Abstract
The classification of breast neuroendocrine neoplasms (Br-NENs) was modified many times over the years and is still a matter of discussion. In the present study, we aimed to evaluate the diagnostic reproducibility and impact on patient outcomes of the most recent WHO 2019 edition of breast tumor classification, namely, for neuroendocrine tumors (NETs) and neuroendocrine carcinomas (NECs). This multicentric observational study included 287 breast neoplasms with NE differentiation. The cases were blindly classified by three independent groups of dedicated breast and/or endocrine pathologists following the 2019 guidelines. Diagnostic concordance and clinical impact were assessed. We observed only a moderate overall diagnostic agreement across the three centers (Cohen’s kappa 0.4532) in distinguishing NET from solid papillary carcinomas (SPCs) and no special type carcinomas (NST) with NE differentiation. Br-NENs were diagnosed in 122/287 (42.5%) cases, subclassified as 11 NET G1 (3.8%), 84 NET G2 (29.3%), and 27 NEC (9.4%), the latter group consisting of 26 large-cell and 1 small-cell NECs. The remaining 165/287 (57.5%) cases were labeled as non-NEN, including SPC, mucinous, NST, and mixed NE carcinomas. While NET and non-NEN cases had a comparable outcome, the diagnosis of NECs showed negative impact on disease-free interval compared to NETs and non-NENs (p = 0.0109). In conclusion, the current diagnostic classification of Br-NENs needs further adjustments regarding morphological and immunohistochemical criteria to increase the diagnostic reproducibility among pathologists. Our data suggest that, apart from high-grade small- and large-cell NECs, Br-NENs behave like non-NEN breast carcinomas and should be managed similarly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the years, the classification of neuroendocrine neoplasms of the breast (Br-NENs) has been updated by the multiple WHO editions and is still under discussion. These changing diagnostic criteria reflect the uncertainty about the real clinical impact of these rare tumors.
In 2018, Rindi et al. [1] and the latest WHO classification of endocrine and neuroendocrine tumors proposed a common classification framework for neuroendocrine neoplasms (NEN) found in any anatomical location [2]. The intent was to allow consistent patient management, while acknowledging organ-specific differences in classification criteria, tumor biology, and prognostic factors. Following this proposal, the updated NEN classification was also introduced in the 2019 WHO breast tumor fascicle [3], recommending pathologists adopt the terms “neuroendocrine tumor” (NET) and “neuroendocrine carcinoma” (NEC) in cases showing the morphology and immunoprofile typical of, respectively, well-differentiated and poorly differentiated NENs. According to the Elston and Ellis grading system, Br-NENs are graded as well-differentiated tumor (G1), intermediate differentiated tumor (G2), or poorly differentiated (G3) carcinoma. To completely adhere with this classification framework, special-type breast carcinomas (BCs) expressing neuroendocrine markers, such as solid papillary carcinomas (SPCs) and mucinous carcinomas, were removed from the NEN category [3].
Although a uniform classification of all NENs from different organ systems may represent an ideal approach for both pathologists and clinicians, its application to breast neoplasms has raised several uncertainties [4, 5]. Indeed, there is still a lack of clear-cut standards to differentiate real Br-NENs from solid BCs having some degree of neuroendocrine differentiation. An effective differential diagnosis requires validated and reproducible morphological criteria, in addition to well-defined qualitative and quantitative thresholds for neuroendocrine marker assessment [4]. Regarding prognosis, only few studies described the impact of NE differentiation in BC, reporting contrasting results [6, 7]. From a clinical perspective, a diagnosis of Br-NET, despite the term tumor, implies an identical treatment to any BC of comparable grade, stage, and hormonal profile, being most Br-NETs estrogen (ER) and progesterone (PgR) receptor positive.
In this complex background, the aim of our study was twofold: first, we evaluated the diagnostic agreement for Br-NENs among dedicated breast and endocrine pathologists strictly adhering to the latest WHO edition, reviewing a series of BC diagnosed as “neuroendocrine” or “with neuroendocrine features” from 2001 to 2019. Second, we analyzed the impact of the diagnosis of Br-NET and Br-NEC on patient outcomes to better understand the clinical relevance of these peculiar entities.
Materials and methods
Case series
To select a series of BC with NE differentiation, we searched the electronic medical records for keywords such as “breast,” “carcinoma,” “neoplasm,” “infiltrative,” and “neuroendocrine” and included surgical specimens with available hematoxylin and eosin (H&E)–stained glass slide and at least one general neuroendocrine marker immunostain. Specifically, we collected and included 100 specimens of BC with NE differentiation from Città della Salute e della Scienza Hospital (Turin, Italy), 23 from Candiolo Cancer Institute (Candiolo, Italy), 75 from University of Insubria (Varese, Italy), and 89 from Sacro Cuore Don Calabria Hospital (Verona, Italy), totaling 287 cases.
For each case, available demographic and clinical data such as age, disease stage, type of surgery, type of therapy, and follow-up data were obtained from clinical charts. Pathological report information included tumor diameter, histological grade, mitotic count, surgical margins status, vascular invasion, Ki67 proliferation index, as well as ER, PgR, and human epidermal growth factor receptor 2 (HER2) status.
Both ER and PgR were considered positive if more than 1% of tumor cells had a nuclear immunostaining [8]. HER2 status was classified as negative (score 0, 1 + , or 2 + not amplified) or positive (score 3 + by IHC or HER2 amplified by FISH) according to the recommended guidelines for invasive carcinoma [9].
The data collection also included surrogate molecular profiles based on immunohistochemical/FISH status of ER, PgR, HER2, and Ki67 according to the recommendations of St. Gallen [10]. Immunohistochemical data regarding the percentage of positivity of chromogranin A (CgA) and synaptophysin (SYN) were also recorded.
The study was approved by the Research Ethics Committee for Human Biospecimen Utilization (Department of Medical Sciences—ChBU) of the University of Turin (n°5/2020) and by The Ethics Committee of Candiolo Cancer Institute (“Neurobreast” project). Written consent was not required considering the retrospective nature of the study and no impact on patients’ care. The study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). All cases were de-identified, and all clinical-pathological data were accessed anonymously.
Case review
For each of the 287 cases, a representative H&E-stained section and at least one neuroendocrine marker (CgA and/or SYN) were included in the blind review process (of the original diagnosis) by three independent groups of experts from Turin, Candiolo, and Varese (henceforth regarded as A, B, and C research centers, respectively). The groups were composed of breast and/or endocrine dedicated pathologists, instructed to strictly follow the criteria stated in the 2019 WHO fifth edition of breast tumor classification [3].
The presence of convincing neuroendocrine morphology in > 90% of the tumor area was required for the diagnosis of NET or NEC. The diagnosis of NET was rendered in cases of invasive tumor with low- to intermediate-grade neuroendocrine morphology, showing organoid growth patterns (solid nests, trabeculae, pseudoglands) and typical cytology, while NEC cases were bearing high-grade neuroendocrine morphology, almost indistinguishable from their pulmonary small-/large-cell counterpart. Diffuse and uniform immunoreactivity for neuroendocrine markers was required in support of diagnosis, both for NET and for NEC.
Cases with general NE marker expression, in which clear and indicative NE morphology according to the latest WHO criteria was not observed, were defined as non-NENs and included no special type (NST) carcinomas and special types of BC, including SPC and mucinous carcinoma. Cases showing co-existence of NEN and non-NEN where both components comprised 10 to 90% of the tumor area, were designated as mixed carcinomas. Before the revision process started, we agreed that a diagnosis was acceptable when at least two reviewer groups selected the same diagnostic category. Cases with complete discordance between three groups were reviewed by a fourth, independent reviewer, followed by a consensus meeting among all participants using digital images, to reach agreement.
Statistical analysis
Statistical analyses were carried out using Stata 15.0 software (StataCorp, College Station, TX, USA).
To evaluate the consensus between rendered diagnoses, the “overall agreement” was calculated, i.e., the sum of the true positives and true negatives was compared to the total number of cases by calculating Cohen’s kappa index to eliminate the random component. The interpretation of the kappa values was performed according to the following guidelines: k 0.01–0.20 = none to slight agreement; k 0.21–0.40 = fair agreement; k 0.41–0.60 = moderate; k 0.61–0.80 = substantial; k 0.81–1.00 = almost perfect agreement [11].
The predominant NEN histotype diagnosis was used to study the clinical impact. The differences in the clinical and pathological variables were analyzed using parametric and non-parametric tests (Student’s t test, Pearson’s chi-square test, Bonferroni’s correction, Wilcoxon’s rank test).
Disease-free survival (DFS) was assessed from the date of diagnosis to the date of relapse or the date of the last checkup. Overall survival (OS) was assessed from the date of diagnosis to the date of death from any cause or to the date of the last checkup. All deceased patients were considered events. The survival analysis was determined by the Kaplan–Meier curves, and the Mantel log-rank test was used to compare the statistical differences. Univariable and multivariable Cox regression analyses were carried out on DFS and OS to calculate HRs and 95% CIs for the different study groups. The proportional hazard assumption was assessed with the Schoenfeld residuals. This did not give reasons to suspect violation of this assumption. All statistical tests were two sided. P values < 0.05 were considered significant.
Results
According to the latest WHO classification, Br-NENs were diagnosed in 122/287 (42.5%) cases. These were subclassified as NET G1 (11 cases, 3.8%), NET G2 (84, 29.3%), and NEC (27, 9.4%). The NEC group consisted of 26 large-cell and 1 small-cell carcinomas. The remaining 165/287 (57.5%) cases included tumors that did not meet the strict morphological NEN criteria such as NST (58 cases, 20.2%), SPC (30 cases, 10.5%), mucinous carcinomas (50 cases, 17.4%), and mixed-type BCs (27 cases, 9.4%) (Table 1, Fig. 1).
A case of NET G2, demonstrating organoid growth pattern, with solid nests separated by a thin fibrovascular stroma (a, 100 ×). High-grade NECs (b, c, 200 ×) showing large/pleomorphic (b) and small/lymphocyte-like cells (c) with hyperchromatic nuclei, and high mitotic index. Solid papillary carcinoma (d, 100 ×) with typical solid-growth pattern, delicate fibro-vascular cores, and mild nuclear atypia. Sheets of neoplastic cells suspended in abundant extracellular mucin, typically seen in mucinous breast carcinoma (e, 100 ×). Invasive carcinoma of no special type (f, 100 ×) showing neuroendocrine differentiation as revealed by chromogranin A immunohistochemical analysis (insert, 150 ×). A case of mixed breast carcinoma neatly divided into two components, upper NST and lower NET G2 (g, 10 ×), showing absence and strong expression of chromogranin A, respectively (h, 10 ×)
Multicentric case revision: inter-reviewers’ agreement
The three centers (A, B, and C) reached complete agreement in 122/287 (42.5%) cases, partial agreement in 126/287 (43.9%) cases (where two of three centers rendered the same diagnosis), and completely discordant diagnoses in 39/287 (13.6%) cases, most of them showing solid growth and/or mucinous foci. The diagnostic discrepancies among centers are shown in detail in Supplementary Table 1.
After the consensus discussion among all reviewers using digitalized glass slides, the discrepant cases were independently reviewed by a fourth pathologist (AP), using a relative diagnostic majority to evaluate the clinical relevance of the Br-NEN histotype (see “Materials and methods” section).
We observed a moderate diagnostic agreement across all three centers (Cohen’s kappa 0.4532) (Table 2). Comparing each center, we noted substantial agreement between centers A and B (Cohen’s kappa 0.6329, 70.4%), while a fair agreement was observed between centers A and C (Cohen’s kappa 0.4004, 50.87%), and centers B and C (Cohen’s kappa 0.3442, 46.7%) (Table 2).
Clinico-pathological characteristics of the case series
Table 3 shows the clinico-pathological characteristics of the case series. We observed a significantly larger median diameter in NECs (19 mm), compared to NET (12 mm) and non-NENs (15 mm) (p = 0.042). Likewise, a significantly higher median mitotic count in NECs (13 mitoses/mm2), compared to NETs (4 mitoses/mm2) and non-NENs (5 mitoses/mm2) (p < 0.001).
Almost one-third of NECs’ surgical margins were positive (28%, 7/25), while the surgical margin involvement was seldomly seen in NETs (5.7%, 5/87) and non-NENs (13.2%, 19/144) (p = 0.006).
The median value of SYN expression was significantly higher in NECs (90, interval 50–100), compared to NETs (90, interval 0–100) and non-NENs (70, interval 0–100) (p = 0.003), being positive in nearly all NEC cases with available staining. We did not observe significant differences in the expression of CgA across the groups (Table 3).
Regarding surrogate molecular classification, virtually all Br-NENs in our series belonged to luminal subtypes. In detail, luminal A subtype was more frequent in NETs (41/63, 65.1%), compared to NECs (5/23, 21.7%) and non-NENs (67/122, 54.9%). In contrast, luminal B subtype was more frequent in NECs (17/23 cases, 73.9%) than NETs (20/63, 31.7%) and non-NENs (48/122, 39.2%) (p = 0.008). All BCs, regardless of the presence or absence of neuroendocrine differentiation, were treated according to the surrogate molecular classification.
In comparison to NECs, which show recurrent disease in one-third of affected patients (7/22, 31.8%), recurrence was less common in NETs (8/73, 11%) and non-NENs (18/126, 14.3%) (p = 0.043) (Table 3).
Impact of WHO 2019 Br-NEN classification on the outcome
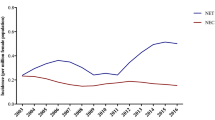
In univariate analyses and Kaplan–Meier estimates, no differences were observed in OS. However, when DFS was considered, the diagnosis of NEC (HR 3.67, CI 1.33–10.1, p = 0.012) was associated with an adverse prognosis (Supplementary Table 2; Fig. 2a, b).
In detail, as shown in Supplementary Table 3, there is a significant declining trend in 5- and 10-year DFS. At 5-year assessment, 91.1% of NETs and 90.2% of non-NENs avoided recurrent disease, compared to 69.6% of NECs. Furthermore, 81.9% of patients with NETs and 79.5% of non-NENs were disease free at 10-year follow-up, versus 50.3% of patients with NECs (p = 0.0109) (Supplementary Table 3).
However, DFS multivariate analyses that included tumor diameter, grade, and classification according to the WHO 2019 did not show a significant impact of the latter on the outcome (Supplementary Table 4).
Discussion
Intending to increase diagnostic reproducibility, WHO’s latest edition of breast tumors classification [3] employs a uniform scheme for all human NE tumors across the different organ systems, as proposed by the International Agency for Research on Cancer [1]. However, when applied to the diagnostic practice, this classification has proven to be difficult to manage, and the existence of a pure Br-NEN is still controversial and widely debated in the literature, except for the extremely rare primary small cell carcinoma [12,13,14]. Indeed, in our experience, we can apply the category of pure Br-NENs in a small subset of cases, whereas mixed forms, in which NEN component co-exists with a NST or special type BC, are more common. However, although mixed neoplasms (mixed neuroendocrine/non-neuroendocrine neoplasms, MiNEN) are considered an integral part of NENs in the digestive tract [15] and other organs [1, 2], the WHO classification of breast tumors does not include this entity as a NEN [3].
As recently discussed in several studies [4, 13, 14], fitting Br-NENs into the rigid two-tiered scheme of NEN classification (well-differentiated NETs vs. poorly differentiated NECs) poses various relevant diagnostic and conceptual challenges. In fact, the attempt to align the classification criteria of Br-NEN with those of other organs has been questioned, mainly due to the overlapping clinical behavior and treatment approach with that of non-NE conventional breast cancers.
Moreover, although Br-NENs are currently described as neoplasms with a morphology and immunophenotype (CgA SYN, INSM1, etc.) similar to that of gastroenteropancreatic and pulmonary NENs, the nuclear features typically seen in extra-mammary organs (“salt and pepper” stippled chromatin and small nucleoli) are not frequently noted in Br-NENs [4, 16].
Considering the difficulties in rendering a diagnosis of Br-NEN, to the best of our knowledge, we performed the first multicentric study on the diagnostic reproducibility of a relatively large series of breast neoplasms with NE differentiation. We observed a moderate diagnostic agreement across all three centers (Cohen’s kappa 0.4532), although in approximately 13% of cases, a uniform diagnostic interpretation among the three research centers was not obtained. This suggests that the diagnostic criteria for recognizing Br-NENs, including NET and NEC histologic types, may not be unanimously interpreted. Our study also suggests that a different professional background may influence the subjective interpretation of these tumors, differing between dedicated breast pathologists and those more specifically involved in endocrine tumor diagnostics. Furthermore, following the last WHO edition criteria [3], less than half of cases were diagnosed as NENs (122/287, 42.5%), confirming the rarity of these breast neoplasms, at least in their “pure” forms.
Immunohistochemical analysis of NE biomarkers represent the gold standard in the diagnosis of these tumors [5, 17]. Together with the most sensitive and specific markers, CgA and SYN [18], a novel biomarker, INSM1, has been proposed as an accurate indicator of NE differentiation of BC to support NEN diagnosis [5, 19, 20]. However, these immunomarkers are not routinely assessed, reserved for when pathologists identify or suspect a NE morphology in H&E-stained slides. Moreover, none of these markers proved useful in distinguishing pure Br-NENs from other mammary carcinomas with NE differentiation [3]. As a result, the diagnostic criteria, as well as proposed cut-off for NE immunomarkers, and terminology of Br-NEN still vary in recent studies [6], making data comparison impractical.
From a prognostic point of view, high-grade NEC cases demonstrated a shorter DFS, compared to NETs and non-NENs (p = 0.0109). However, these data were not confirmed in multivariate analyses. Moreover, all cases of NECs were G3 neoplasms, demonstrating larger tumor diameter, higher mitotic and Ki67 index, more frequently positive surgical margins, and lower PgR levels than NETs and non-NENs, all features associated with an aggressive disease. The DFS between NETs and non-NENs were similar, and there were no significant OS differences detected across all histological groups. These data suggest that NENs behave similar to other invasive BCs and clinically should be managed equivalently. Our results should be interpreted considering some limitations: (1) the retrospective nature of the study design, offset by a large multicentric case series; (2) the exclusion of mixed carcinomas from Br-NEN category, due to the lack of clear criteria to diagnose this entity in the breast, in contrast to the MiNEN category used in other organs.
In conclusion, the classification of Br-NENs went through multiple modifications over the years and is still a matter of debate. The current WHO (3) diagnostic proposal for Br-NEN needs further adjustments to facilitate accurate recognition of these neoplasms and increase the diagnostic reproducibility among pathologists. To date, it is important to emphasize that Br-NENs, in regard to clinical management, should be treated as conventional BCs.
In line with this, extreme caution should be adopted when making treatment decisions at a multidisciplinary level. In fact, as remarked by WHO 2019 (3), the term NET should be interpreted as cancer and not simply as tumor, implying the same clinical, therapeutic, and prognostic characteristics of NST carcinomas of identical grade and stage, to avoid unjustified treatment de-escalation, with a potential impact on patients’ health.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, Busam KJ, de Krijger RR, Dietel M, El-Naggar AK, Fernandez-Cuesta L, Klöppel G, McCluggage WG, Moch H, Ohgaki H, Rakha EA, Reed NS, Rous BA, Sasano H, Scarpa A, Scoazec J-Y, Travis WD, Tallini G, Trouillas J, van Krieken JH, Cree IA (2018) A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol 31:1770–1786. https://doi.org/10.1038/s41379-018-0110-y
Rindi G, Mete O, Uccella S, Basturk O, La Rosa S, Brosens LAA, Ezzat S, de Herder WW, Klimstra DS, Papotti M, Asa SL (2022) Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol 33:115–154. https://doi.org/10.1007/s12022-022-09708-2
Rakha EA, Reis-Filho JS, Sasano H, Wu Y (2019) Neuroendocrine neoplasms: introduction, neuroendocrine tumor, neuroendocrine carcinoma. In: WHO Classification of Tumours Editorial Board. Breast tumours, 5th edn. International Agency for Research on Cancer, Lyon, France, pp 155–161
Uccella S (2022) The classification of neuroendocrine neoplasms of the breast and its clinical relevance. Virchows Arch 481:3–12. https://doi.org/10.1007/s00428-021-03223-1
Metovic J, Castellano I, Marinelli E, Osella-Abate S, Sapino A, Cassoni P, Papotti M (2021) INSM1 expression in breast neoplasms with neuroendocrine features. Endocr Pathol 32:452–460. https://doi.org/10.1007/s12022-021-09682-1
Krawczyk N, Röwer R, Anlauf M, Muntanjohl C, Baldus SE, Neumann M, Banys-Paluchowski M, Otten S, Luczak K, Ruckhäberle E, Mohrmann S, Hoffmann J, Kaleta T, Jaeger B, Esposito I, Fehm T (2022) Invasive breast carcinoma with neuroendocrine differentiation: a single-center analysis of clinical features and prognosis. Geburtshilfe Frauenheilkd 82:68–84. https://doi.org/10.1055/a-1557-1280
Papotti M, Macrì L, Finzi G, Capella C, Eusebi V, Bussolati G (1989) Neuroendocrine differentiation in carcinomas of the breast: a study of 51 cases. Semin Diagn Pathol 6:174–188
Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR, Chavez-MacGregor M, Perlmutter J, Perou CM, Regan MM, Rimm DL, Symmans WF, Torlakovic EE, Varella L, Viale G, Weisberg TF, McShane LM, Wolff AC (2020) Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol Off J Am Soc Clin Oncol 38:1346–1366. https://doi.org/10.1200/JCO.19.02309
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. Arch Pathol Lab Med 142:1364–1382. https://doi.org/10.5858/arpa.2018-0902-SA
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn H-J, Panel members (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol Off J Eur Soc Med Oncol 24:2206–2223. https://doi.org/10.1093/annonc/mdt303
Viera AJ, Garrett JM (2005) Understanding interobserver agreement: the kappa statistic. Fam Med 37:360–363
Hasbay B, Aytaç HÖ, Aka Bolat F (2022) Neuroendocrine tumors of the breast: single-center experience. Eur J Breast Health 18:30–36. https://doi.org/10.4274/ejbh.galenos.2021.6349
Rakha E, Toss M, Quinn C (2022) Specific cell differentiation in breast cancer: a basis for histological classification. J Clin Pathol 75:76–84. https://doi.org/10.1136/jclinpath-2021-207487
Rakha E, Tan PH (2022) Head to head: do neuroendocrine tumours in the breast truly exist? Histopathology 81:2–14. https://doi.org/10.1111/his.14627
WHO Classification of Tumours Editorial Board (2019) Digestive system tumours, 5th edn. International Agency for Research on Cancer, Lyon, France
Tang F, Wei B, Tian Z, Gilcrease MZ, Huo L, Albarracin CT, Resetkova E, Zhang H, Sahin A, Chen J, Bu H, Abraham S, Wu Y (2011) Invasive mammary carcinoma with neuroendocrine differentiation: histological features and diagnostic challenges. Histopathology 59:106–115. https://doi.org/10.1111/j.1365-2559.2011.03880.x
Annaratone L, Medico E, Rangel N, Castellano I, Marchiò C, Sapino A, Bussolati G (2014) Search for neuro-endocrine markers (chromogranin A, synaptophysin and VGF) in breast cancers. An integrated approach using immunohistochemistry and gene expression profiling. Endocr Pathol 25:219–228. https://doi.org/10.1007/s12022-013-9277-4
Righi L, Sapino A, Marchiò C, Papotti M, Bussolati G (2010) Neuroendocrine differentiation in breast cancer: established facts and unresolved problems. Semin Diagn Pathol 27:69–76. https://doi.org/10.1053/j.semdp.2009.12.003
Kawasaki T, Kaira K (2021) Insulinoma-associated protein 1 (INSM1) expression in breast carcinomas with neuroendocrine morphologies: application and future prospective. Virchows Arch 479:191–194. https://doi.org/10.1007/s00428-020-02935-0
Seijnhaeve E, Galant C, Van Bockstal MR (2021) Nuclear insulinoma-associated protein 1 expression as a marker of neuroendocrine differentiation in neoplasms of the breast. Int J Surg Pathol 29:496–502. https://doi.org/10.1177/1066896920985938
Acknowledgements
The authors would like to thank Dr. Rebecca McConnell (Bronson Healthcare Group & Western Michigan University Homer Stryker M.D. School of Medicine, Kalamazoo, MI, USA) for English language editing.
Funding
Open access funding provided by Università degli Studi di Torino within the CRUI-CARE Agreement. No funding was received for conducting this study. CM and AS are funded in part by the project SEE-HER under FPRC 5X1000 MS2017 PTCRC.
Author information
Authors and Affiliations
Contributions
Conceptualization: MP, AS. Methodology: MP, IC, AS, CM, SU. Case collection: JM, EC, GB, GQ, SU, RM. Formal analysis and investigation: MP, IC, AS, CM, SU, JM, EC, RM, AP. Database management: JM, EC, GQ, RM. Writing—original draft preparation: MP, IC, JM, AS, CM, SU. Statistical analysis: SO-A. Writing—review and editing: all authors.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Research Ethics Committee for Human Biospecimen Utilization (Department of Medical Sciences—ChBU) of the University of Turin (n°5/2020) and by The Ethics Committee of Candiolo Cancer Institute (“Neurobreast” project). The study was conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). Written consent was not required considering the retrospective nature of the study and no impact on patients’ care. All cases were de-identified, and all clinical-pathological data were accessed anonymously.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Metovic, J., Cascardi, E., Uccella, S. et al. Neuroendocrine neoplasms of the breast: diagnostic agreement and impact on outcome. Virchows Arch 481, 839–846 (2022). https://doi.org/10.1007/s00428-022-03426-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-022-03426-0