Abstract
High-grade osteosarcoma, a primary malignant bone tumour, is experiencing a global increase in reported incidence with varied prevalence. Despite advances in management, which include surgery and neoadjuvant chemotherapy often an unsatisfactory outcome is found due to poor or heterogeneous response to chemotherapy. Our study delved into chemotherapy responses in osteosarcoma patients and associated molecular expressions, focusing on CD95 receptor (CD95R), interferon (IFN)-γ, catalase, heat-shock protein (Hsp)70, and vascular endothelial growth factor (VEGF). Employing immunohistochemistry and Huvos grading of post-chemo specimens, we analysed formalin-fixed paraffin-embedded (FFPE) osteosarcoma tissue of resected post-chemotherapy specimens from Dr. Soetomo General Academic Hospital in Surabaya, Indonesia (DSGAH), spanning from 2016 to 2020. Results revealed varied responses (poor 40.38%, moderate 48.08%, good 11.54%) and distinct patterns in CD95R, IFN-γ, catalase, Hsp70, and VEGF expression. Significant differences among response groups were observed in CD95R and IFN-γ expression in tumour-infiltrating lymphocytes. The trend of diminishing CD95R expression from poor to good responses, accompanied by an increase in IFN-γ, implied a reduction in the count of viable osteosarcoma cells with the progression of Huvos grading. Catalase expression in osteosarcoma cells was consistently elevated in the poor response group, while Hsp70 expression was highest. VEGF expression in macrophages was significantly higher in the good response group. In conclusion, this study enhances our understanding of immune-chemotherapy interactions in osteosarcoma and identifies potential biomarkers for targeted interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-grade osteosarcoma is a primary malignant bone tumour characterized by the formation of immature bone or osteoid. It predominantly occurs in the long bone metaphysis, such as the distal femur, proximal tibia, fibula, proximal humerus, and pelvis, where the epiphyseal growth plate is highly active [1]. The reported incidence of osteosarcoma tends to increase globally, with varying prevalence in different regions [2]. In countries with less developed cancer registries, or public health care, the incidence remains unclear. For instance, a study at Dr. Cipto Mangunkusumo National Central General Hospital, Jakarta, (CMNCGH) reported an average of 16.8 cases per year with a total of 219 cases from 1995—2008 [3]. The National Health Survey in 2018 indicated a cancer prevalence of 1.79 per thousand people in Indonesia [4]. In contrast in the United States, the annual incidence ranges from 4 to 5 cases per million individuals [1].
The management of osteosarcoma involves surgery and chemotherapy. Chemotherapy protocols typically include combinations of cisplatin, doxorubicin, methotrexate, and ifosfamide [5]. Neoadjuvant chemotherapy, given before definitive surgery, often yields unsatisfactory results, with a significant number of patients exhibiting a poor histological response [6, 7].
At CMNCGH, among 20 stage IIB osteosarcoma cases, 12 cases (60%) showed unresponsiveness based on the Huvos grading system I and II during a time window from 1995 to 2008 [3, 8]. International data also reveals variations, with studies indicating substantial rates of unresponsiveness. In the United States, a study found a 31% response rate to induction chemotherapy (MAP regimen involving Methotrexate, Cisplatin, and Doxorubicin) and a 36% response rate with the addition of Ifosfamide (MAPI regimen) [9]. Meta-analyses in China and studies in Korea demonstrated varying response rates to intensified and conventional chemotherapy doses [10,11,12]. Similarly, studies in Tunisia indicated a high percentage (78%) of poor responses to chemotherapy indicated by tumour necrosis < 90% [13]. These findings raise concerns about the potential ability of cancer cells to protect themselves from chemotherapy agents. Osteosarcoma at first sight is not hallmarked by immune infiltrate, though when one observes more carefully, in the background of this tumour numerous macrophages can be found. This is also reflected in the expression signature in cDNA expression arrays [14]. Potentially this pathway can also be used to target the tumour as well as via an augmentation of the immune system for instance via the augmentation of natural killer cell activity [15]. Alternatively, the intact interferon signalling in leukocytes of osteosarcoma patients gives potential adjuvant treatment options [16].
Understanding the mechanisms behind the differences in the response of osteosarcoma to therapy is crucial for determining treatment success. Efforts to investigate these differences are necessary for the development of effective therapies and prognosis determination.
Materials and Methods
In this study, we employed an observational approach with a retrospective design to assess the expression of CD95 receptor (CD95R), interferon (IFN)-γ, catalase, heat-shock protein (Hsp)70, and vascular endothelial growth factor (VEGF) in osteosarcoma patients. The evaluation involved a comparison based on the Huvos grading system (I, II, and III-IV) and immunohistochemical assessment methods. Our analysis utilized stored formalin-fixed paraffin-embedded (FFPE) osteosarcoma tissue from patients diagnosed with osteosarcoma with or without metastasis at DSGAH. Diagnoses were histopathologically confirmed at DSGAH during the 5 years of inclusion from 2016 to 2020.
The criteria for sample selection included lesion location in long bones, surgical procedures involving resection or amputation, receipt of neoadjuvant chemotherapy with Doxorubicin and Cisplatin only, sufficient tumour tissue in paraffin blocks for immunohistochemical examination, and the evaluation of chemotherapy response using the Huvos grading system. Exclusion criteria comprised non-representative samples in terms of the number of cells to be evaluated, biopsy as the sole type of surgery, patients undergoing life-saving amputation without preoperative chemotherapy, and patients receiving radiotherapy. The evaluation of Huvos grading [8] involved categorizing responses as per Garcia-Castelano’s classification [17]. Huvos grade I indicated a poor response with no necrosis or less than 50% necrosis, grade II reflected a moderate response with necrosis ranging from 50 to 90%, grade III signified a good response with necrosis between 90 and 99%, and Grade IV represented a total response with 100% necrosis.
We have evaluated FFPE tissue from 52 osteosarcoma patients treated by surgery both amputation and limb-sparing surgery. No biopsy specimens were used. For the assessment of IFN-γ expression in osteosarcoma cells, positive reactions were determined using anti-human IFN-γ (Cat. No. A12450, ABclonal, Woburn, MA, United States). Similarly, the examination of CD95R expression involved evaluating positive reactions in lymphocyte cytoplasm with the use of anti-CD95R (Cat. No. A19582, ABclonal, Woburn, MA, United States). Catalase activity in the tumour cell cytoplasm was analysed through the application of anti-Catalase (Cat. No. A11220, ABclonal, Woburn, MA, United States). The assessment of Hsp70 expression entailed the examination of positive reactions in the tumour cell cytoplasm using anti-Hsp70 (Cat. No. CM 407 A, Biocare Medical, Concord, CA, United States). Meanwhile, the analysis of VEGF expression involved evaluating positive reactions in macrophage cytoplasm using anti-VEGF (Cat. No. A17877, ABclonal, Woburn, MA, United States). Each section was treated with a labelled antibody, and diaminobenzidine served as the chromogen (Cat. No. BDB2004, Biocare Medical, Pacheco, CA, United States). After counterstaining, the sections underwent dehydration with increasing concentrations of alcohol and were mounted. All quantifications were conducted by evaluating five high-power field hotspots on lymphocytes through an Olympus CX41 light microscope with a magnification of 600 × and on tumour tissue through an Olympus CX41 light microscope with a magnification of 400x. The positive result indicated a continued activity of certain proteins associated with proliferation in malignant cells or mechanism of tumour resistance. Positive staining was only considered when cell bound; indeed there was sometimes some aspecific staining present in the extracellular matrix, but this was not considered positive or relevant.
The normality of the data was examined using the Shapiro–Wilk test. To compare the differences between groups, the Kruskal–Wallis test was employed, followed by Dunn's multiple comparisons tests. Additionally, Spearman's correlation test was executed. A p-value below 0.05 was deemed statistically significant. All statistical analyses were performed using GraphPad Prism for Windows, Version 9.3.0, San Diego, CA, United States.
Results
The characteristics of the observed subjects are presented in Table 1. Chemotherapy responses in osteosarcoma patients were categorized as poor (Huvos grade I) at 40.38%, moderate (Huvos grade II) at 48.08%, and good (Huvos grade III) at 11.54%. The occurrence of osteosarcoma in the subjects of this study was found to be highest in the age range of 10–19 years and lowest in those above 30 years. The results of the Chi-square analysis for gender and age data revealed homogeneity, as evidenced by a p-value > 0.05. This suggests that gender and age did not serve as confounding factors within the scope of this study. Table 2 shows that in the poor response group, the highest number was the chondroblastic variant, with more females than males. This supports previous observations in studies that prove the chondroblastic type osteosarcoma has a poorer histological response to chemotherapy [18, 19]. This was followed by the osteoblastic variant, which was also more common in females. In the moderate response group, the osteoblastic and chondroblastic variants were balanced, with more males than females for both variants, followed by the fibroblastic variant, which was more prevalent in females. In the good response group, the osteoblastic and giant cell-rich variants were equally distributed in males, while in female patients, none of them showed a good response.
Histological examination based on Huvos grading indicates that in Huvos grade I, viable tumour cells constitute 80%. Huvos grade II displays viable tumour cells at 40%, accompanied by a necrotic area of 60%. In Huvos grade III, viable tumour cells are observed at 10%, with a predominant necrotic area of 90% (Fig. 1).
Histopathological examination based on Huvos grading. Huvos grade I shows viable tumour cells at 80% (a). Huvos grade II exhibits viable tumour cells at 40% with a necrotic area of 60% (b). Huvos grade III reveals viable tumour cells at 10% with a necrotic area of 90% (c). Haematoxylin–eosin stain, magnification 400x
Morphological changes that are pronounced in the pre-operative chemotherapy patients’ tissue can be grouped as damaged tumour cells (completely necrotic or degenerative, vascular lesions (engorged, cystic, damaged blood vessels, associated with haemorrhages) and reparative changes involving fibroblast, angioblast, and osteoblast. In the case of complete destruction of tumour cells, the cells are absent or barely visible and sometimes recognised as bizarre cells. Although pre-operative chemotherapy samples show different changes from non-chemotherapy samples, no complete discrimination between those two can be reached. Probably the changes observed in pre-operative chemotherapy samples are a sum of spontaneous and chemotherapy-induced necrosis; we categorized all as the result of the chemotherapy [20].
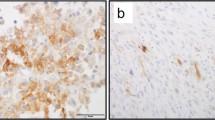
A significant difference (p < 0.05) in CD95R expression in lymphocytes was identified between poor and good chemotherapy responses in osteosarcoma. CD95R expressions were higher in the poor response group (74.09 ± 23.30) compared to the moderate (72.60 ± 17.32) and good (44.53 ± 25.19) response groups, and the moderate response group exhibited higher levels than the good response group (Fig. 2).
Immunohistochemistry of CD95 receptor (CD95R) in osteosarcoma cell. The number of CD95R-expressing cells was evaluated using immunohistochemistry specimens and a light microscope at 400 × magnification. Significant differences were observed between Huvos grade I and Huvos grade III groups (*p < 0.05) (a). The representative immunohistochemistry results illustrate Huvos grade I (b), Huvos grade II (c), and Huvos grade III groups (d). Green arrows indicate positive staining cells
Significant differences (p < 0.05) in IFN-γ expression in lymphocytes were observed among the chemotherapy response groups in osteosarcoma, including between poor and moderate responses, as well as poor and good responses. Higher IFN-γ expressions were noted in the good response group (35.15 ± 11.36) compared to the moderate (22.69 ± 7.439) and poor (16.17 ± 10.66) response groups, and the moderate response group exhibited higher levels than the poor response group (Fig. 3).
Immunohistochemical expression of interferon (IFN)-γ in lymphocytes. The number of IFN-γ-expressing cells was counted using immunohistochemistry specimens and a light microscope at 400 × magnification. Significant differences were observed between groups (*p < 0.05, **p < 0.01) (a). The representative immunohistochemistry results illustrate Huvos grade I (b), Huvos grade II (c), and Huvos grade III groups (d). Green arrows indicate positive staining cells
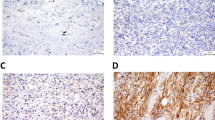
No significant differences (p > 0.05) in catalase expression in osteosarcoma cells were observed among the chemotherapy response groups in osteosarcoma. Catalase expressions were 30.68 ± 24.40 in the poor response group, 30.06 ± 21.95 in the moderate response group, and 32.70 ± 4.856 in the good response group (Fig. 4).
Immunohistochemical expression of catalase in osteosarcoma cells. The number of catalase-expressing cells was evaluated using immunohistochemistry specimens and a light microscope at 400 × magnification. There were no significant differences between groups (a). The representative immunohistochemistry results illustrate Huvos grade I (b), Huvos grade II (c), and Huvos grade III groups (d). Green arrows indicate positive staining cells
A significant difference (p < 0.05) was observed in Hsp70 expression in osteosarcoma cells between poor and moderate chemotherapy responses in osteosarcoma. The highest Hsp70 expression was observed in the poor response group (26.11 ± 18.40) compared to the moderate (17.73 ± 13.58) and good (20.12 ± 3.549) response groups (Fig. 5).
Immunohistochemical expression of heat-shock protein (Hsp)70 in osteosarcoma cells The number of Hsp70-expressing cells was evaluated using immunohistochemistry specimens and a light microscope at 400 × magnification. There was a significant difference between Huvos grade I and Huvos grade II groups (*p < 0.05) (a). The representative immunohistochemistry results illustrate Huvos grade I (b), Huvos grade II (c), and Huvos grade III groups (d). Green arrows indicate positive staining cells
A significant difference (p < 0.05) in VEGF expression in macrophages was found between the poor and good chemotherapy responses in osteosarcoma, with higher VEGF expression in the good response group. VEGF expressions were 18.49 ± 15.80 in the poor response group, 18.77 ± 7.481 in the moderate response group, and 28.45 ± 3.917 in the good response group (Fig. 6).
Immunohistochemical expression of vascular endothelial growth factor (VEGF) in macrophages. The number of VEGF-expressing cells was evaluated using immunohistochemistry specimens and a light microscope at 400 × magnification. There was a significant difference between Huvos grade I and Huvos grade II groups (*p < 0.05) (a). The representative immunohistochemistry results illustrate Huvos grade I (b), Huvos grade II (c), and Huvos grade III groups (d). Green arrows indicate positive staining cells
Discussion
The incidence of osteosarcoma in this study was highest in the age range of 10–19 years and lowest above 30 years. This pattern aligns with another study that noted most osteosarcoma cases typically occur in children aged 12–16 years [19] and aligns with the notion that most osteosarcoma cases develop between the ages of 14–18 years old, corresponding to the acceleration of pubertal growth. The annual incidence rate is 4.4 cases/1 million population for people aged 0–24 years, with males affected more frequently and the M:F ratio 1.3: 1 [21].
Osteosarcoma is highly malignant and shows strong invasive capabilities, progressive disease development, and a very high mortality rate [22]. Before the discovery of chemotherapy in the 1970s, the prognosis for osteosarcoma patients was deemed very poor, with a survival rate of < 20%. The management involving surgical resection with adequate surgical margins combined with neoadjuvant chemotherapy has increased the survival rate to 60–70% and has reached a plateau. The survival rate for patients with localized osteosarcoma is 65%, while for patients with metastatic osteosarcoma, it is < 20% [23, 24]. In selective cases, patients can be rescued by metastasectomy [25].
Recent approaches using multimodal strategies, including systemic chemotherapy before surgery (neoadjuvant chemotherapy), followed by local surgical procedures and supplemented with postoperative chemotherapy (adjuvant chemotherapy), have improved long-term success rates to 60–70%, enhancing overall survival [26]. However, current therapeutic modalities have various disadvantages, such as the lack of a significant improvement in survival rates despite decades of chemotherapy use, and the side effects associated with high-dose chemotherapy. Given the advancements in scientific knowledge, there is a fundamental need to identify new biological indicators for patient prognosis and chemotherapy response detection. The aim is also to develop innovative and specific therapeutic approaches targeting specific molecular targets (targeted therapy) to improve outcomes for osteosarcoma patients with a poor prognosis [27].
Only four chemotherapy agents have shown objective responses in osteosarcoma: Doxorubicin at 43%, Ifosfamide at 33%, Methotrexate at 32%, and Cisplatin at 26%. Studies utilizing three active agents have demonstrated better outcomes than those using only two regimens [28]. The first-line chemotherapy includes Cisplatin and Doxorubicin (category 1), MAP (high-dose Methotrexate, Cisplatin, and Doxorubicin), and the combination of Doxorubicin, Cisplatin, Ifosfamide, and high-dose Methotrexate (MAPI) [5]. In this study, patients received neoadjuvant chemotherapy following the National Comprehensive Cancer Network (NCCN) guidelines, consisting of Cisplatin and Doxorubicin only. The mean 5-year event-free survival (EFS) and 5-year overall survival (OAS) are 48% and 62% for two-drug regimens, and 58% and 70% respectively for three or more drug regimens. Patients treated with chemotherapy have lower 5y-EFS compared to those who are treated with surgery and chemotherapy, showing that patients with localised high-grade osteosarcoma cannot be treated by chemotherapy alone [29]. Intraarterial cisplatin is more effective than methotrexate. Multidrug regimen without methotrexate achieves similar survival rates to methotrexate-based regimens. The meta-analysis on EFS shows significantly improved survival by administering three drug-regimen compared to a two-drug regimen but shows no significant difference with a 4 drug-regimen [29].
The long-term success of traditional chemotherapy and targeted anticancer agents often relies on immunological effects. Immunogenicity results from a combination of antigenicity and adjuvanticity, and many anticancer drugs activate adaptive stress responses in malignant cells, serving as immunological adjuvants [30]. In some malignancies, both innate and adaptive immune cells play a role in the tumour microenvironment, involving natural killer (NK) cells, antigen-presenting cells (APC) like macrophages and dendritic cells (DC), and lymphocytes, effectively controlling tumours [31].
Cell death plays a pivotal role in cancer, encompassing both programmed cell death and non-programmed cell death. Programmed cell death involves apoptosis, autophagy, and necroptosis (programmed necrosis) [32]. The apoptosis process unfolds through two pathways: extrinsic (receptor-mediated apoptosis) and intrinsic (mitochondria-mediated apoptosis) [33]. The extrinsic pathway is initiated by the binding of death receptors, such as CD95R (and analogous receptors like tumour necrosis factor (TNF) receptor 1 and its counterparts), on the plasma membrane with its extracellular ligand, CD95L [32]. Upon stimulation, CD95L binds to CD95R, forming a death signal complex. Following its formation, the death-inducing signalling complex (DISC) initiates, recruiting various proapoptotic factors, including caspase-8. Subsequent cleavage of caspase-8 and effector caspase-3 leads to proteolytic protein breakdown and apoptosis [34,35,36].
In this study, a significant difference was found between poor and good responses, with the highest CD95R expression in poor responses, suggesting that higher CD95R expression corresponds to increased chemotherapy resistance. This contradicts previous studies indicating that low CD95R expression is associated with a worse prognosis in osteosarcoma patients [37]. CD95R expression is inversely related to tumour metastasis potential, with increased CD95R expression correlated with decreased tumour cell metastasis potential [38]. This is supported by studies showing prolonged survival trends in patients with CD95R expression [39].
CD95R, expressed on the tumour cell surface, contributes to immune cell-mediated cytotoxicity. Patients with a poor chemotherapy response have low CD95R levels, making it easier for tumour cells to metastasise. Those with high CD95R levels have the potential for longer survival and a lower probability of death [39]. Another study investigated the effects of Doxorubicin and Edelfosine lipid nanoparticles on osteosarcoma. The study revealed that this chemotherapy combination increased CD95R expression on tumour cell surfaces, triggering apoptosis [40].
CD95R is associated with the TNF receptor family and mediates apoptosis when bound to its natural ligand, CD95L (CD178/TNFSF6), or stimulated with agonistic antibodies [35]. CD95L is predominantly expressed in activated T lymphocytes and NK cells, the primary subset of innate immune cells responsible for antiviral and antitumor responses [41, 42]. NK cells, defined as CD56+/CD3.− lymphocytes, exhibit cytotoxicity, and cytokine production, particularly in response to exogenous cytokines like IL-2, IL-12, IL-15, IL-18, and IL-21 [43,44,45,46]. Among the most prominent cytokines produced by NK cells are TNF-α and IFN-γ [47].
IFN-γ, a crucial cytokine, enhances tumour cell death mediated by CD95R, playing a vital role in immunomodulation and antitumor activity [48]. IFN-γ is an immunomodulatory cytokine that promotes apoptosis directly or through induced lipid peroxidation and ferroptosis, contributing to an effective antitumor immune response [49]. This is supported by a study that evaluated the influence of IFN-γ on ligand expression in various NK cells on cancer cells, with none of these ligands being downregulated by IFN-γ. However, CD274/PD-L1, CD54/ICAM-1, HLA-DR, MHC class I, CD95/FasR, and CD270/HVEM are regulated in various tumour types [34]. More specifically, in osteosarcoma, a study mentioned that IFN-γ sensitizes osteosarcoma cells to CD95R, inducing apoptosis through the upregulation of CD95R and caspase-8 [50, 51].
This study revealed significant differences in IFN-γ expression among chemotherapy response groups. This suggests that IFN-γ might play a key role in the effectiveness of chemotherapy, with lower levels associated with increased resistance. The results are in line with a previous study that suggested a correlation between elevated PSMD14 expression and a higher-risk category (younger age group, ≤ 20.83 years of age), metastasis within five years, and a higher tumour grade in osteosarcoma patients [37]. PSMD14 is a component of the 26S proteasome. The group with increased PSMD14 expression exhibited decreased immune responses, including reduced levels of IFN-γ [52].
A previous study emphasized the significant contribution of IFN-γ to antitumor and antimetastatic effects, triggering FAS ligand formation and apoptosis induction in cancer cells [50]. Interestingly, previous studies highlighted the positive impact of chemotherapy on IFN-γ levels, demonstrating its correlation with good treatment response in osteosarcoma [53]. Additionally, a previous study underscores the potential preventive role of NK cells in osteosarcoma development, as evidenced by higher circulating NK cell counts in normal controls compared to osteosarcoma patients [54, 55]. Natural Killer (NK) cells release IFN-γ as one of the most potent effector cytokines [47].
Our study suggests that in a good response, the immune system remains resilient against chemotherapy, marked by an increase in IFN-γ. The phenomenon of decreasing CD95R expression from poor to good responses, accompanied by an increase in IFN-γ, suggests a declining number of viable osteosarcoma cells as the Huvos grading advances.
Excessive reactive oxygen species (ROS) leading to oxidative stress plays a pivotal role in carcinogenesis [56]. Cells maintain intracellular homeostasis by developing an extensive antioxidant system, including catalase, superoxide dismutase (SOD), and glutathione peroxidase (GPx). Catalase facilitates the decomposition of hydrogen peroxide into water and oxygen (2H2O2 → 2H2O + O2), crucial for degrading H2O2 and preserving cells against oxidative damage. The expression of catalase varies in tumour cells [57]. Low catalase expression correlates with high H2O2 production, influencing signalling pathway activation to induce proliferation, migration, and invasion in cancer cells [58,59,60]. Changes in catalase expression after short-term H2O2 exposure are influenced by factors such as exposure time, H2O2 concentration, basal antioxidant enzyme capacity, and the cellular model used [57]. High catalase expression has been observed in certain human cancer cell lines, including gastric cancer exposed to cisplatin chemotherapy [61].
No significant difference in catalase expression was observed among the chemotherapy response groups, implying that catalase may not play a decisive role in chemotherapy outcomes. Similarly, a previous study conducted on male patients under 20 years old with osteosarcoma did not find a significant association between catalase level and chemotherapy response [62]. However, its consistently elevated expression in poor responders raises the need for further investigation. Catalase expression did not exhibit the capacity to protect against ROS attacks induced by chemotherapy.
The role of Hsp in maintaining cellular homeostasis and protection is vital, with their heightened production occurring under stressful conditions [63]. These proteins play a crucial role in safeguarding cells from stress-related injuries and are often overexpressed in various malignancies [64]. Among them, Hsp70, an ATP-dependent molecular chaperone, regulates protein conformation, stability, and interactions, including essential proteins for cell survival, growth, and immune responses [65, 66]. Excessive Hsp70 expression in cancer cells is implicated in various oncogenic events, such as anti-apoptotic responses, antitumor immune responses, tumour growth, and cell migration [67].
The poor response group exhibited the highest Hsp70 expression. Previous studies on Hsp in osteosarcoma revealed prognostic associations, with Hsp27, Hsp60, and Hsp70 linked to poor prognosis [68]. Additionally, Hsp27 and Hsp70 were identified as potential markers to distinguish conventional and low-grade central osteosarcoma [64]. Lower Hsp70 expression correlated with higher tumour cell necrosis rate (TCNR), indicating its utility in predicting and evaluating the neoadjuvant chemotherapy's effectiveness in inhibiting osteosarcoma cell proliferation [69]. These findings contribute valuable insights into the complex interplay of Hsp70 in osteosarcoma and its response to chemotherapy, providing avenues for further study and potential therapeutic interventions.
Angiogenesis is a crucial aspect of tumorigenesis, influencing tumour growth and metastatic potential [70]. Vascular endothelial growth factor (VEGF), a key player in angiogenesis and vasculogenesis, primarily acts on various cell types, particularly endothelial cells [71]. VEGF is considered a major mediator of angiogenesis and has been implicated in carcinogenesis and metastasis [72]. Tumour malignancy requires a persistent supply of new blood vessels to sustain growth and facilitate metastasis [73]. In osteosarcoma, VEGF-A expression, as demonstrated by immunohistochemistry, has been associated with a higher risk of lung metastasis and poorer survival [74].
In our study, VEGF expression exhibited distinct patterns, notably higher in good response cases compared to both moderate and poor responses. This variability may be attributed to significant cell death in osteosarcoma, hindering the formation of new blood vessels. Despite numerous studies exploring the correlation between VEGF overexpression and clinical outcomes in osteosarcoma patients, the results remain inconclusive. Meta-analyses assessing the connection between VEGF expression and overall survival in osteosarcoma patients have consistently indicated that elevated VEGF expression is associated with poorer overall survival, with no significant heterogeneity among studies [72]. The positive expression of VEGF in surviving tumour cells following neoadjuvant chemotherapy emerges as a prognostic factor in osteosarcoma, offering valuable insights for identifying potential angiogenic targets in the pursuit of personalized future therapies [75].
We studied the expression on resected post-chemo specimens. Osteosarcoma is an extremely heterogeneous tumour and diagnostic (core-needle) biopsies are only small specimens, which are taken for diagnosis and do not necessarily reflect the expression of molecules throughout the whole tumour. Our study focuses on the response to chemotherapy correlated with immunological response. Including paired biopsy specimens would not result in reliable data given the tumour heterogeneity, so the results reflect the expression profile of the surviving cells post-chemotherapy. Pre-chemo expression in the tumours and changes over time during chemotherapy treatment are not assessed in this study.
The novelty of this study lies in the conventional chemotherapy's mode of action, typically involving DNA synthesis inhibition through folate metabolism disruption (methyl tetrahydrofolate). However, it appears that chemotherapy can also influence the immune response, as evidenced by the findings of increased IFN-γ expression and decreased CD95R expression in a good response to chemotherapy.
Considering our findings, suggestions for further study and clinical implications emerge. There is a need for further investigations to delve into the specific functions of lymphocytes in the pathogenesis of osteosarcoma malignancy. Understanding the intricate interplay between the immune system and osteosarcoma progression can pave the way for targeted therapeutic interventions and personalized treatment strategies. Expanding our knowledge in these areas will undoubtedly contribute to refining prognostic assessments and advancing the development of more effective therapeutic approaches for osteosarcoma patients.
Conclusion
Our study on osteosarcoma chemotherapy responses and the associated molecular expressions provides valuable insights into mechanisms of resistance and potential routes for exploration for additional treatment options. The findings contribute to the growing understanding of the complex interactions between the immune system, antioxidants, and chemotherapy responses in osteosarcoma patients. The identification of specific biomarkers associated with treatment response provides a foundation for potential targeted therapeutic interventions in the pursuit of improved patient outcomes.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
References
Kumar V, Abbas A, Aster JC (2017) Robbins & Cotran Pathologic Basis of Disease, 10th ed. Elsevier. Amsterdam
Mirabello L, Troisi RJ, Savage SA (2009) Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the surveillance, epidemiology, and end results program. Cancer 115:1531–1543. https://doi.org/10.1002/cncr.24121
Kamal AF, Ismail I, Mi’raj F, Hutagalung EU (2011) Outcomes of stage IIB osteosarcoma treated by limb salvage surgery using extracorporeally irradiated (ECI) autograft. Med J Indonesia 20:131–137
Basic Health Research (2018) Riskesdas national report 2018, health research and development agency 198
Yu D, Zhang S, Feng A, Xu D, Zhu Q et al (2019) Methotrexate, doxorubicin, and cisplatinum regimen is still the preferred option for osteosarcoma chemotherapy: a meta-analysis and clinical observation. Medicine 98(19):e15582. https://doi.org/10.1097/MD.0000000000015582
Berner K, Johannesen TB, Berner A et al (2015) Time-trends on incidence and survival in a nationwide and unselected cohort of patients with skeletal osteosarcoma. Acta Oncol 54:25–33. https://doi.org/10.3109/0284186X.2014.923934
Maleki Dana P, Sadoughi F, Asemi Z, Yousefi B (2021) Anti-cancer properties of quercetin in osteosarcoma. Cancer Cell Int 21:1–9. https://doi.org/10.1186/s12935-021-02067-8
Huvos AG (1990) Bone Tumors: Diagnosis, Treatment and Prognosis. WB Saunders, Philadelphia, USA
Schwartz CL, Wexler LH, Krailo MD et al (2016) Intensified Chemotherapy With Dexrazoxane Cardioprotection in Newly Diagnosed Nonmetastatic Osteosarcoma: A Report From the Children’s Oncology Group. Pediatr Blood Cancer 63:54–61. https://doi.org/10.1002/pbc.25753
Zhang Y, He Z, Duan Y et al (2018) Does intensified chemotherapy increase survival outcomes of osteosarcoma patients? A meta-analysis. J Bone Oncol 12:54–60. https://doi.org/10.1016/j.jbo.2018.04.001
Kang JM, Ju HY, Joo J, Sung JY, Park SY, Kim JH et al (2020) Histologic response and toxicity following interval-compressed four-drug therapy given preoperatively in children and young adults with Osteosarcoma: a retrospective study. Oncology 98(2):81–90. https://doi.org/10.1159/000502548
Kim SH, Shin KH, Moon SH et al (2017) Location of residual viable tumor cells after neoadjuvant chemotherapy: A new concept with high prognostic performance in osteosarcoma. J Surg Oncol 115:752–759. https://doi.org/10.1002/jso.24571
Letaief F, Khrouf S, Yahiaoui Y et al (2020) Prognostic factors in High-Grade Localized Osteosarcoma of the Extremities: The Tunisian Experience. J Orthop Surg 28(3):2309499020974501. https://doi.org/10.1177/2309499020974501
Buddingh EP, Kuijjer ML, Duim RAJ et al (2011) Tumor - Infiltrating Macrophages Are Associated with Metastasis Suppression in High Grade Osteosarcoma: A Rationale for Treatment with Macrophage Activating Agents. Clin Cancer Res 17:2110–2119. https://doi.org/10.1158/1078-0432.CCR-10-2047
Pahl JHW, Ruslan SEN, Buddingh EP et al (2012) Anti-EGFR Antibody Cetuximab Enhances the Cytolytic Activityof Natural Killer Cells toward Osteosarcoma. Clin Cancer Res 18:432–441. https://doi.org/10.1158/1078-0432.CCR-11-2277
Buddingh EP, Ruslan SEN, Berghuis D et al (2012) Intact Interferon signaling in peripheral blood leukocyte of high-grade osteosarcoma patients. Cancer Immunol Immunother 61:941–947. https://doi.org/10.1007/s00262-012-1232-6
García-Castellano JM, Atallah Yordi N, Reyes C, Healey JH (2012) Histopathologic and radiologic assessment of chemotherapeutic response in Ewing’s sarcoma: a review. Sarcoma 2012:357424. https://doi.org/10.1155/2012/357424
Smeland S, Bielack SS, Whelan J, Bernstein M, Hoogendoorn P, Krailo MD, Gorlick R, Janeway KA, Ingleby FC, Anninga J, Antal I, Arndt C, Brown KLB, Butterfass-Bahloul T, Calaminus G, Capra M, Dhooge C, Eriksson M, Flanagan AM, Friedel G, Gebhardt MC, Gelderblom H, Goldsby R, Grier HE, Grimer R, Hawkins DS, Hecker-Nolting S, Sundby Hall K, Isakoff MS, Jovic G, Kühne T, Kager L, von Kalle T, Kabickova E, Lang S, Lau CC, Leavey PJ, Lessnick SL, Mascarenhas L, Mayer-Steinacker R, Meyers PA, Nagarajan R, Randall RL, Reichardt P, Renard M, Rechnitzer C, Schwartz CL, Strauss S, Teot L, Timmermann B, Sydes MR, Marina N (2019) Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in EURAMOS-1 (European and American Osteosarcoma Study) cohort. Europ J Cancer 109:36–50. https://doi.org/10.1016/j.ejca.2018.11.027
Hauben EI, Weeden S, Pringle J et al (2002) Does the histological subtype of high-grade central osteosarcoma influence the response to treatment with chemotherapy and does it affect overall survival? A study on 570 patients of two consecutive trials of the European Osteosarcoma Intergroup. Eur J Cancer 38:1218–1225. https://doi.org/10.1016/S0959-8049(02)00037-0
Misdorp W, Hart G, Delemarre JFM, Voute PA, Van Der Eijken JW (1988) An analysis of spontaneous and chemotherapy-associated changes in skeletal osteosarcomas. J Pathol 156(2):119–128
WHO Classification of Tumours Editorial Board (2020) Soft Tissue and Bone Tumours WHO Classification of Tumors, 5th edn. International Agency for Research on Cancer, Lyon, France
Zhao X, Wu Q, Gong X et al (2021) Osteosarcoma: a review current and future therapeutic approaches. Biomed Eng Online 20:1–14. https://doi.org/10.1186/s12938-021-00860-0
Buddingh EP, Schilham MW, Ruslan SEN et al (2011) Chemotherapy-resistant osteosarcoma is highly susceptible to IL-15-activated allogenic and autologous NK cells. Cancer Immunol Immunother 60:575–586. https://doi.org/10.1007/s00262-010-0965-3
Miwa S, Takeuchi A, Ikeda H et al (2013) Prognostic Value of Histological Response to Chemotherapy in Osteosarcoma Patients Receiving Tumor-Bearing Frozen Autograft. PLoS ONE 8:e71362. https://doi.org/10.1371/journal.pone.0071362
Buddingh EP, Anninga JK, Versteegh MIM et al (2010) Prognostic factors in pulmonary metastasized high-grade osteosarcoma. Pediatr Blood Cancer 54:216–221. https://doi.org/10.1002/pbc.22293
Durfee RA, Mohammed M, Luu HH (2016) Review of Osteosarcoma and Current Management. Rheumatol Ther 3(2):221–243. https://doi.org/10.1007/s40744-016-0046-y
Fernandes I, Melo-Alvim C, Lopes-Brás R et al (2021) Osteosarcoma pathogenesis leads the way to new target treatments. Int J Mol Sci 22:1–19. https://doi.org/10.3390/ijms22020813
Meyers PA (2015) Systemic Therapy for Osteosarcoma and Ewing Sarcoma. American Society of Clinical Oncology Educational Book. e644-e647. https://doi.org/10.14694/EdBook_AM.2015.35.e644
Anninga JK, Gelderblom H, Fiocco M et al (2011) Chemotherapeutic adjuvant treatment for osteosarcoma: Where do we stand? Eur J Cancer 47(16):2431–2445. https://doi.org/10.1016/j.ejca.2011.05.030
Galluzzi L, Buqué A, Kepp O et al (2015) Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 28:690–714. https://doi.org/10.1016/j.ccell.2015.10.012
Gajewski TF, Schreiber H, Fu YX (2013) Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 14:1014–1022. https://doi.org/10.1038/ni.2703
Ouyang L, Shi Z, Zhao S et al (2012) Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif 45:487–498. https://doi.org/10.1111/j.1365-2184.2012.00845.x
Circu ML, Aw TY (2010) Reactive oxygen species, cellular redox systems, and apoptosis. Free Rad Biol Med 48:749–762. https://doi.org/10.1016/j.freeradbiomed.2009.12.022c
Kuo HM, Tseng CC, Chen NF, et al (2018) MSP-4, an Antimicrobial Peptide, Induces Apoptosis via Activation of Extrinsic Fas/FasL- and Intrinsic Mitochondria-Mediated Pathways in One Osteosarcoma Cell Line. Marine Drugs 16(1). https://doi.org/10.3390/md16010008
Peter ME, Hadji A, Murmann AE et al (2015) The role of CD95 and CD95 ligand in cancer. Cell Death Differ 22:549–559. https://doi.org/10.1038/cdd.2015.3
Qadir AS, Ceppi P, Brockway S et al (2017) CD95/Fas Increases Stemness in Cancer Cells by Inducing a STAT1-Dependent Type I Interferon Response. Cell Rep 18:2373–2386. https://doi.org/10.1016/j.celrep.2017.02.037
Kansara M, Teng MW, Smyth MJ, Thomas DM (2014) Translational biology of osteosarcoma. Nat Rev Cancer 14:722–735. https://doi.org/10.1038/nrc3838
Gordon N, Kleinerman ES (2009) The role of Fas/FasL in the Metastatic Potential of Osteosarcoma and Targeting this Pathway for the Treatment of Osteosarcoma lung metastases. Pediatric and Adolescent Osteosarcoma: 497–508. https://doi.org/10.1007/978-1-4419-0284-9_29
Casanova JM, Almeida JS, Reith JD et al (2021) Tumor-infiltrating lymphocytes and cancer markers in osteosarcoma: Influence on patient survival. Cancers (Basel) 13(23):6075. https://doi.org/10.3390/cancers13236075
González-Fernández Y, Imbuluzqueta E, Zalacain M et al (2017) Doxorubicin and edelfosine lipid nanoparticles are effective acting synergistically against drug-resistant osteosarcoma cancer cells. Cancer Lett 388:262–268. https://doi.org/10.1016/j.canlet.2016.12.012
Strauss G, Lindquist JA, Arhel N et al (2009) CD95 co-stimulation blocks activation of naive T cells by inhibiting T cell receptor signaling. J Exp Med 206:1379–1393. https://doi.org/10.1084/jem.20082363
Perera Molligoda Arachchige AS (2021) Human NK cells: From development to effector functions. Innate Immun 27:212–229. https://doi.org/10.1177/17534259211001512
Aquino-López A, Senyukov VV, Vlasic Z et al (2017) Interferon gamma induces changes in Natural Killer (NK) cell ligand expression and alters NK cell-mediated lysis of pediatric cancer cell lines. Front Immunol 8:391. https://doi.org/10.3389/fimmu.2017.00391
Kim TD, Lee SU, Yun S et al (2011) Human microRNA-27a*targets Prf1 and GzmB expression to regulate NK-cell cytotoxicity. Blood 118:5476–5486. https://doi.org/10.1182/blood-2011-04-347526
Bryceson YT, Chiang SCC, Darmanin S et al (2011) Molecular mechanisms of natural killer cell activation. J Innate Immun 3:216–226. https://doi.org/10.1159/000325265
Seo H, Jeon I, Kim BS et al (2017) IL-21-mediated reversal of NK cell exhaustion facilitates anti-Tumour immunity in MHC class I-deficient tumours. Nat Commun 8:1–14. https://doi.org/10.1038/ncomms15776
Fauriat C, Long EO, Ljunggren HG, Bryceson YT (2010) Regulation of human NK-cell cytokine and chemokine production by target cell recognition. Blood 115:2167–2176. https://doi.org/10.1182/blood-2009-08-238469
Shadrin N, Saphira MG, Khalfin B, Uppalapati L, Parola AH, Nathan I (2015) Serine protease inhibitors interact with IFN-γ through upregulation of FasR, a novel therapeutic strategy against cancer. Exp Cell Res 330:223–239. https://doi.org/10.1016/j.yexcr.2014.11.005
Jorgovanovic D, Song M, Wang L, Zhang Y (2020) Roles of IFN-γ in tumor progression and regression: a review. Biomarker Research. https://doi.org/10.1186/s40364-020-00228-x
Li Z, Xu Q, Peng H et al (2011) IFN-γ enhances HOS and U2OS cell lines susceptibility to γδ T cell-mediated killing through the Fas/Fas ligand pathway. Int Immunopharmacol 11:496–503. https://doi.org/10.1016/j.intimp.2011.01.001
Inaba H, Glibetic M, Buck S et al (2004) Interferon-γ sensitizes osteosarcoma cells to Fas-induced apoptosis by up-regulating Fas receptors and caspase-8. Pediatr Blood Cancer 43:729–736. https://doi.org/10.1002/pbc.20151
Gong Y, Wei ZR (2022) Identification of PSMD14 as a potential novel prognosis biomarker and therapeutic target for osteosarcoma. Cancer Reports 5(7):e1522. https://doi.org/10.1002/cnr2.1522
Kawano M, Tanaka K, Itonaga I et al (2016) Dendritic cells combined with doxorubicin induces immunogenic cell death and exhibits antitumor effects for osteosarcoma. Oncol Lett 11:2169–2175. https://doi.org/10.3892/ol.2016.4175
Tarek N, Lee DA (2014) Natural killer cells for osteosarcoma. Adv Experimantal Med Biol 804:341–353. https://doi.org/10.1007/978-3-319-04843-7_19
Markiewicz K, Zeman K, Kozar A et al (2012) Evaluation of selected parameters of cellular immunity in children with osteosarcoma at diagnosis. Medicyna wieku rozwojoweko 16:212–221
Quan X, Lim SO, Jung G (2011) Reactive oxygen species downregulate catalase expression via methylation of a CpG Island in the Oct-1 promoter. FEBS Lett 585:3436–3441. https://doi.org/10.1016/j.febslet.2011.09.035
Glorieux C, Zamocky M, Sandoval JM et al (2015) Regulation of catalase expression in healthy and cancerous cells. Free Radical Biol Med 87:84–97. https://doi.org/10.1016/j.freeradbiomed.2015.06.017
Wu WS (2006) The signaling mechanism of ROS in tumor progression. Cancer and Metastasis Review 25:695–705. https://doi.org/10.1007/s10555-006-9037-8
Wu D, Yotnda P (2011) Production and detection of reactive oxygen species (ROS) in cancers. Journal of Visualized Experiments (57):3357. https://doi.org/10.3791/3357
Sen S, Kawahara B, Chaudhuri G (2012) Maintenance of higher H2O2 levels, and its mechanism of action to induce growth in breast cancer cells: Important roles of bioactive catalase and PP2A. Free Radical Biol Med 53:1541–1551. https://doi.org/10.1016/j.freeradbiomed.2012.06.030
Xu H, Choi SM, An CS et al (2005) Concentration-dependent collateral sensitivity of cisplatin-resistant gastric cancer cell sublines. Biochem Biophysical Res Comm 328:618–622. https://doi.org/10.1016/j.bbrc.2005.01.015
Mustokoweni S, Hidayat M, Kalim H, Mintaroem K, Fitri LE (2023) Catalase-expressing cell count and necrotic area in the chemotherapeutic response of osteosarcoma. Europ Chem Bulletin 12(10):8441–8450. https://doi.org/10.4804/ecb/2022.12.10.594
Wang X, Xie L, Zhu L (2021) Clinicopathological significance of HSP70 expression in gastric cancer: a systematic review and meta-analysis. BMC Gastroenterol 21(1):1–12. https://doi.org/10.1186/s12876-021-01990-4
Moon A, Bacchini P, Bertoni F et al (2010) Expression of heat shock proteins in osteosarcomas. Pathology 42:421–425. https://doi.org/10.3109/00313025.2010.493866
Ding L, He S, Sun X (2014) HSP70 desensitizes osteosarcoma cells to baicalein and protects cells from undergoing apoptosis. Apoptosis 19:1269–1280. https://doi.org/10.1007/s10495-014-0995-y
Goloudina AR, Demidov ON, Garrido C (2012) Inhibition of HSP70: a challenging anti-cancer strategy. Cancer Lett 325:117–124. https://doi.org/10.1016/J.CANLET.2012.06.003
Elmallah MIY, Cordonnier M, Vautrot V (2020) Membrane-anchored heat-shock protein 70 (Hsp70) in cancer. Cancer Lett 469:134–141. https://doi.org/10.1016/J.CANLET.2019.10.037
Uozaki H, Ishida T, Kakiuchi C et al (2000) Expression of heat shock proteins in osteosarcoma and its relationship to prognosis. Pathol Res Pract 196:665–673. https://doi.org/10.1016/S0344-0338(00)80118-1
Ji F, Lv R, Zhao T (2017) A correlation analysis between tumor imaging changes and p-AKT and HSP70 expression in tumor cells after osteosarcoma chemotherapy. Oncol Lett 14:6749–6753. https://doi.org/10.3892/ol.2017.7005
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: The next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Assi T, Watson S, Samra B, et al (2021) Targeting the vegf pathway in osteosarcoma. Cells 10. https://doi.org/10.3390/cells10051240
Yu XW, Wu TY, Yi X et al (2014) Prognostic significance of VEGF expression in osteosarcoma: A meta-analysis. Tumor Biology 35:155–160. https://doi.org/10.1007/s13277-013-1019-1
Yang SY, Yu H, Krygier JE, et al (2007) High VEGF with rapid growth and early metastasis in a mouse osteosarcoma model. Sarcoma 2007. https://doi.org/10.1155/2007/95628
Yang J, Yang D, Sun Y et al (2011) Genetic amplification of the vascular endothelial growth factor (VEGF) pathway genes, including VEGFA, in human osteosarcoma. Cancer 117:4925–4938. https://doi.org/10.1002/cncr.26116
Bajpai J, Sharma M, Sreenivas V et al (2009) VEGF expression as a prognostic marker in osteosarcoma. Pediatr Blood Cancer 53:1035–1039. https://doi.org/10.1002/pbc.22178
Acknowledgements
The authors wish to acknowledge the Musculoskeletal team of Dr. Soetomo General Academic Hospital.
Author information
Authors and Affiliations
Contributions
SM: study design, histological review, scoring of immunohistochemistry, data analyses and drafting of the manuscript; FM: orthopedic clinical data, study design, contributing to manuscript; RS: Radiological data and review, contributing to manuscript; DN: contributing to study design, editing the manuscript; MH: study design, orthopaedic and follow up data contributing to literature and writing of the manuscript; HK: Immunological review, study design, contributing to literature search and writing, KM: scoring histology, selection of antibodies and study design; LEF: comments on study design, results validation and comments in manuscript, PCWH: comments on study design, literature search, immunology, presentation of data and writing of the manuscript. All authors have read and agreed to the submitted version of the manuscript.
Corresponding authors
Ethics declarations
Declarations
The authors declare no competing interests or funding. The research was funded by the institutes where the authors are employed. The research was performed following ethical standards of the treating institutes with specific study Ref.No.: 0689/LOE/301.4./XI/2021.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mustokoweni, S., Mahyudin, F., Setiawati, R. et al. Correlation of High-Grade Osteosarcoma Response to Chemotherapy with Enhanced Tissue Immunological Response: Analysis of CD95R, IFN-γ, Catalase, Hsp70, and VEGF. Virchows Arch 484, 925–937 (2024). https://doi.org/10.1007/s00428-024-03801-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-024-03801-z