Abstract
Main conclusion
The dormancy release by KAR1 is associated with a reduction of coleorhiza and radicle sensitivity to ABA as well as with reduction the ABA/GAs ratio in the coleorhiza, by a decrease content of ABA, and in the radicle, by a decrease the ABA and an increase of the GAs contents.
Abstract
Both, karrikin 1 (KAR1) and gibberellin A3 (GA3), release dormancy in Avena fatua caryopses, resulting in the emergence of coleorhiza (CE) and radicle (RE). Moreover, KAR1 and GA3 stimulate CE and RE in the presence of abscisic acid (ABA), the stimulation being more effective in CE. The stimulatory effects of KAR1 and GA3 involve also the CE and RE rates. A similar effect was observed at KAR1 concentrations much lower than those of GA3. KAR1 increased the levels of bioactive GA5 and GA6 in embryos and the levels of GA1, GA5, GA3, GA6 and GA4 in radicles. The stimulatory effect of KAR1 on germination, associated with increased levels of gibberellins (GAs) and reduced levels of ABA in embryos, was counteracted by paclobutrazol (PAC), commonly regarded as a GAs biosynthesis inhibitor. Consequently, KAR1 decreased the ABA/GAs ratio, whereas PAC, used alone or in combination with KAR1, increased it. The ABA/GAs ratio was reduced by KAR1 in both coleorhiza and radicle, the effect being stronger in the latter. We present the first evidence that KAR1-induced dormancy release requires a decreased ABA/GAs ratio in coleorhiza and radicle. It is concluded that the dormancy-releasing effect of KAR1 in A. fatua caryopses includes (i) a reduction of the coleorhiza and radicle sensitivity to ABA, and (2) a reduction of the ABA/GAs ratio (i) in the coleorhiza, by decreasing the ABA content, and (ii) in the radicle, by decreasing the ABA and increasing the content GAs, particularly GA1. The results may suggest different mechanisms of dormancy release by KAR1 in monocot and dicot seeds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
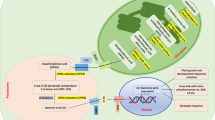
Harvested intact viable seeds of many plant species from both monocotyledonous and dicot plants are not able to complete germination under suitable species-specific conditions (water, temperature, air, light). Such seeds are regarded as primarily dormant (Bewley 2013). Primary dormancy established during seed maturation is very important in the wild plant life, as it facilitates survival and dispersal of the species. On the other hand, seed dormancy in agricultural crop weeds makes their control difficult. It is widely accepted that the balance between ABA and GAs, which are synthesized in cytosol (Fig. 1), is responsible for the state of seed dormancy. ABA plays a key role in the induction and maintenance of dormancy, and GAs participate in dormancy release and/or germination (Finkelstein et al. 2008). Avena fatua is an example of an annual weed grass which infests major cereals in the world, including Poland, and produces primarily dormant caryopses which show physiological dormancy. This dormancy is expressed as the absence of complete germination at warmer temperatures at which non-dormant caryopses can germinate. Dormant caryopses of the grass in question, able to remain viable in a soil bank for several years, have been used as a model material in the study of the dormancy release mechanism in monocots (Simpson 1990; Kępczyński 2018, 2023).

Adapted from Kępczyńska and Orlowska (2021)
Diagram illustrating the metabolic pathways of ABA and GAs. The compounds analyzed in this work are shown as grey fields.
Dormancy of A. fatua caryopses/florets can be removed by various treatments. It is released by after-ripening during dry storage of florets (Kępczyński et al. 2021) as well as by treatments involving GA3 (Kępczyński 2018) or nitric oxide (NO) (Kępczyński et al. 2021). The NO treatment was observed to be ineffective with respect to the content of GAs from non-13-hydroxylation and 13-hydroxylation pathways considered as bioactive (Hedden and Phillips 2000), but strongly decreased the content of ABA (Kępczyński et al. 2021). In addition, although the dormancy-releasing effect of NO was counteracted by paclobutrazol (PAC), a GAs biosynthesis inhibitor which blocks the GAs biosynthesis by inhibiting ent-kaurene oxidation (Desta and Amare 2021), PAC did not affect the GAs contents, however it increased the ABA content (Kępczyński et al. 2021). The dormancy release by NO has been concluded to involve a decrease in the ABA/GAs ratios and a reduction of caryopsis sensitivity to ABA. Other factors such as plant-derived smoke, smoke water and KAR1 isolated from smoke are also known to stimulate seed germination in numerous species, including dormant caryopses of A. fatua (Kępczyński 2023). KAR1 has been found to be more effective in releasing dormancy than GA3. Interaction between KAR1 and PAC suggested that induction of germination in dormant A. fatua caryopses involves endogenous GAs (Ruduś et al. 2019). As shown by Kępczyński and Van Staden (2012), although exogenous ethylene did not release caryopsis dormancy completely, to remove dormancy due to KAR1 endogenous ethylene action was required. Also, as reported by Ruduś et al. (2019), the effect of KAR1 was associated with non-transcriptional and transcriptional activation of ACC synthase and ACC oxidase and with modulation of the sensitivity to ethylene by regulation synthesis of ethylene receptors.
It is known that, following dormancy release, the sensu stricto germination of both monocot and dicot non-dormant seeds has been completed when the radicle or other embryonic tissue emerges through the structure covering it (Bewley et al. 2013). In the case of caryopses of, e.g., barley, Brachypodium distachyon and A. fatua, the radicle is sheathed by coleorhiza and germination is considered to involve two stages (González-Calle et al. 2015; Holloway et al. 2020; Kępczyński et al. 2021). During the first stage, the coleorhiza breaks through the surrounding structures, the second stage being associated with radicle emergence through the coleorhiza. Therefore, the sensu stricto germination is completed when the coleorhiza in monocots is punctured by radicle. So far, studies involving caryopses have used different germination criteria: the coleorhiza emergence from the surrounding tissues (Gubler et al. 2008; Kępczyński 2023), the radicle emergence through the coleorhiza (Gendreau et al. 2008) or both criteria simultaneously (Jacobsen et al. 2013; González-Calle et al. 2015; Holloway et al. 2020; Kępczyński et al. 2021). Previously, the coleorhiza was considered as responsible for protecting the emerging radicle (Sargent and Osborne 1980), whereas at present it is recognized as playing also a key role in grass caryopsis dormancy (Millar et al. 2006; Barrero et al. 2009), including in A. fatua (Holloway et al. 2020). Moreover, it was postulated that ABA reduction in the coleorhiza is very important in controlling caryopsis dormancy and germination of barley (Millar et al. 2006; Barrero et al. 2009) and A. fatua (Kępczyński et al. 2021).
The present study was aimed at highlighting the relationship between KAR1 and gibberellins (GAs) as well as ABA in dormancy release in A. fatua caryopses. This was done by studying the effect of KAR1 and GA3 on the final percentage and speed of the dormant caryopses’ coleorhiza and radicle emergence in the absence or in the presence of ABA. The relationship between KAR1 and endogenous GAs was investigated by determining contents of bioactive GAs from the non-13-hydroxylation pathway (GA4 and GA7) and from the 13-hydroxylation pathway (GA1, GA5, GA3 and GA6) in embryos from caryopses germinated in the presence of KAR1. In addition, effects of PAC, KAR1 and PAC + KAR1 on the GAs and ABA contents were explored. Moreover, the GAs level was determined in the coleorhiza and radicle of embryos isolated from caryopses treated with KAR1. Further, changes in the ABA/GAs ratio in embryos, coleorhiza and radicle in KAR1-treated caryopses were investigated. The results provide new information on the role of KAR1 in releasing caryopsis dormancy of grasses in relation to ABA/GAs ratios in the coleorhiza and radicle before germination is completed.
Material and methods
Avena fatua L. spikelets, containing florets, collected in 2015, were dried to a constant moisture content (ca. 11%) and stored at – 20 ℃ until used.
Germination assays
Air-dried dormant caryopses (florets without the lemma and palea) (25 in 3 replicates) were incubated in the dark at 20 °C in Petri dishes (6 cm diameter) on a single layer of filter paper (Whatman No.1) moistened with 1.5 ml distilled water, KAR1 (10–9, 3 × 10–9,10–8 M), GA3 (10–5, 10–4, 10–3 M), ABA (10–5, 3 × 10–5, 10–3 M), KAR1 + ABA, GA3 + ABA, PAC (10–4 M),, and KAR1 (3 × 10–9 M) + PAC. Chemicals, except for PAC which was dissolved in acetone, were dissolved in water at room temperature (KAR1) or in water heated to ca. 40 ℃. Caryopses with CE over the coat and with RE through the coleorhiza were counted every day until day 7 of germination (Fig. 2). All manipulations were performed under green light which did not affect germination. Effects of the compounds used on dormancy release were characterized by the final percentage of caryopses and Timson´s index (Σ7), calculated by summing up the CE or RE percentages over 7 days (Timson 1965).
Determination of GAs and ABA contents
Dormant caryopses (25 in 3 replicates) were incubated in the dark at 20 ℃ in Petri dishes (6 cm diameter) on a single layer of filter paper (Whatman No. 1) moistened with 1.5 ml distilled water, KAR1 (3 × 10–9 M) for 18, 24, 30 or 36 h or KAR1 + PAC (10–4 M) for 30 h. Upon completion of incubation, the embryos (after 18, 24 and 36 h) or coleorhizae and radicles (after 24 h) were isolated, and GAs and ABA were analyzed as described previously (Kępczyński et al. 2021, 2023). In Fig. 4 and 5 the GAs contents in embryos incubated in water or PAC, demonstrated previously, were used. Likewise, the data of ABA contents obtained in previous studies (Kępczyński et al. 2021, 2023) were used for calculating the ABA/GAs ratios.
Statistical treatment
The mean ± standard deviation (SD) of three replicates was calculated. Significance of differences between the means was tested using one- or two-way analysis of variance (ANOVA; Statistica for Windows v. 10.0, Stat-Soft Inc., Tulsa, OK, USA). Duncan’s multiple range test was used to identify significantly different (P ≤ 0.05) mean values.
Results
Effects of KAR1 and GA3 on the emergence of coleorhiza and radicles of A. fatua caryopses in the absence or in the presence of ABA
Both, coleorhizae and radicles, of the primarily dormant A. fatua caryopses incubated in water or ABA solutions were almost or totally unable to emerge (0–5%) (Fig. 3). KAR1 stimulated the coleorhiza emergence (CE) in all the concentrations used (Fig. 3A). The highest and similar levels of stimulation were found after KAR1 was applied at concentrations of 3 × 10–9 and 10–8 M; ca. 90% of caryopses observed show CE. KAR1 applied at concentrations of 10–9- 10–8 M produced a similar CE despite the presence of ABA at 10–5 M (ca. 80%). Application of KAR1 at 3 × 10–9 or 10–8 M markedly enhanced CE in the presence of 3 × 10–5 and 10–4 M ABA; ca. 50% of coleorhizae emerged. KAR1 used 3 × 10–9 and 10–8 M resulted in ca. 90% RE (Fig. 3B). The highest antagonizing KAR1 effect was found when ABA was used at the lowest concentration (10–5 M). KAR1 at all concentrations did not affect germination in the presence of ABA at the highest concentration of 10–4 M (ca. 3%). KAR1 at 10 −8 M increased Timson´s index (Σ7) by the factor of 21 and 23 in the coleorhiza and radicle, respectively (Table 1A). In the presence of ABA at 10–5 M, KAR1 at 10 −8 M did enhance Timson´s index in both the coleorhiza and radicle by the factor of 39 and 31, respectively.
Effects of KAR1 and GA3 on the emergence of coleorhiza (A, C) and radicle (B, D) of A. fatua in the absence or in the presence of ABA after 7 days of germination. One way ANOVA with Duncan´s post hoc test was used to test for significance of differences. Means denoted by different letters differ significantly (P < 0.05, n = 3)
Likewise, GA3 stimulated CE and RE at all the concentrations used (Fig. 3C, D). The highest stimulatory effect on CE and RE of dormant caryopses was observed at GA3 concentrations of 10–4 and 10–3 M; almost all the caryopses (90–100%) showed CE and RE. When used at the concentrations mentioned, GA3 resulted in 90% of the caryopses showing CE, despite the presence of ABA at 10–5- 3 × 10–5 M (Fig. 3C). When used at the two highest concentrations in the presence of 10–5 M ABA, GA3 produced an almost complete RE (90–95%) (Fig. 3D). The stimulatory effect of GA3 was not evident in the presence of 10–4 M ABA (ca. 3%). GA3 at10−4 M increased Timson´s index calculated for the coleorhiza and radicle by the factor of 24 and 27, respectively (Table 1B). When used together with ABA, GA3 resulted in a Timson’s index increase by the factor of 34 and 20 in the coleorhiza and radicle, respectively.
Effects of KAR1 on GAs contents in embryos from germinated caryopses
Contents of GAs from non-13-hydroxylation, GA4 and GA7, and 13-hydroxylation pathway, GA1, GA5, GA3 and GA6 in embryos isolated from caryopses germinated for various periods of time in water or KAR1 solutions was determined (Fig. 4). After 18 h of germination, KAR1 did not affect the contents of GA1, GA4 and GA7 (not shown) and decreased the GA5 content, whereas the contents of GA3 and GA6 increased (ca. 2 times). When the period of germination in the presence of KAR1 was extended to 36 h, the GA5 and GA6 contents in embryos were higher than in embryos from caryopses germinated in water; the increase was 1.5–2.2 times.
Effects of KAR1 on the GAs contents in embryos of A. fatua caryopses after 18 and 36 h of germination. After 36 h, coleorhiza emerged in 49% of caryopses. Vertical bars indicate ± SD. One way ANOVA with Duncan´s post hoc test was used to test for significance of differences. Means denoted by different letters differ significantly (P < 0.05, n = 3)
Effects of PAC, KAR1 and PAC + KAR1 on radicle emergence as well as ABA and GAs contents and ABA/GAs ratio in embryos
Radicles of dormant caryopses were almost unable to emerge when kept in water or in PAC solutions; ca. 2–5% of the radicles emerged (Fig. 5A). In contrast, KAR1-treated caryopses germinated nearly completely (ca. 90% showed emerged radicles). PAC applied in combination with KAR1 almost totally counteracted the stimulatory effect of the latter; ca. 6% of the caryopses showed emerging radicles. Contents of ABA, GA5, GA3 and GA6 (the 13-hydroxylation pathway) were determined in embryos isolated from caryopses germinated for 30 h in water, in the presence of PAC, KAR1 and the combination of PAC and KAR1. PAC was found to enhance, by 30%, the ABA content compared to embryos from water-germinated caryopses (Fig. 5B). In contrast, KAR1 applied alone decreased (by 60%) the ABA content. When PAC was applied simultaneously with KAR1, the ABA level was lower (by 30%) than that in embryos from water-germinated caryopses, but was higher than that in KAR1-treated ones. PAC did not affect the GAs content, whereas KAR1 increased it by 40% (Fig. 5C). The GAs content was similar in embryos from caryopses incubated in water, in the presence of PAC or KAR1 applied simultaneously with PAC. The ABA/GAs ratios showed large treatment-dependent differences; the ratio of 1.6 to 2.9 was associated with the absence of germination, the ratio of 0.6 being characteristic of germination resulting from KAR1 treatment (Fig. 5D).
Effects of KAR1, PAC and KAR1 + PAC on the final radicle emergence of A. fatua caryopses (A) as well as on GAs (GA5, GA3, GA6) (C) and ABA (B) contents in embryos after 30 h of caryopsis germination (presented as % of control content) and the ABA/GAs ratios (D) in embryos. One way ANOVA with Duncan´s post hoc test was used to test for significance of differences. Means denoted by different letters differ significantly (P < 0.05, n = 3)
Effects of KAR1 on the GAs content and ABA/GAs ratio in coleorhiza and radicle
KAR1 did not affect the GA9, GA4, GA7, GA20, GA1, GA8, GA5 and GA6 contents in the coleorhiza of caryopses germinated for 24 h, but decreased the GA3 content (Fig. 6A). The content of all GAs except for GA9 and GA7 was increased in radicles of KAR1-treated caryopses, the highest effect being seen in GA1; the GA1 content increased by the factor of ca. 6. The total content of all the bioactive GAs from both GAs biosynthesis pathways was similar in the coleorhiza from untreated and KAR1-treated caryopses (Fig. 6B). When caryopses were treated with KAR1, the bioactive GAs content in the radicles was 1.7 times higher than that in the radicles of untreated caryopses. The ABA/GAs ratios in the coleorhiza and radicles showed KAR1 to decrease the ratio by the factor 1.5 and 3, respectively (Fig. 6C).
Effects of KAR1 on the GAs (GA9, GA4, GA7, GA20, GA1, GA8, GA5) (A) contents, total GAs content (B) and the ABA/GAs ratios (C) in coleorhiza and radicle after 24 h germination of A. fatua caryopses. One way ANOVA with Duncan´s post hoc test was used to test for significance of differences. Means denoted by different letters differ significantly (P < 0.05, n = 3)
Discussion
Relationship between exogenous KAR1 or GA3 and exogenous ABA
The results presented confirm the findings, reported by previous studies, that KAR1 applied at very low concentrations is able to remove dormancy in caryopses of A. fatua (Kępczyński 2018, 2023; Fig. 3), thus enabling almost all the caryopses to complete germination. The time course of germination in grasses, e.g. Brachypodium distachyon (González-Calle et al. 2015) and A. fatua (Kępczyński et al. 2021) involves two stages: the coleorhiza emergence (CE) at the first stage and the radicle emergence (RE) at the second, either stage or the two combined being used as a criterion for caryopsis germination (Gubler et al. 2008; Gendreau et al. 2008; Jacobsen et al. 2013; González-Calle et al. 2015). The coleorhiza is recognized as being mainly responsible for dormancy control in caryopses of barley (Barrero et al. 2009), B. distachyon (González-Calle et al. 2015) and A. fatua (Holloway et al. 2020; Kępczyński et al. 2021), and plays a role similar to that of the endosperm in dicot seeds. Radicle emergence through the endosperm or coleorhiza is regarded as a completed germination (Bewley et al. 2013). KAR1 turned out to be very effective in inducing CE and RE of dormant caryopses, not only when applied alone (Kępczyński et al. 2021; Fig. 3A, B), but also in the presence of ABA (Fig. 3A, B), a compound which inhibits germination of non-dormant A. fatua caryopses (Kępczyński et al. 2021). However, in an experiment with Arabidopsis seeds, KAR1 was unable to overcome ABA inhibition of seed germination (Nelson et al. 2009). Like in previous studies, GA3, similarly to KAR1, released dormancy in A. fatua caryopses, but much higher concentrations were needed (Kępczyński 2018; Fig. 3C, D). This is consistent with findings from other seeds, e.g. those of Arabidopsis and Brassica tournefortii, more sensitive to KAR1 than to gibberellin (Daws et al. 2007; Stevens et al. 2007; Nelson et al. 2009). In the presence of ABA, a higher concentration of GA3 than KAR1 was required for dormant A. fatua caryopses to germinate (Fig. 3). Antagonistic effects of both KAR1 and GA3 towards ABA were more evident in CE than in RE. This indicates that, in the presence of ABA, the coleorhiza is more sensitive to KAR1 and GA3 than the radicle, which is consistent with previous results showing a stronger response of dormant caryopses to KAR1 in the coleorhiza than in the radicle (Kępczyński et al. 2021). Not only did both, KAR1 and GA3, increase the percentage germination in the presence of ABA, but they also accelerated caryopsis germination, even when the final germination was not affected (Table 1). Taking into account the final germination and the germination speed, it can be concluded that KAR1 and GA3 reduced the sensitivity of both the coleorhiza and radicle to ABA; however, the effect was more effective in the coleorhiza. It was demonstrated earlier (Kępczyński et al. 2023) that NO, another dormancy-releasing agent, was able to reduce the sensitivity of A. fatua caryopses to ABA.
The relationship between exogenous KAR1 and endogenous GAs and ABA
Embryos
A. fatua embryos showed the presence of GAs from the non-13-hydroxylation and 13-hydroxylation pathways ( Kępczyński et al. 2021; Fig. 4), recognized as bioactive (Hedden 2016). The dormancy releasing effect of KAR1 was associated only with an increase in the 13-hydroxylation pathway GAs (Fig. 4), in contrast to the dormancy releasing effect of NO, which did not affect the contents of GAs from both pathways in the embryos (Kępczyński et al. 2023). This suggests a difference between these compounds in the dormancy release mechanisms. The stimulating effect of KAR1 and NO on germination of dormant caryopses was strongly counteracted by PAC (Kępczyński 2018; Ruduś et al. 2019; Kępczyński et al. 2023; Fig. 5A) regarded as a GAs biosynthesis inhibitor (Desta and Amare 2021), which suggests a requirement for endogenous GAs in response to both compounds. PAC applied alone to caryopses was reported to not affect GAs content in embryos (Kępczyński et al. 2023; Fig. 5C), nor did it affect the GAs content when caryopses were treated with NO (Kępczyński et al. 2023). However, PAC reduced the GAs content when applied simultaneously with KAR1, the decline being associated with a reduced stimulatory effect of KAR1 on germination of dormant caryopses (Fig. 5A, C). It was also reported earlier that PAC, in addition to influencing the GAs content, can increase the content of ABA by increasing its synthesis and/or inhibiting its catabolism (Yamaguchi et al. 2007; Desta and Amare 2021). The ABA content, which was reduced by KAR1 (Kępczyński et al. 2021; Fig. 5B) as a result of degradation to phaseic acid (Kępczyński 2023), and by NO (Kępczyński et al. 2023), was increased due to PAC being used alone (Kępczyński et al. 2023) or simultaneously with KAR1 (Fig. 5B). Taken together, this might confirm different mechanisms involved in dormancy release in A. fatua caryopses by KAR and NO.
It is widely accepted that the ABA/GAs ratio is mainly responsible for the dormancy level and seed germination (Rodriguez et al. 2015; Tuan et al. 2018). A high ABA/GAs ratio associated with a high ABA and low GAs contents is responsible for dormancy. A low ratio, associated with low ABA and high GAs levels, allows germination. ABA plays a crucial role in the induction and maintenance of seed dormancy (Rodriguez-Gacio et al. 2009). GAs act antagonistically to ABA, promote dormancy release and are required for germination (Kucera et al. 2005; Bewley et al. 2013). Taking into account the ABA/GAs ratio in A. fatua embryos, it can be seen that the KAR1-effected dormancy release is associated with a marked decrease in the ratio (Fig. 5D). Its high level in embryos from caryopses germinated in water or in the presence of PAC or PAC + KAR1 is presumably responsible for inability of the caryopses to transit from dormancy to germination. Reduction of the ABA/GAs ratio by KAR1 involves a decrease in the ABA content and a simultaneous increase in GAs contents (Fig. 5B, C), while in the case of NO the effect is associated only with a decrease in the ABA content (Kępczyński et al. 2023). Interestingly, the ABA levels in Arabidopsis seeds were not affected by KAR1 prior to radicle emergence, although KAR1 did effectively remove dormancy (Nelson et al. 2009). Moreover, KAR1 only slightly increased the GA4 level. It has also been shown that KAR1 can delay germination of non-dormant soybean seeds by a change in the ABA/GA4 ratio due to the biosynthesis of ABA and GA4 being enhanced and impaired, respectively (Meng et al. 2016).
Coleorhiza
Since the coleorhiza is considered to be responsible for the dormancy state of barley (Millar et al. 2006; Barrero et al. 2009) and A. fatua (Holloway et al. 2020) caryopses, GAs from the non-13-hydroxylation and 13-hydroxylation pathways were determined not only in embryos, but also in the coleorhiza. KAR1 was found to not increase the contents of bioactive GAs from both pathways (Fig. 6A, B). Likewise, the GA9, substrate for GA4 and GA7, as well as GA20, the substrate for GA1 and GA5, were not affected by KAR1. KAR1 was earlier demonstrated to be capable of reducing the ABA content in the coleorhiza (Kępczyński et al. 2021). Taking these results into account, it can be concluded that the KAR1-effected control of the ABA but not GAs contents in the coleorhiza plays a vital role in releasing caryopsis dormancy. It has been previously postulated that endogenous GAs in the coleorhiza are not involved in dormancy release of barley caryopses since expression of the genes involved in the GAs synthesis (KAURENOIC ACID OXIDASE1) was upregulated only in the coleorhiza of dormant caryopses, and expression of GIBBERELLIN 2-OXIDASE1 responsible for GAs inactivation was upregulated in coleorhiza of non-dormant caryopses (Barrero et al.2009). Thus, ABA in the coleorhiza plays an essential role in the control of caryopsis dormancy in barley (Barrero et al. 2009) and A. fatua (Holloway et al. 2020; Kępczyński et al. 2021), GAs not being involved. The ABA/GAs ratio showed that, like in the embryos, KAR1 reduces the ratio in the coleorhiza (Fig. 6C), but in contrast to embryos the effect is associated only with a reduction in the ABA content, which probably could allow an increase in the activity of enzymes responsible for weakening the coleorhiza, as was postulated for barley (Barrero et al. 2009), Brachypodium distachyon (Gonzalez-Calle et al. 2015) and A. fatua (Holloway et al. 2020).
Radicle
KAR1 increased the total content of bioactive GAs from both pathways (Fig. 6B), which was related to an increase in the content of four gibberellins (GA1, GA5, GA3 and GA6) from the 13-hydroxylation pathway and one, GA4, from the non-13-hydroxylation pathway (Fig. 6A). The largest impact of KAR1 was recorded in GA1 the content of which was increased by ca. 470%, suggesting that it is mainly GA1 that is required for radicle growth. The contents of GA4, GA5, GA3 and GA6 increased by 50–70%. Moreover, GA20—a substrate for GA1 biosynthesis—was markedly, by ca. 40%, increased by KAR1, which may suggest the potential to synthesize GA1, enabling a further increase of its content and, perhaps, also the remaining GAs of this pathway. The increase of the content of GA8, a GA1 catabolite, by KAR1 may indicate the regulation of the bioactive gibberellin concentrations also through deactivation. The presented data could indicate that GAs from the 13-hydroxylation pathway play the main role in radicle growth; GA4 from the non-13-hydroxylation pathway seems to be less important (Fig. 6A). In contrast to KAR1 effect on the GAs content, KAR1 reduced the content of ABA (Kępczyński et al. 2021), a GAs antagonist, considered as a positive regulator of dormancy and negatively affecting seed germination (Hilhorst 1995). Thus, the balance between ABA and GAs was altered by KAR1, like in the coleorhiza. It is worth emphasizing that the reduction of the ABA/GAs ratio by KAR1 in the coleorhiza is associated only with a reduction in the ABA content, whereas in the radicle, in addition to the reduction of the ABA content, there is also an increase in the GAs content. Considering the antagonistic effects of ABA and GAs, a change in the ABA/GAs ratio possibly enables the radicle to grow and break through the coleorhiza, allowing germination to be completed. Thus, KAR1-associated dormancy release, involves—in addition to a reduction of the ABA/GAs ratio in the coleorhiza—probably also an increase of the radicle’s expansive force by a decrease of the ABA/GAs ratio, making it easier for the radicle to penetrate the coleorhiza. It was earlier proposed that RE may depend not only on weakening of the coleorhiza, but also on the expansive force of the radicle (Barrero et al. 2009). Our previous study (Kępczyński et al. 2021) allowed to suggest that the inability of dormant caryopses to complete germination, probably mainly due to the endogenous ABA concentration being too high, might involve inhibition of the cell-cycle activation.
To summarize, like in previous studies (Kępczyński 2018, 2023), KAR1 and GA3, very actively induced dormancy release in A. fatua caryopses. Both compounds reduced the sensitivity of the coleorhiza and radicle to exogenous ABA. The dormancy releasing KAR1 effect was associated with a decrease in the ABA/GAs ratios in embryos, coleorhiza and radicle before germination was completed. The mechanism of dormancy release by KAR1 is related to a reduction of the coleorhiza sensitivity to ABA and a decrease in the ABA/GAs ratios in the coleorhiza, regarded as playing a key role in maintaining caryopsis dormancy, by a decrease in the ABA level. A KAR1-induced reduction of the radicle ABA/GAs ratio, involving a large increase in the content of bioactive GAs, particularly GA1, as well as a reduction of the ABA level are probably required for the radicle to grow and break through the coleorhiza. Thus, caryopsis dormancy release under the influence of KAR1 requires a reduction of the ABA/GAs ratio in the coleorhiza and also in the radicle, which probably allows the radicle expansion force to increase, thus facilitating the coleorhiza puncture. It has been shown for the first time here that a reduction of the ABA/GAs ratio is necessary for dormancy release by KAR1. The results presented may also indicate that the mode of KAR1-effected dormancy release differs between monocot and dicot seeds.
Data availability
The data sets generated and/or analysed during the current study are available from the corresponding author on a reasonable request.
Abbreviations
- CE:
-
Coleorhiza emergence
- GAs :
-
Gibberellins
- KAR1 :
-
Karrikin 1
- NO:
-
Nitric oxide
- PAC:
-
Paclobutrazol
- RE:
-
Radicle emergence
References
Barrero JM, Talbot MJ, White RG, Jacobsen JV, Gubler F (2009) Anatomical and transcriptomic studies of the coleorhiza reveal the importance of this tissue in regulating dormancy in barley. Plant Physiol 150:1006–1021
Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H (2013) Germination. In: Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H (eds) Physiology of development, germination and dormancy. Springer, New York, pp 133–181
Daws MI, Davies J, Pritchard HW, Brown NAC, Van Staden J (2007) Butenolide from plant-derived smoke enhances germination and seedling growth of arable weed species. Plant Growth Regul 51:73–82
Desta B, Amare G (2021) Paclobutrazol as a plant growth regulator. Chem Biol Technol Agric 8:1
Finkelstein R, Reeves W, Ariizumi T, Steber C (2008) Molecular aspects of seed dormancy. Annu Rev Plant Biol 59:387–415
Gendreau E, Romaniello S, Barad S, Leymarie J, Benech-Arnold R, Corbineau F (2008) Regulation of cell cycle activity in the embryo of barley seeds during germination as related to grain hydration. J Exp Bot 59:203–212
González-Calle V, Barrero-Sicilia C, Carbonero P, Iglesias-Fernández R (2015) Mannans and endo-β-mannanases (MAN) in Brachypodium distachyon: expression profiling and possible role of the BdMAN genes during coleorhiza-limited seed germination. J Exp Bot 66:3753–3764
Gubler F, Hughes T, Waterhouse P, Jacobsen J (2008) Regulation of dormancy in barley by blue light and after-ripening: effects on abscisic acid and gibberellin metabolism. Plant Physiol 147:886–896
Hedden P (2016) Gibberellin biosynthesis in higher plants. Annu Plant Rev 49:73–94
Hilhorst WM (1995) A critical update on seed dormancy. Seed Sci Res 5:61–73
Holloway T, Steinbrecher T, Perez M, Seville A, Stock D, Nakabashi K, Leubner-Metzger G (2020) Coleorhiza-enforced seed dormancy: a novel mechanism to control germination in grasses. New Phytol 229:2179–2191
Jacobsen JV, Barrero JM, Hughes T, Julkowska M, Taylor JM, Xu Q, Gubler F (2013) Roles for blue light, jasmonate and nitric oxide in the regulation of dormancy and germination in wheat grain (Triticum aestivum L.). Planta 238:121–138
Kępczyńska E, Orlowska A (2021) Profiles of endogenous ABA, bioactive GAs, IAA and their metabolites in Medicago truncatula Gaertn. non-embryonic and embryonic tissues during induction phase in relation to somatic embryo formation. Planta. 253:67
Kępczyński J (2018) Induction of agricultural weed seed germination by smoke and smoke-derived karrikin (KAR1), with a particular reference to Avena fatua L. Acta Physiol Plant 40:87
Kępczyński J (2023) Induction of dormancy release in agricultural weed seeds by plant-derived smoke and smoke-derived Karrikin1 (KAR1). A relationship with plant hormones. In: Soumya M, Aftab T (eds) Strigolactones karrikins and alkamides in plants. Taylor & Francis, Boca Raton, pp 225–240
Kępczyński J, Van Staden J (2012) Interaction of karrikinolide and ethylene in controlling germination of dormant Avena fatua L. caryopses. Plant Growth Regul 67:185–190
Kępczyński J, Wójcik A, Dziurka M (2021) Avena fatua caryopsis dormancy release is associated with changes in KAR1 and ABA sensitivity as well as with ABA reduction in coleorhiza and radicle. Planta 253:52
Kępczyński J, Wójcik A, Dziurka M (2023) NO-mediated dormancy release of Avena fatua caryopses is associated with decrease in abscisic acid sensitivity, content and ABA/GAs ratios. Planta. 257:101
Kucera B, Cohn MA, Leubner-Metzger G (2005) Plant hormone interactions during seed dormancy release and germination. Seed Sci Res 15:281–307
Meng Y, Chen F, Shuai H, Luo X, Ding J et al (2016) Karrikins delay soybean seed germination by mediating abscisic acid and gibberellin biogenesis under shaded conditions. Sci Rep 6:22073
Millar AA, Jacobsen JV, Ross JJ, Helliwell CA, Poole AT, Scofield G, Reid JB, Gubler F (2006) Seed dormancy and ABA metabolism in Arabidopsis and barley: the role of ABA 80—hydroxylase. Plant J 45:942–954
Nelson DC, Riseborough JA, Flematti GR, Stevens J, Ghisalberti EL, Dixon KW, Smith SM (2009) Karrikins discovered in smoke trigger Arabidopsis seed germination by a mechanism requiring gibberellic acid synthesis and light. Plant Physiol 149:863–873
Rodríguez MV, Barrero JM, Corbineau F, Gubler F, Benech-Arnold RL (2015) Dormancy in cereals (not too much, not so little): about the mechanisms behind this trait. Seed Sci Res 25:99–119
Rodríguez-Gacio MC, Matilla-Vázquez MA, Matilla AJ (2009) Seed dormancy and ABA signaling. Plant Signal Behav 4:1035–1048
Ruduś I, Cembrowska-Lech D, Jaworska A, Kępczyński J (2019) Involvement of ethylene biosynthesis and perception during germination of dormant Avena fatua L. caryopses induced by KAR1 or GA3. Planta 249:719–738
Sargent JA, Osborne DJ (1980) A comparative study of the fine structure of coleorhiza and root during the early hours of germination of rye embryos. Protoplasma 104:91–103
Simpson GM (1990) Seed dormancy in grasses. Cambridge University Press, Cambridge
Stevens JC, Merritt DJ, Flematti GR, Ghisalberti EL, Dixon KW (2007) Seed germination of agricultural weeds is promoted by the butenolide 3-methyl-2H-furo[2,3-c]pyran-2-one under laboratory and field conditions. Plant Soil 298:113–124
Timson J (1965) New method of recording germination data. Nature 207:216–217
Tuan PA, Kumar R, Rehal PK, Toora PK, Ayle BT (2018) Molecular mechanism underlying abscisic acid/gibberellin balance in the control of seed dormancy and germination in cereals. Front Plant Sci 9:668
Yamaguchi S, Kamiya Y, Nambara E (2007) Regulation of ABA and GA levels during seed development and germination in Arabidopsis. In: Bradford KJ, Nonogaki H (eds) Seed development, dormancy and germination. Wiley-Blackwell, Hoboken, pp 224–247
Acknowledgements
We would like to express our gratitude to Prof. Ewa Kępczyńska for reading and commenting on the manuscript. The authors thank Emilia Jesionowska, M.Sc. for technical assistance. We are indebted to Dr Teresa Radziejewska for linguistic assistance.
Author information
Authors and Affiliations
Contributions
JK: conceived and designed the research, interpreted results and wrote the manuscript; AW: conducted the physiological experiments; MD: carried out the GC analysis. All authors read, reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kępczyński, J., Dziurka, M. & Wójcik, A. KAR1-induced dormancy release in Avena fatua caryopses involves reduction of caryopsis sensitivity to ABA and ABA/GAs ratio in coleorhiza and radicle. Planta 259, 126 (2024). https://doi.org/10.1007/s00425-024-04387-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-024-04387-1