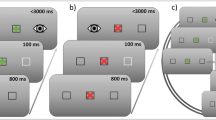
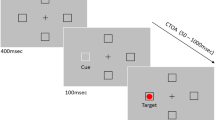
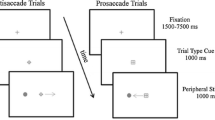
Abstract
Prolonged fixation can lead to the generation of tiny and fast eye movements called microsaccades, whose dynamics can be associated with higher cognitive mechanisms. Saccade preparation is also reflected in microsaccadic activity, but the few studies on this topic provided mixed results. For instance, fewer microsaccades have been observed when participants were asked to prepare for an anti-saccade (i.e., a saccade in the opposite direction to the target) as compared to a pro-saccade (i.e., a saccade executed towards a target), but null results have also been reported. In the attempt to shed new light on this topic, two experiments were carried out in which the context of presentation of pro- and anti-saccade trials was manipulated. Pupil size was also recorded, as a further index of cognitive load. In Experiment 1, participants were asked to prepare and perform pro- and anti-saccades in response to a peripheral target, according to a central instruction cue provided at the beginning of each trial (intermixed condition). In Experiment 2, the same task was employed, but pro- and anti-saccade trials were delivered in two distinct blocks (blocked condition). In both experiments, greater saccadic latencies and lower accuracy emerged for anti- than for pro-saccades. However, in the intermixed condition, a lower microsaccadic rate and a greater pupil size emerged when participants prepared for anti- rather than pro-saccades, whereas these differences disappeared in the blocked condition. These results suggest that contextual factors may play a key role in shaping oculomotor dynamics linked to saccade preparation.









Similar content being viewed by others
Notes
Some authors reported that, in video-based eye-tracking systems, changes in pupil size can influence the computation of eye-gaze direction, leading to potential artefactual results (e.g., Choe, Blake, & Lee, 2016; Nyström, Hooge, & Andersson, 2016). However, Gautier, Bedell, Siderov and Waugh (2016; Appendix C)—who recorded pupil size and microsaccades through an EyeLink 1000—concluded that pupil size is unlikely to affect microsaccade detection.
References
Albares, M., Criaud, M., Wardak, C., Nguyen, S. C. T., Ben Hamed, S., & Boulinguez, P. (2011). Attention to baseline: Does orienting visuospatial attention really facilitate target detection? Journal of Neurophysiology, 106, 809–816.
Antoniades, C., Ettinger, U., Gaymard, B., Gilchrist, I., Kristjánsson, A., Kennard, C., et al. (2013). An internationally standardised antisaccade protocol. Vision Research, 84, 1–5.
Betta, E., Galfano, G., & Turatto, M. (2007). Microsaccadic response during inhibition of return in a target-target paradigm. Vision Research, 47, 428–436.
Betta, E., & Turatto, M. (2006). Are you ready? I can tell by looking at your microsaccades. Neuroreport, 17, 1001–1004.
Cherkasova, M. V., Manoach, D. S., Intriligator, J. M., & Barton, J. J. (2002). Antisaccades and task-switching: Interactions in controlled processing. Experimental Brain Research, 144, 528–537.
Choe, K. W., Blake, R., & Lee, S. H. (2016). Pupil size dynamics during fixation impact the accuracy and precision of video-based gaze estimation. Vision Research, 118, 48–59.
Collewijn, H., & Kowler, E. (2008). The significance of microsaccades for vision and oculomotor control. Journal of Vision, 8, 1–21.
Corneil, B. D., & Munoz, D. P. (2014). Overt responses during covert orienting. Neuron, 82, 1230–1243.
Costela, F. M., Otero-Millan, J., McCamy, M. B., Macknik, S. L., Troncoso, X. G., & Jazi, A. N., et al. (2014). Fixational eye movement correction of blink-induced gaze position errors. PLoS One, 9, e110889.
Dalmaso, M., Castelli, L., Scatturin, P., & Galfano, G. (2017). Working memory load modulates microsaccadic rate. Journal of Vision, 17, 1–12. https://doi.org/10.1167/17.3.6.
Engbert, R., & Kliegl, R. (2003). Microsaccades uncover the orientation of covert attention. Vision Research, 43, 1035–1045.
Engbert, R., & Kliegl, R. (2004). Microsaccades keep the eyes’ balance during fixation. Psychological Science, 15, 431–436.
Everling, S., Dorris, M. C., Klein, R. M., & Munoz, D. P. (1999). Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. Journal of Neuroscience, 19, 2740–2754.
Everling, S., & Fischer, B. (1998). The antisaccade: A review of basic research and clinical findings. Neuropsychologia, 36, 885–899.
Everling, S., & Munoz, D. P. (2000). Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. Journal of Neuroscience, 20, 387–400.
Gao, X., Yan, H., & Sun, H.-J. (2015). Modulation of microsaccade rate by task difficulty revealed through between- and within-trial comparisons. Journal of Vision, 15, 1–15. https://doi.org/10.1167/15.3.3.
Gautier, J., Bedell, H. E., Siderov, J., & Waugh, S. J. (2016). Monocular microsaccades are visual-task related. Journal of Vision, 16, 1–16.
Gilbert, S. J., Spengler, S., Simons, J. S., Steele, J. D., Lawrie, S. M., Frith, C. D., et al. (2006). Functional specialization within rostral prefrontal cortex (area 10): A meta-analysis. Journal of Cognitive Neuroscience, 18, 932–948.
Hafed, Z. M., Chen, C. Y., & Tian, X. (2015). Vision, perception, and attention through the lens of microsaccades: Mechanisms and implications. Frontiers in Systems Neuroscience, 9, 167.
Hafed, Z. M., Goffart, L., & Krauzlis, R. J. (2009). A neural mechanism for microsaccade generation in the primate superior colliculus. Science, 323, 940–943.
Hafed, Z. M., & Ignashchenkova, A. (2013). On the dissociation between microsaccade rate and direction after peripheral cues: Microsaccadic inhibition revisited. Journal of Neuroscience, 33, 16220–16235.
Hermens, F., Zanker, J. M., & Walker, R. (2010). Microsaccades and preparatory set: A comparison between delayed and immediate, exogenous and endogenous pro-and anti-saccades. Experimental Brain Research, 201, 489–498.
Hyönä, J., Tommola, J., & Alaja, A. M. (1995). Pupil dilation as a measure of processing load in simultaneous interpretation and other language tasks. Quarterly Journal of Experimental Psychology, 48A, 598–612.
Jainta, S., Vernet, M., Yang, Q., & Kapoula, Z. (2011). The pupil reflects motor preparation for saccades—Even before the eye starts to move. Frontiers in Human Neuroscience, 5, 97.
Johnston, K., & Everling, S. (2009). Task-relevant output signals are sent from monkey dorsolateral prefrontal cortex to the superior colliculus during a visuospatial working memory task. Journal of Cognitive Neuroscience, 21, 1023–1038.
Just, M. A., Carpenter, P. A., & Miyake, A. (2003). Neuroindices of cognitive workload: Neuroimaging, pupillometric and event-related potential studies of brain work. Theoretical Issues in Ergonomics Science, 4, 56–88.
Kahneman, D., & Beatty, J. (1966). Pupil diameter and load on memory. Science, 154, 1583–1585.
Kliegl, R., Rolfs, M., Laubrock, J., & Engbert, R. (2009). Microsaccadic modulation of response times in spatial attention tasks. Psychological Research Psychologische Forschung, 73, 136–146.
Klinger, J., Tversky, B., & Hanrahan, P. (2011). Effects of visual and verbal presentation on cognitive load in vigilance, memory, and arithmetic tasks. Psychophysiology, 48, 323–332.
Ko, H.-K., Poletti, M., & Rucci, M. (2010). Microsaccades precisely relocate gaze in a high visual acuity task. Nature Neuroscience, 13, 1549–1553.
Krauzlis, R. J., Goffart, L., & Hafed, Z. M. (2017). Neuronal control of fixation and fixational eye movements. Philosophical Transactions of the Royal Society B: Biological Sciences, 372, 20160205.
Krejtz, K., Duchowski, A. T., Niedzielska, A., Biele, C., & Krejtz, I. (2018). Eye tracking cognitive load using pupil diameter and microsaccades with fixed gaze. PLoS One, 13, e0203629.
Lange, E. B., Zweck, F., & Sinn, P. (2017). Microsaccade-rate indicates absorption by music listening. Consciousness and Cognition, 55, 59–78.
Lisi, M., Bonato, M., & Zorzi, M. (2015). Pupil dilation reveals top-down attentional load during spatial monitoring. Biological Psychology, 112, 39–45.
Martinez-Conde, S., & Macknik, S. L. (2017). Unchanging visions: The effects and limitations of ocular stillness. Philosophical Transactions of the Royal Society B: Biological Sciences, 372, 20160204.
Martinez-Conde, S., Macknik, S. L., Troncoso, X. G., & Dyar, T. A. (2006). Microsaccades counteract fading during fixation. Neuron, 49, 297–305.
Martinez-Conde, S., Otero-Millan, J., & Macknik, S. L. (2013). The impact of microsaccades on vision: Towards a unified theory of saccadic function. Nature Reviews Neuroscience, 14, 83–96.
Mathôt, S., Fabius, J., Van Heusden, E., & Van der Stigchel, S. (2018). Safe and sensible preprocessing and baseline correction of pupil-size data. Behavior Research Methods, 50, 94–106.
McCamy, M. B., Macknik, S. L., & Martinez-Conde, S. (2014). Different fixational eye movements mediate the prevention and the reversal of visual fading. Journal of Physiology, 592, 4381–4394.
McCamy, M. B., Otero-Millan, J., Di Stasi, L. L., Macknik, S. L., & Martinez-Conde, S. (2014). Highly informative natural scene regions increase microsaccade production during visual scanning. Journal of Neuroscience, 34, 2956–2966.
Miyake, A., & Shah, P. (Eds.). (1999). Models of working memory: Mechanisms of active maintenance and executive control. Cambridge: Cambridge University Press.
Munoz, D. P., & Everling, S. (2004). Look away: The anti-saccade task and the voluntary control of eye movement. Nature Reviews Neuroscience, 5, 218–228.
Nyström, M., Hooge, I., & Andersson, R. (2016). Pupil size influences the eye-tracker signal during saccades. Vision Research, 121, 95–103.
Otero-Millan, J., Macknik, S. L., Serra, A., Leigh, R. J., & Martinez-Conde, S. (2011). Triggering mechanisms in microsaccade and saccade generation: A novel proposal. Annals of the New York Academy of Sciences, 1233, 107–116.
Pastukhov, A., & Braun, J. (2010). Rare but precious: Microsaccades are highly informative about attentional allocation. Vision Research, 50, 1173–1184.
Peel, T. R., Hafed, Z. M., Dash, S., Lomber, S. G., & Corneil, B. D. (2016). A causal role for the cortical frontal eye fields in microsaccade deployment. PLoS Biology, 14, e1002531.
Pierce, J. E., McCardel, J. B., & McDowell, J. E. (2015). Trial-type probability and task-switching effects on behavioral response characteristics in a mixed saccade task. Experimental Brain Research, 233, 959–969.
Piquado, T., Isaacowitz, D., & Wingfield, A. (2010). Pupillometry as a measure of cognitive effort in younger and older adults. Psychophysiology, 47, 560–569.
Poletti, M., & Rucci, M. (2016). A compact field guide to the study of microsaccades: Challenges and functions. Vision Research, 118, 83–97.
Privitera, C. M., Carney, T., Klein, S., & Aguilar, M. (2014). Analysis of microsaccades and pupil dilation reveals a common decisional origin during visual search. Vision Research, 95, 43–50.
Richer, F., & Beatty, J. (1985). Pupillary dilations in movement preparation and execution. Psychophysiology, 22, 204–207.
Rolfs, M. (2009). Microsaccades: Small steps on a long way. Vision Research, 49, 2415–2441.
Rolfs, M., Engbert, R., & Kliegl, R. (2005). Cross- modal coupling of oculomotor control and spatial attention in vision and audition. Experimental Brain Research, 166, 427–439.
Rolfs, M., Kliegl, R., & Engbert, R. (2008). Toward a model of microsaccade generation: The case of microsaccadic inhibition. Journal of Vision, 8, 1–23.
Schaeffer, D. J., Chi, L., Krafft, C. E., Li, Q., Schwarz, N. F., & McDowell, J. E. (2015). Individual differences in working memory moderate the relationship between prosaccade latency and anti- saccade error rate. Psychophysiology, 52, 605–608.
Shen, K., Bezgin, G., Selvam, R., McIntosh, A. R., & Ryan, J. D. (2016). An anatomical interface between memory and oculomotor systems. Journal of Cognitive Neuroscience, 28, 1772–1783.
Siegenthaler, E., Costela, F. M., McCamy, M. B., Di Stasi, L. L., Otero-Millan, J., Sonderegger, A., et al. (2014). Task difficulty in mental arithmetic affects microsaccadic rates and magnitudes. European Journal of Neuroscience, 39, 287–294.
Sirois, S., & Brisson, J. (2014). Pupillometry. Interdisciplinary Reviews: Cognitive Science, 5, 679–692.
Theeuwes, J., Olivers, C. N., & Chizk, C. L. (2005). Remembering a location makes the eyes curve away. Psychological Science, 16, 196–199.
Unsworth, N., & Robison, M. K. (2018). Tracking working memory maintenance with pupillometry. Attention, Perception, & Psychophysics, 80, 461–484.
Valsecchi, M., Betta, E., & Turatto, M. (2007). Visual oddballs induce prolonged microsaccadic inhibition. Experimental Brain Research, 177, 196–208.
Valsecchi, M., & Turatto, M. (2009). Microsaccadic responses in a bimodal oddball task. Psychological Research Psychologische Forschung, 73, 23–33.
van der Wel, P., & van Steenbergen, H. (2018). Pupil dilation as an index of effort in cognitive control tasks: A review. Psychonomic Bulletin and Review(in press). https://doi.org/10.3758/s13423-018-1432-y.
Wang, C. A., Blohm, G., Huang, J., Boehnke, S. E., & Munoz, D. P. (2017). Multisensory integration in orienting behavior: Pupil size, microsaccades, and saccades. Biological Psychology, 129, 36–44.
Wang, C. A., Boehnke, S. E., White, B. J., & Munoz, D. P. (2012). Microstimulation of the monkey superior colliculus induces pupil dilation without evoking saccades. Journal of Neuroscience, 32, 3629–3636.
Wang, C. A., Brien, D. C., & Munoz, D. P. (2015). Pupil size reveals preparatory processes in the generation of pro-saccades and anti-saccades. European Journal of Neuroscience, 41, 1102–1110.
Wardak, C., Ramanoël, S., Guipponi, O., Boulinguez, P., & Ben Hamed, S. B. (2012). Proactive inhibitory control varies with task context. European Journal of Neuroscience, 36, 3568–3579.
Watanabe, M., Matsuo, Y., Zha, L., Munoz, D. P., & Kobayashi, Y. (2013). Fixational saccades reflect volitional action preparation. Journal of Neurophysiology, 110, 522–535.
Xue, L., Huang, D., Wang, T., Hu, Q., Chai, X., Li, L., et al. (2017). Dynamic modulation of the perceptual load on microsaccades during a selective spatial attention task. Scientific Reports, 7, 16496.
Zeligman, L., & Zivotofsky, A. Z. (2017). Back to basics: The effects of block vs. interleaved trial administration on pro-and anti-saccade performance. PLoS ONE, 12, e0172485.
Zhou, X., & Constantinidis, C. (2017). Fixation target representation in prefrontal cortex during the antisaccade task. Journal of Neurophysiology, 117, 2152–2162.
Zuber, B. L., Stark, L., & Cook, G. (1965). Microsaccades and the velocity-amplitude relationship for saccadic eye movements. Science, 150, 1459–1460.
Acknowledgements
This work was funded by the Italian Ministry of Education, University, and Research (Futuro in Ricerca 2012, Grant number RBFR12F0BD to Giovanni Galfano) and by the University of Padova (Bando Giovani Ricercatori 2015 “Assegno Senior”, Grant number GRIC15QDDH to Mario Dalmaso). The authors are grateful to Ralf Engbert for his valuable suggestions on data analysis, to Daniela Toffoletto for her help during data collection, and to two anonymous reviewers for their advice and constructive criticisms on a previous version of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the studies.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dalmaso, M., Castelli, L. & Galfano, G. Microsaccadic rate and pupil size dynamics in pro-/anti-saccade preparation: the impact of intermixed vs. blocked trial administration. Psychological Research 84, 1320–1332 (2020). https://doi.org/10.1007/s00426-018-01141-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00426-018-01141-7