, Aggarwal M (2014) In vivo magnetic resonance imaging of the human limbic white matter. Front Aging Neurosci 6:321
Moriarty J, Varma AR, Stevens J, Fish M, Trimble MR, Robertson MM (1997) A volumetric MRI study of Gilles de la Tourette’s syndrome. Neurology 49:410–415
Mufson EJ, Benoit R, Mesulam MM (1988) Immunohistochemical evidence for a possible somatostatin-containing amygdalostriatal pathway in normal and Alzheimer’s disease brain. Brain Res 453:117–128
Neary TJ (1995) Afferent projections to the hypothalamus in ranid frogs. Brain Behav Evol 46:1–13
Noback CR, Strominger NL, Demarest RJ, Ruggiero DA (2005) The human nervous system. Structure and function, 6th edn. Humana Press, New Jersey
Nolte J (2002) The human brain. An introduction to its functional anatomy, 5th edn. Mosby, Missouri
Paré D, Smith Y (1994) GABAergic projection from the intercalated cell masses of the amygdala to the basal forebrain in cats. J Comp Neurol 344:33–49
Post S, Mai JK (1980) Contribution to the amygdaloid projection field in the rat. A quantitative autoradiographic study. J Hirnforsch 21:199–225
Saygin ZM, Osher DE, Augustinack J, Fischl B, Gabrieli JD (2011) Connectivity-based segmentation of human amygdala nuclei using probabilistic tractography. Neuroimage 56:1353–1361
Scalia F, Gallousis G, Roca S (1991) Differential projections of the main and accessory olfactory bulb in the frog. J Comp Neurol 305:443–461
Schmahmann JD, Smith EE, Eichler FS, Filley CM (2008) Cerebral white matter: neuroanatomy, clinical neurology, and neurobehavioral correlates. Ann N Y Acad Sci 1142:266–309
Schultz RT (2005) Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci 23:125–141 (review)
Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A (2007) A validated network of effective amygdala connectivity. NeuroImage 36:736–745
Stephens DN, Duka T (2008) Review. Cognitive and emotional consequences of binge drinking: role of amygdala and prefrontal cortex. Philos Trans R Soc Lond B Biol Sci 363:3169–3179
Stoddard-Apter SL, MacDonnell MF (1980) Septal and amygdalar efferents to the hypothalamus which facilitate hypothalamically elicited intraspecific aggression and associated hissing in the cat. An autoradiographic study. Brain Res 193:19–32
Tanaka Y, Miyazawa Y, Akaoka F, Yamada T (1997) Amnesia following damage to the mammillary bodies. Neurology 48:160–165
Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M, Anderson J, Nutile L, Renshaw P, Weiss R, Becerra L, Borsook D (2010) Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain 133:2098–2114
Usunoff KG, Schmitt O, Itzev DE, Haas SJ, Lazarov NE, Rolfs A, Wree A (2009) Efferent projections of the anterior and posterodorsal regions of the medial nucleus of the amygdala in the mouse. Cells Tissues Organs 190:256–285
Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, Pandya DN, Hagmann P, D’Arceuil H, de Crespigny AJ (2008) Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage 41:1267–1277
Wood RI, Swann JM (2005) The bed nucleus of the stria terminalis in the Syrian hamster: subnuclei and connections of the posterior division. Neuroscience 135:155–179
Zarei M, Patenaude B, Damoiseaux J, Morgese C, Smith S, Matthews PM, Barkhof F, Rombouts SA, Sanz-Arigita E, Jenkinson M (2010) Combining shape and connectivity analysis: an MRI study of thalamic degeneration in Alzheimer’s disease. Neuroimage 49:1–8
Zeineh MM, Holdsworth S, Skare S, Atlas SW, Bammer R (2012) Ultra-high resolution diffusion tensor imaging of the microscopic pathways of the medial temporal lobe. Neuroimage 62:2065–6582
Acknowledgments
This work is funded by the American National Institutes of Health (NIH)-Institute for Neurological Diseases and Stroke (NIH-NINDS: R01-NS052505-04) and the Dunn Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamali, A., Sair, H.I., Blitz, A.M. et al. Revealing the ventral amygdalofugal pathway of the human limbic system using high spatial resolution diffusion tensor tractography. Brain Struct Funct 221, 3561–3569 (2016). https://doi.org/10.1007/s00429-015-1119-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-015-1119-3