Abstract
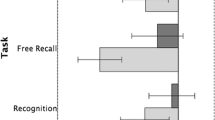
It is well known that memory affects eye movements. However, the role of individual eye fixations for recognition memory processes has hardly been investigated. Recent findings show that second fixations are especially relevant for recollection, a process associated with the retrieval of context information, but less for recognition based solely on item familiarity. The aim of the present study was to overcome limitations of a previous study (Schwedes and Wentura in Memory, 2019. https://doi.org/10.1080/09658211.2019.1567789) and to provide further evidence that second fixations are especially relevant for recollection-based recognition. Whereas recollection- and familiarity-based recognition was an unconstrained quasi-experimental variable in a previous study, here we manipulated the depth of stimulus processing in the encoding phase to experimentally manipulate the probability of subsequent item recollection. In the old/new recognition memory test, presentation of test probes was terminated after one or two stimulus fixations. “Old” responses in the recognition test were followed by a remember/know/guess procedure to assess recollection-based versus familiarity-based recognition. We found the expected depth of processing effect, with better recognition and more recollection-based responses after deep encoding. This effect, however, was significantly larger if two fixations instead of just one were allowed. There were no corresponding effects for familiarity-based recognition. Thus, a second fixation seems to play an important role only for recollection-based recognition.



Similar content being viewed by others
Notes
Note that this interaction test is equivalent to a paired samples t test (with t = squareroot(F)) that compares the dependent variable “deep advantage” (i.e., the difference between Pr for deeply encoded items minus Pr for shallowly encoded items) for one and two fixations; this t test, however, allows for adequate one-tailed testing, t(30) = 2.03, p = .026 (one-tailed), dZ = .36.
The correlation between total duration and number of fixations was r = .56. That is, the degree of collinearity is not so extreme that it would preclude this analysis from the outset.
We thank the anonymous Reviewer 2 for his hint to this alternative interpretation.
This model resulted in a singular fit. Therefore, we reduced the number of variance–covariance parameters by running a random intercept model. The results were essentially the same (t = 1.21, p = .226).
References
Bates, D., Maechler, M., Bolker, B., & Walker, S. (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67, 1–48. https://doi.org/10.18637/jss.v067.i01.
Barr, D. J., Levy, R., Scheepers, C., & Tily, H. J. (2013). Random effects structure for confirmatory hypothesis testing: keep it maximal. Journal of Memory and Language, 68, 255–278. https://doi.org/10.1016/j.jml.2012.11.001.
Bower, G. H., & Karlin, M. B. (1974). Depth of processing pictures of faces and recognition memory. Journal of Experimental Psychology, 103, 751–757.
Craik, F. I., & Lockhart, R. S. (1972). Levels of processing: A framework for memory research. Journal of Verbal Learning and Verbal Behavior, 11, 671–684.
Craik, F. I., & Tulving, E. (2004). Depth of processing and the retention of words in episodic memory. In D. A. Balota & E. J. Marsh (Eds.), Cognitive psychology: Key readings in cognition (pp. 296–308). New York: Psychology Press.
Evans, L. H., & Wilding, E. L. (2012). Recollection and familiarity make independent contributions to memory judgments. The Journal of Neuroscience, 32, 7253–7257.
Faul, F., Erdfelder, E., Lang, A.-G., & Buchner, A. (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191.
Gardiner, J. M., & Richardson-Klavehn, A. (2000). Remembering and knowing. In E. Tulving & F. I. M. Craik (Eds.), The oxford handbook of memory (pp. 229–244). New York: Oxford University Press.
Hannula, D. E., Althoff, R. R., Warren, D. E., Riggs, L., Cohen, N. J., & Ryan, J. D. (2010). Worth a glance: Using eye movements to investigate the cognitive neuroscience of memory. Frontiers in Human Neuroscience, 4, 1–16.
Hsiao, J. H., & Cottrell, G. (2008). Two fixations suffice in face recognition. Psychological Science, 19, 998–1006.
Jarmasz, J., & Hollands, J. G. (2009). Confidence intervals in repeated measures designs: The number of observations principle. Canadian Journal of Experimental Psychology, 63, 124–138.
Kafkas, A., & Montaldi, D. (2012). Familiarity and recollection produce distinct eye movement, pupil and medial temporal lobe responses when memory strength is matched. Neuropsychologia, 50, 3080–3093.
Liepmann, D., Beauducel, A., Brocke, B., & Nettelnstroth, W. (2012). Intelligenz-Struktur-test: Screening—(IST-screening). Manual. Göttingen: Hogrefe.
Mäntylä, T., & Holm, L. (2006). Gaze control and recollective experience in face recognition. Visual Cognition, 13, 365–386.
Moscovitch, M., & Craik, F. I. (1976). Depth of processing, retrieval cues, and uniqueness of encoding as factors in recall. Journal of Verbal Learning & Verbal Behavior, 15, 447–458. https://doi.org/10.1016/S0022-5371(76)90040-2.
Marinkovic, K., Oscar-Berman, M., Urbam, T., O’Reilly, C. E., Howard, J. A., Sawyer, K., & Harris, G. J. (2009). Alcoholism and dampened temporal limbic activation to emotional faces. Alcoholism, Clinical and Experimental Research, 33, 1880–1892.
Mueller, J. H., Bailis, K. L., & Goldstein, A. G. (1979). Depth of processing and anxiety in facial recognition. British Journal of Psychology, 70, 511–515.
Peirce, J. W. (2007). PsychoPy—Psychophysics software in Python. Journal of Neuroscience Methods, 162, 8–13.
Richardson-Klavehn, A., Gardiner, J. M., & Java, R. I. (1996). Memory: Task dissociations, process dissociations and dissociations of consciousness. In G. Underwood (Ed.), Implicit cognition (pp. 85–158). New York: Oxford University Press.
Rugg, M. D., & Curran, T. (2007). Event-related potentials and recognition memory. Trends in Cognitive Sciences, 11, 251–257.
Ryan, J. D., Hannula, D. E., & Cohen, N. J. (2007). The obligatory effects of memory on eye movements. Memory, 15, 508–525.
Schwedes, C., & Wentura, D. (2012). The revealing glance: Eye gaze behavior to concealed information. Memory & Cognition, 40, 642–651.
Schwedes, C., & Wentura, D. (2016). Through the eyes to memory: Fixation durations as an early indirect index of concealed knowledge. Memory & Cognition, 44, 1244–1258.
Schwedes, C., & Wentura, D. (2019). The relevance of the first two eye fixations for recognition memory processes. Memory. https://doi.org/10.1080/09658211.2019.1567789.
Snodgrass, J. G., & Corwin, J. (1988). Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology, 117, 34–50.
Tulving, E. (1985). Memory and consciousness. Canadian Psychology, 26, 1–12.
Wig, G. S., Miller, M. B., Kingstone, A., & Kelley, W. M. (2004). Separable routes to human memory formation: Dissociating task and material contributions in the prefrontal cortex. Journal of Cognitive Neuroscience, 16, 139–148.
Wixted, J. T., & Mickes, L. (2010). A continuous dual-process model of remember/know judgments. Psychological Review, 117, 1025–1054.
Yonelinas, A. P. (1994). Receiver-operating characteristics in recognition memory: Evidence for a dual-process model. Journal of Experimental Psychology, Learning, Memory, and Cognition, 20, 1341–1354.
Yonelinas, A. P. (2002). The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language, 46, 441–517.
Yonelinas, A. P., & Jacoby, L. L. (1995). The relation between remembering and knowing as bases for recognition: Effects of size congruency. Journal of Memory and Language, 34, 622–643.
Acknowledgements
We thank Ullrich Ecker for valuable comments on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix A
Below we provide information about the loci of first and second fixations. Note, since these analyses were not the focus of the reported study, the used materials were not normed, that is, there was small variance in the exact position of the eyes and the nose. To report the loci of first and second fixations, we used a normalization procedure that post hoc minimized the differences in fixation locations due to the differences in the used material.
Normalization procedure
In a first step, we generated an average face of all faces, by averaging each pixel over all faces. We then took the x and y position of the right eye, the left eye, and the tip of the nose from this average face (target face) and from each of the faces used in the experiment (hereinafter source faces). To norm the fixation locations (x and y position) of the source face, we calculated four scaling factors for each source face. One for the distance in the y-dimension between left eye and the tip of the nose, one for the distance in the y-dimension between right eye and the tip of the nose, one for the distance in the x-dimension between left eye and the tip of the nose, and one for the distance in the x-dimension between right eye and the tip of the nose. Each scaling factor was computed by the relation of the respective distance in the source face and the target face. The x and y values of each fixation were then multiplied by the corresponding scaling factor. For example, for the x position of a fixation that landed to the right of the nose, the x scaling factor for the right side (for the distance in the x-dimension between right eye and the tip of the nose) was used and vice versa. We used these normalized data for visualization and analyses of the loci.
Results
As can be seen from Fig. 4, first fixations (plot B) are more distributed than second fixations (plot C). Plot A also shows, that the typical locations of a first and a second fixation differ between individuals.
a Shows the grand mean (big dot) of the location of the first (red) and second (green) fixation and the mean locations of first and second fixations of each participant (small dots). b Shows the 2d density distribution of all single first fixations and c the 2d density distribution of all single second fixations (color figure online)
To analyze whether the x and y dimensions between first and second fixations differ significantly, we run two multilevel linear regression (using the lmer function of the lme4 package). The difference of the x-dimension and y-dimension values, respectively, of first and second fixation served as the dependent variable (comparable to Hsiao & Cottrell, 2008), participants as random factor, and the test for the regression constant as the decisive result. The results show that first and second fixations differ significantly in the x (t = 7.87, p < .001) and y (t = − 11.16, p < .001) dimension. Second fixations are more right (closer to the nose) and lower (more in the center of the face) than first fixations.
In addition, we analyzed, whether the (Euclidian) distance between first and second fixation is predictive for, first, the correctness of the response after two allowed fixations and, second, for the probability of a remember (vs. know) response.
Regarding the correctness of the response after two allowed fixations, we ran a multilevel logistic regression (using the glmer function of the lme4 package; with participants as random factor incl. random slopes) with the correctness of the response as the dependent variable and the objective old/new status of the face as well as the distance between first and second fixation as the predictors with participants as random factor. The results show no influence of the distance between first and second fixations on the correctness of the response (main effect distance: z = 1.142, p = .253; interaction effect: z = − 0.051, p = .959).
Regarding the response type (remember vs. know response), we ran a multilevel logistic regression (using the glmer function of the lme4 package; with participants as random factor incl. random slopes) with the type of response (remember = 1, know = 0) as the dependent variable and the distance as the predictor with participants as random factor. Only trials with old stimuli were analyzed. The results show no influence of the distance between first and second fixations on the response type (main effect distance: z = 0.75, p = .454). These results show that the distance between first and second fixations is not predictive for the correctness of the response and not predictive for the remember/know distinction.
To provide information whether second fixations land closer to diagnostic face parts for deeply encoded faces, we, first, ran two multilevel linear regressions to test whether locations for deeply and shollowly encoded faces differ at all: One with the x position as the dependent variable and a second with y positions as the dependent variable and in both models LOP (deep = 1 and shallow = − 1) as the predictor and participants as random factor. Regarding the x coordinates, LOP had no significant influence, t = − 0.36, p = .724. The same was the case for the y dimension, t = 1.49, p = .137. Thus, the location of second fixations does not differ for deeply and shallowly encoded items. Hence, by inference it cannot be the case that second fixations land closer to diagnostic face parts for deeply encoded faces.
In a last analysis we looked whether second fixation locations (x and y coordinates) differ between “remember” and “know” responses. Therefore, we again ran two multilevel linear regressions (using the lmer function of the lme4 package). One with the x position as the dependent variable and a second one with y positions as the dependent variable and in both models the remember/know response (remember = 1 and know = − 1) as the predictor and participant as random factor. Regarding the x coordinates, the remember/know response had no significant influence, t = 0.95, p = .350. The same was the case for the y dimension,Footnote 4t = 1.17, p = .242. Thus, the locations of second fixations do not differ between remember and know responses.
Appendix B
Since the LOP effects represent the difference in the hit rates (see data preparation), we only report effects in responses bias that include the factor fixation number. A 2 (fixation number: one vs. two) × 2 (LOP condition: deep vs. shallow) ANOVA for repeated measures with Br as the dependent variable yielded a main effect of fixation number that missed the criterion of significance, F(1,30) = 3.29, p = .080, \(\eta_{p}^{2} = .099\) as well as a significant fixation number × LOP interaction effect, F(1,30) = 11.57, p = .002, \(\eta_{p}^{2} = .278\). For deeply encoded items, the bias became more liberal when two fixations were allowed, t(30) = 3.00, p = .005, dZ = .54. That is, after deep encoding participants are more cautious in responding “old” if only one stimulus information is available. With an additional input their reservation to respond “old” declines. This was not the case for shallowly encoded items, t(30) = 0.15, p = .882.
Rights and permissions
About this article
Cite this article
Schwedes, C., Scherer, D. & Wentura, D. Manipulating the depth of processing reveals the relevance of second eye fixations for recollection but not familiarity. Psychological Research 84, 2237–2247 (2020). https://doi.org/10.1007/s00426-019-01218-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00426-019-01218-x