Abstract
Main conclusion
The Na+/Ca2+ ratio of 1/5 ameliorated the inhibitory action of NaCl and improved the germination and growth of Vicia faba. Addition of Rhizobium also enhanced nodulation and nitrogen fixation.
Abstract
Casting light upon the impact of salinity stress on growth and nitrogen fixation of Vicia faba supplemented with Rhizobium has been traced in this work. How Ca2+ antagonizes Na+ toxicity and osmotic stress of NaCl was also targeted in isosmotic combinations of NaCl and CaCl2 having various Na+:Ca2+ ratios. Growth of Vicia faba (cultivar Giza 3) was studied at two stages: germination and seedling. At both experiments, seeds or seedlings were exposed to successively increasing salinity levels (0, 50, 100, 150, and 200 mM NaCl) as well as isosmotic combinations of NaCl and CaCl2 (Na+:Ca2+ of 1:1, 1:5, 1:10, 1:15, 1:18, and 1: 20), equivalent to 150 mM NaCl. Inocula of the local nitrogen-fixing bacteria, Rhizobium leguminosarum (OP715892) were supplemented at both stages. NaCl salinity exerted a negative impact on growth and metabolism of Vicia faba; inhibition was proportional with increasing salinity level up to the highest level of 200 mM. Seed germination, shoot and root lengths, fresh and dry weights, chlorophyll content, and nodules (number, weight, leghemoglobin, respiration, and nitrogenase activity) were inhibited by salinity. Ca2+ substitution for Na+, particularly at a Na/Ca ratio of 1:5, was stimulatory to almost all parameters at both stages. Statistical correlations between salinity levels and Na/Ca combinations proved one of the four levels (strong- or weak positive, strong- or weak negative) with most of the investigated parameters, depending on the parameter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food, fodder, and energy are currently insufficient and magnified further by the increasing population all over the globe. Such utilizations of plants are linked to water availability as a detrimental factor in agriculture and crop production. Freshwater is in shortage in many areas of the world and consequent to such limitation; sunlight utilization by plants is inefficient and accounts for less than 1% (Hussein and Lambert 2020).
Concurrent to water deficit or drought, soil salinity is a worldwide naturally occurring barricade limiting the distribution of natural flora and hampering growth and productivity of agricultural crops. Approximately, 19.5% of all irrigated land and 2.1% of dry land is affected by salt stress (FAO 2021), continuing to increase because of mishandled irrigation (Shrivastava and Kumar 2015). In arid and semi-arid regions, the salinization process occurs because of inadequate amounts of precipitation along with high evaporation for considerable leaching (Dai et al. 2011). Besides, irrigation using wastewater resulted in high salt concentrations of N, P, and K in the topsoil, which decrease within depth, and the increase of salts in topsoil was proportional to its content in the wastewater used (El-Zohri et al. 2014; Wang et al. 2021). Global warming is also enhancing soil drought and, thus, salinization as a result of enhanced evapotranspiration, which imposes an additive burden on soil structure and plants, e.g., stomata are closed and their photosynthetic gas exchange with the atmosphere (CO2 uptake and O2 evolution) is inhibited.
Salinity exerts three types of stress: ion toxicity (of Na+ and Cl−), water deficit stress, and ion imbalance stress due to disturbed uptake of key elements (e.g., N and P); these collectively inhibit plants growth and productivity. Saline conditions lead to the production of reactive oxygen species (ROS) in chloroplasts, mitochondria, as well as in the apoplastic space (Juan et al. 2021) causing membrane peroxidation, ion leakage, and damage to nucleic acids, and cellular structure and ultimately reduces the quality and total yield of the affected crop. Lotfi et al. (2022) recorded increased K content in drought-stressed wheat plants, which plays a role in osmotic adjustment.
High salt can directly impair the biological nitrogen fixation (BNF) via impairing the interactions between Rhizobium and the host plant inhibiting nodule formation (Singleton and Bohlool 1984; Zahran 1999) and indirectly affecting the symbiosis by reducing the growth of the host plant. BNF and legumes are crucial not only for agriculture and crop yield but also for the environment, at least in terms of CO2 uptake and O2 release. Otherwise, the use of N fertilizers has degraded huge land extensions around the world and BNF is required to replace tons of synthetic fertilizers (Cassman and Dobermann 2022). Ca2+ deficiency is a typical feature of salt stress; the addition of Ca2+ to legumes grown under high NaCl concentrations had positive effects on nitrogen fixation (El-Hamdaoui et al. 2003).
Counteracting soil salinity is urgent in many countries having salinity-affected soils and limited freshwater resources; their population increases and actually suffering food shortage. Attempts conducted to overcome salinity-related adverse effects are numerous and diverse including repair of soil factors, adopting relevant halotolerant/halophytic plants, phytohormones, genetic manipulations (transgenic plants), etc. In this concert, calcium has long been used to overcome the adverse effects of numerous types of stresses, which widely vary in effects and nature (Abdel-Basset and Issa 1994; Abdel-Basset and Matsumoto 2008; Briffa et al. 2020). In this respect, Cramer et al. (1990) reported mitigation by calcium of progressively inhibited root elongation and osmolality of two corn (Zea mays L.) cultivars differing in their salt tolerance. Also, Ahmed et al. (1989) has found that certain Na–Ca combinations enhanced growth of the salinized cultures of the green alga Chlorella vulgaris. The maxima of cell number, dry matter and pigments, with the lowest value of respiration and the stress marker proline were recorded at an optimal Na+/Ca2+ ratio of 13.5. The calcium-induced alleviation of the harmful effects of NaCl may be due to the reduced uptake of Na and to the subsequent elevation of water content of hypocotyls and cotyledonary of regenerated tomato shoot under Na–Ca combination (El-Enany et al. 2001). The utilization of the tissue culture technique to derive cell lines tolerant to NaCl stress is one approach to the improvement of salt tolerance in tomatoes (Cuartero et al. 2006). It has been reported that increasing calcium concentration of the culture medium increases the frequency of somatic embryogenesis in salinized carrot and the organogenesis of shoots and roots (Dogan 2020). Calcium acts both at the intracellular level (Poovaiah and Reddy 1987) and at the apoplast level as an ion responsible for membrane stability, ion transport, and wall rigidity. It has also been reported that Ca is essential for K/Na selectivity.
Subsequently, the main objective of this work was to elucidate the potential ameliorative role played by calcium to diminish the negative impacts exerted by salinity in Vicia faba. Salinity is an inherent intrinsic characteristic in many areas of the world. Furthermore, most of the water on earth is saline. The case study plant V. faba was studied at two stages of growth: germination for 7 days in Petri dishes as well as seedlings in soil pots (45-day-old plants). Germination, fresh and dry mass, nodulation, chlorophyll, nitrogenase activity, leghemoglobin and respiration were assessed as measurables to the calcium-induced ameliorations in the salinity-strained Vicia faba plants. The insight of the herein study plan included early stages of plant development (seed germination and seedling growth), which is indicative for plant performance at its older stages (seed germination and seedling growth). Unique calcium ameliorative properties, ascribed to its ubiquitous interferences in cellular structures and metabolism, were also monitored. The case study plant, Vicia faba, is a major legume in Egypt; its importance as food and fodder are documented. It also may be useful in land reclamation, as it forms nitrogen-fixing nodules, which are important to overcome one of the major problems of salt-affected soils, i.e., nitrogen limitation and the subsequent need of excessive synthetic fertilizers. Remediation of soil salinity problems by calcium application at such laboratory work may be modeled to cultivable areas in Egypt and elsewhere in the world.
Materials and methods
Germination and seedling experiment
Seeds of Vicia faba (Giza 3) were obtained from the Faculty of Agriculture, Assiut University, Assiut, Egypt, and germinated in the presence of successively increasing NaCl-salinity levels in addition to various Na+/Ca2+ ratios. Germination was performed in 3 replicates in a Petri dish experiment in a growth chamber at 25 °C and followed for 7 days and terminated upon full germination of control seeds.
Treatments
Four levels of salinity (50, 100, 150, and 200 mM NaCl) and Na–Ca combinations having Na+/Ca2+ ratios of 1:1, 1:5, 1:10, 1:15, 1:18, and 1:20, iso-osmotically equivalent to 150 mM NaCl, were applied to the pot grown plants same as in germination test in Petri dishes.
The Na+/Ca2+ ratios were calculated according to the following equation:
where 2 and 3 are constants represent the number of dissociating ions from NaCl and CaCl2, respectively; α is the reciprocal of a certain (needed) Na+/Ca2+ ratio and β is the number of ions at a certain (needed) molarity of NaCl (= mM*2). “X” is the sum number of Na+, and Cl− ions at a certain molar combination of NaCl and CaCl2.
Concentration of NaCl (Y mM) = X/2,
Concentration of CaCl2 (Z mM) = (β– X)/3.
a) Germination assessment
Seed germination was assessed by the following parameters:
-
–Germination percentage (GP):
GP = (number of germinated seeds ÷ total number of seeds sown) × 100,
-
–Germination index (GI): GI = Σ(Gt/Tt),
where Gt is the number of seeds germinated on day t, and Tt is the number of days (Hakim et al. 2010; Keshavarizi and Mohammed 2012).
Mean germination time (MGT):
-
–MGT = Σ(Ti × Ni)/ΣNi,
where Ni is the number of newly germinated seeds at time Ti (Ruan et al. 2002).
b) Seedling assessment
Seedling growth was assessed by fresh weight, dry weight and seedling vigor index (SVI). For seedling growth, ten seedlings were randomly selected from each Petri dish at the end of the germination period. After selection, shoot length (SL) and root length (RL) were measured in cm. Seedlings’ fresh weight (FW) and dry weight (DW), after drying the samples in an oven for 72 h at 80 °C, were recorded. In addition, SVI (Mahender et al. 2015) was assessed as follows:
SVI = mean germination percentage × mean seedling length.
Experimental set-up for plant sowing
Seeds of faba bean (Vicia faba L. Giza 3 cultivar) were surface sterilized by ethanol (70% for 30 s), NaOCl (5% for 3 min), and washed 5 times with sterilized water. Ten sterilized seeds were then planted in soil pots and sown for 60 days at plastic pots of 7 kg capacity; each pot contained 5 kg of sand/clay (1:2) soil from around Assiut city area. Seedlings were thinned to four plants per pot of comparable height and vigor at 7 days after planting. Table 1 represents the soil mixture analysis. The experiment was laid out randomly with three replicates in the greenhouse and the pots were rotated regularly on the benches to equalize the effect of sunlight intensity at the different times of the day. Plants were irrigated with distilled water up to the field capacity every other day over the 60 days of growth. Inoculation with bacteria was done twice after 15 and 30 days from sowing the seeds. The growth experiment was conducted in the botanical garden, Department of Botany and Microbiology, University of Assiut during the growing season of Vicia faba (November–February) under natural conditions of light, temperature, and humidity. Plants were harvested for analysis at 15, 30, 45, 60 days after planting. The experimental design and analytical procedure were always conducted in three replicates.
Seeds in Petri dishes and seedlings in soil pots were inoculated with a salinity-tolerant strain of Rhizobium leguminosarum; the herein-newly isolated strain from bean nodules cultivated in salinized soil. Control pots received neither of the above treatments nor the bacterium.
Isolation of Rhizobium strain from Vicia faba nodules
Nodules were detached from V. faba roots, washed with sterile water followed by surface sterilization with 95% alcohol and again with sterile water. The nodules were, then, surface sterilized with 0.1% sodium hypochlorite for 2–3 min and again washed for at least 10 times with sterile water. The nodules were transferred into culture tubes half filled with sterile water and crushed with a sterile glass rod to obtain a milky bacterial suspension. After serial dilutions, the suspension was streaked on yeast extract mannitol agar (YEMA) plates and incubated for 2–3 days at 28 °C. A single colony was taken from the agar plates and re-streaked on freshly prepared YEMA plates to obtain the pure culture (Gachande and Khansole 2011), which was grown in YEM broth to be used for DNA extraction.
Molecular identification of rhizobia
The morphological, biochemical, and molecular characteristics of the isolate were used for strain characterization, according to Bergey’s Manual of Systematic Bacteriology (Brenner et al. 2005). The bacterial strain was identified according to the partial 1500 bp sequences of 16Sr RNA of the strains, and comparison in the GenBank databases. The total genomic DNA was extracted and purified from the bacterial samples. The primer set of F (5-AGA GTT TGA TCC TGG CTC AG-3) with a GC clamp and R (5-GGT TAC CTT GTT ACG ACT T-3) at the annealing temperature of 65 °C was used for the PCR amplification of the variable region of 16S rDNA from the purified genomic DNA. Then, PCR clean up to the PCR product was made using GeneJET™ PCR Purification Kit (Thermo Scientific). Loading to 4 µl from the PCR mixture was carried out to examine the PCR product on 1% agarose gel against 1 kb plus ladder (Fermentas). Finally, sequencing of the PCR product on GATC Company using ABI 3730xl DNA sequencer, forward and reverse primers was conducted. Sequence analysis was achieved by searching through the online databases using BLAST. The phylogenetic analysis was performed using MEGA 3.1 software. The phylogenetic tree was constructed by the neighbor joining method. The sequences obtained were compared with the available database sequences using a BLAST search. The sequence of the isolated strain was deposited in the Gen Bank.
Organism and inoculation
The isolated strain was identified as Rhizobium leguminosarum, which was then grown in 250 ml Erlenmeyer flasks containing 100 ml yeast extract mannitol (YEM) broth (Somasegaran and Hoben 1985) in a shaker incubator for 3 days until reaching maximum turbidity (approximately 1 × 109 cells ml−1). Bacterial inoculum population was estimated with plate dilution method and total count (Vincent 1970). Ten ml of the log phase bacterial culture was inoculated into each pot, watered regularly to maintain the soil at field capacity.
Analytical methods
Soil analysis
The used soil was prepared (sand:clay 1:2), air-dried, and soil samples (0–20 cm top layer) were analyzed before addition of salt treatments using standard procedures (Central lab, Faculty of Agriculture, Assiut University). In brief, soil pH was determined (HI 2216; Hanna, Smithfield, RI, USA). Available P was extracted using the Mehlich-3 and determined using the ammonium vanadate method and amount determined using a spectrophotometer (Mehlich 1984). Organic carbon was determined by Walkley and Black (1935) sulfuric acid–dichromate digestion followed by back titration with ferrous ammonium sulfate, whereas nitrogen was determined using the Kjeldahl method (Bremner and Mulvaney 1982).
Plants
The soil was gently washed off the roots under a stream of running tap water; the nodules were then carefully removed from the roots, counted, and weighed. Roots and shoots were separated, and the fresh weight (FW) of each part was recorded for each plant. They were dried inside a paper envelope in an oven at 80 °C for 24 h, and the dry weights (DWs) were recorded.
Germinating seeds were counted, shoot and root lengths, fresh and dry mass were measured and the averages of 10 shoots or roots ± SE at each treatment are presented. Average nodule numbers of 10 plants ± SE were recorded.
Metabolic pools
Chlorophylls (a and b) contents were assessed in ethanol extracts according to Arvola (1981), calculated and expressed as µg/ml according to Metzner et al. (1965).
In water extracts, reducing sugars were estimated as glucose equivalents according to Miller (1959) and soluble protein contents were estimated using the method of Lowry et al. (1951) using UV-120 spectrophotometer (MioTech, Hong Kong, China).
Determination of leghemoglobin in nodule cytosol
One gram of fresh nodules was rinsed thoroughly with distilled water and immediately hand ground in an ice chilled mortar with 5 ml of distilled water. Nodule homogenates were filtered, and the filtrate was centrifuged at 500 g for 2 min to remove nodule debris. The resulting supernatant was centrifuged at 12,000 g for 15 min to sediment the bacteroids. Leghemoglobin levels in the supernatant, the ‘nodule cytosol’, were determined calorimetrically essentially as described by Larue and Child (1979) using Unico UV-2100 spectrophotometer. The colorimetric assay was standardized using freshly prepared Hemetrol reagent (solution of cyanmethemoglobin titrated exactly according to recommendations of BioMerieux (Marcy L'Etoile, France).
Nitrogenase activity
Nitrogenase activity, as acetylene reduction, was determined in excised nodulated roots, using gas chromatograph (Thermo Scientific, TRACE GC Ultra). The roots were placed in 500 ml bottles sealed with a rubber septum; 50 ml of air were taken, and the same volume of acetylene gas was introduced into the bottle, incubated at 37 °C. Then, samples from the root nodules atmosphere in bottles were withdrawn and injected to the gas chromatograph. Afterward, nodules of each individual root were counted, and their fresh and dry mass were determined. A calibration curve was constructed using pure ethylene.
Respiration (oxygen uptake measurements)
Respiration of Rhizobium leguminosarum was measured both in vitro (germination stage) as well as in vivo (nodules). The respiratory oxygen uptake (R) of Rhizobium leguminosarum was monitored daily (hroughout the germination period of 7 days) using a Clark type electrode computerized to an Oxygen Monitoring System (OMS, Hansatech Instruments Inc., donation from the Alexander von Humboldt Foundation Germany to R. Abdel Basset). Two milliliters of bacterial suspension was monitored at 25 °C for 15 min. In seedlings, respiration of excised nodules was also monitored; the rate was calculated and expressed as mmole O2 mg protein−1 h−1.
Membrane stability index and percentage of membrane injury
Twenty leaf discs were placed into l00 ml flasks and washed thoroughly with three changes of deionized water to remove surface adhered electrolytes. Discs were then kept in thirty ml of deionized water for 24 h at 10 °C in the dark. Afterward, the flasks were warmed to 25 °C, shaken well and the electrical conductivity was measured. Following the conductivity measurements, the leaf tissues were autoclaved for 15 min to release all ions from the tissue, cooled to 25 °C and then the electrical conductivity was measured again. Cell membrane stability (CMS) and relative cell injury were calculated with the formula used by Nijabat et al (2020).
Cell membrane stability (%) = 1 − (E1/ E2) × 100,
where E1 and E2 are the electric conductivity before and after autoclaving, respectively.
Relative cell membrane injury (%) = [1 − (CMS2) / 1 − (CMS1) × 100],
where CMS1 is cell membrane stability for control samples, CMS2 is for salinity-treated samples.
Statistical analysis
Each experiment was repeated three times and the mean values of three replicates ± SE (standard errors) are presented. Statistical analysis of the data was conducted using ANOVA one-way test (analysis of variance) by SPSS program version 21, and Duncan values were determined at 0.05 levels.
Results
In this work, Vicia faba seeds and seedlings were exposed to successfully increasing NaCl concentrations of 0, 50, 100, 150, and 200 mM as well as to various Na/Ca ratios of 1:1, 1:5, 1:10, 1:15, 1:18, and 1:20, iso-osmotically equivalent to 150 mM NaCl. Vicia faba displayed symptoms of negative impacts of salinity. At both plant growth stages (germination and seedlings), faba bean plants were negatively affected by increasing salinity level. Salinity reduced all germination aspects of V. faba; namely GP (germination percentage), germination index (GI), mean germination time (MGT) and seedling vigor index (SVI) (Table 2 and Fig. 1). The highest MGT was observed at control seeds and the lowest was found at 150 mM NaCl (Fig. 1). The addition of bacteria did not enhance MGT either at control or salinized (150 mM NaCl) seeds. However, Na+/Ca2+ ratio of 1:5 stimulated MGT and further by the addition of bacteria.
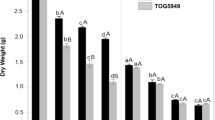
Seedling and plant growth are enhanced by addition of 1:5 Na/Ca treatment compared to control. Fresh and dry mass of shoots are improved significantly at 1:5 supplantation media (Fig. 2). Also, fresh and dry mass of root exhibited similar trend as those of the shoot in presence of 1:5 Na/Ca combination (Fig. 3). Figure 4 summarizes the data in Figs. 2 and 3. It presents the maximum results of growth criteria (fresh and dry mass) that were induced by a certain Na/ratio of 1/5. Figures 2 and 3 present the detailed treatments (salinity levels and Na–Ca combinations) as well as their results, from which the maximum values were selected and presented in Fig. 4. Also, Figs. 2 and 3 set a comparison between the optimum treatment (Na:Ca ratio of 1: 5) that led to the highest growth magnitude with the different salinity levels and other Na/Ca ratios. Chlorophyll content was stimulated at the same ratio of Na/Ca 1:5 (Fig. 5).
Shoot fresh and dry weight of 60-day-old Vicia faba seedlings at successively increasing NaCl levels (i and iii) and at Na: Ca ratios (ii and iv). The ANOVA test was carried out by using SPSS 21 comparisons among means. (n = 3); small letters (a–d) show significance in fresh weights at the different treatments while capital letters (A–C) refer to statistical differences among dry weights, at P = 0.05 level based on Duncan's multiple range test. Letters indicate significance between treatments at their maxima, where they are placed might represent more than one treatment.
Root fresh weight of 60-day-old Vicia faba seedlings at successively increasing NaCl levels (I and iii) and at Na: Ca ratios (ii and iv). The statistics in Fig. 2 applies also to this figure
Maxima after 45 days of growth of variously treated Vicia faba plants at successively increasing NaCl levels and at Na: Ca ratios (only the concentration of 150 mM NaCl and the ratio of 1:5 are shown to conserve space). SFW is shoot fresh weight, SDW is shoot dry weight, RFW is root fresh weight and RDW is root dry weight. The letters (a, b, c) and (α, β, γ), and digits (1, 2, 3) and (i, ii, iii) refer to statistical differences among SFW, SDW, RFW and RDW, respectively; maxima are abstracted from Figs. 2 and 3 subjected to the ANOVA test carried out using SPSS 21 comparisons among means (n = 3)
Maxima after 45 days of growth of variously treated Vicia faba plants at successively increasing NaCl levels and at Na: Ca ratios (only the concentration of 150 mM NaCl and the ratio of 1:5 are shown to conserve space). Chl.a, chlorophyll a; Chl.b, chlorophyll b. The statistics in Fig. 2 applies also to this figure
Nodules number, nodules weight, and leghemoglobin were gradually inhibited by increasing salinity levels (Fig. 6a) and enhanced significantly up to their maximum levels at Na:Ca of 1:5 ratio (Fig. 6b). Nitrogenase activity followed, more or less, the same attitude using the same treatments (Fig. 7).
Maxima of nodule number, nodule weight, and leghemoglobin of 15-day-old Vicia faba plants variously treated at successively increasing NaCl levels and at Na:Ca ratios (only the concentration of 150 mM NaCl and the ratio of 1:5 are shown to conserve space. The statistics in Fig. 2 applies also to this figure
Nitrogenase activity at 60-day-old Vicia faba seedlings of successively increasing NaCl levels (a) and at Na: Ca ratios (b). The statistics in Fig. 2 applies also to this figure
Respiration rates (dark oxygen uptake) of faba seedlings were also inhibited by salinity and maximized by the same ratio (Fig. 8). Enhanced respiration by the optimum Na/Ca ratio of 1/5 indicates the divergence of the generated respiratory energy for growth, i.e., synchronization of energy production with growth.
Respiration of 60-day-old Vicia faba seedlings (a) at successively increasing NaCl levels (a) and at Na: Ca ratios (b). The statistics in Fig. 2 applies also to this figure
Membrane injury (Fig. 9) was elevated by increasing the level of NaCl. The least injury level was recorded at Na–Ca combinations, particularly at 1: 5. Membrane stability index, which is almost the opposite of membrane injury, was calculated as well; it was inhibited by increasing salinity level and improved by calcium (data not shown to conserve space). Minimum injury (ions and metabolites leakage) is in accordance with maximum growth (highest biomass fresh and dry weight).
Percentage of membrane injury of 60-day-old Vicia faba seedlings at successively increasing NaCl levels (a) and at Na: Ca ratios (b). The statistics in Fig. 2 applies also to this figure
Calcium ions were applied to both germination in petri dishes and growth in the soil pots as various Na/Ca ratios of 1:1, 1:5, 1:10, 1:15, 1:18, and 1:20, iso-osmotic to 150 mM NaCl. The partial substitution of Ca2+ for Na+ relieved NaCl stress; particularly, the ratio of Na/Ca 1:5 resulted in the highest relief of seed germination, root length, shoot length, fresh weight of root and shoot, and dry mass of root and shoot, respiration (Table 3).
The bacterial isolate from faba nodules was identified as Rhizobium leguminosarum with similarity 100% and the sequence was deposited in the Gene Bank under the accession number of OP715892. Figure 10 shows the phylogenetic tree of the studied isolate, Rhizobium leguminosarum (OP715892).
-
The correlations between all the assessed criteria in Vicia faba with age, salinity level or sodium/calcium combinations, at which plants grew, were statistically analyzed (Table 4). These criteria included fresh and dry weight of shoots and roots, chlorophyll content, Chl a/Chlb ratios, nodule number, nodule content of leghemoglobin, nodule respiration, and their nitrogenase activity. The statistical analysis between treatments (salinity levels and Na/Ca combinations) with the studied parameters revealed one of four statistical correlations: strong positive, weak positive, strong negative or weak negative, depending on each of the parameters. In summary, the salinity level of 150 mM NaCl induced a strong positive correlation between shoot fresh and dry weight while root fresh and dry weight, nodule number and chlorophyll induced weak positive correlation with time. Nodule weight and respiration induced a strong negative correlation while leghemoglobin and nodule weight induced weak negative correlation with time. The Na/Ca ratio of 1/5 induced a strong positive correlation between shoot fresh and dry weight whereas root fresh and dry weight, nodule number and chlorophyll induced weak positive correlation with time.
-
Although, the other treatments induced correlations with the measured criteria.
-
The statistical analysis revealed statistical correlations between most of the studied parameters with one or both of the treatments (salinity levels or Na/Ca combinations) at one of four correlation levels (strong/ weak positive, strong/ weak negative), specific to each parameter.
Discussion
In this work, growth of Vicia faba was stressed by NaCl salinity at two stages of growth: germination and seedling. In this context, the reaction of plants to salinity in germination and seedling stages seems potential determinants for plants salt tolerance and the production of appropriate crop yields in saline conditions. In accordance with the above statement, Krishnamurthy et al. (2007) concluded that germination and seedling traits can be reliable indices for the final plant performance at salinity conditions. Seed germination was regarded as an important and susceptible stage of plant growth because the duration of this phase determines seedling establishment and future plant growth (Hakim et al. 2010). Tolerance to salt stress at the germination stage and at seedling emergence determines better plant establishment in saline soils (Bojovic et al. 2010; Keshavarizi and Mohammed 2012). Salinity inhibited growth of Vicia faba, while the partial substitution of Ca2+ for Na+ ameliorated the adverse effects of salinity at both stages of growth. Gemination rate index, seedling vigor index (SVI), germination percentage (GP) and mean germination time (MGT) were all inhibited by salinity but enhanced by calcium addition. Shoot-, root length, fresh and dry weight similarly responded. Our results in Vicia faba agree with those of Sorghum (Geressu and Gezahagn 2008; Dehnavi et al. 2020). Germination and emergence stages in sorghum development were the most informative stages of the plant’s lifecycle to evaluate the effect of salinity (Krishnamurthy et al. 2007). Responses in Sorghum varied depending on the genotype (Nimir et al. 2014, 2015; Ali et al. 2020). Also, increasing salinity significantly reduces germination percentage and rate, root and shoots lengths, and fresh and dry weights of the exposed plants (Shrivastava and Kumar 2015). In addition, Mbinda and Kimtai (2019) reported that salinity substantially affects all traits associated with germination and early seedling growth of Sorghum, depending on the variety used and level of salinity stress applied. Salinity has also negative correlation with germination percentage (GP), germination index (GI), and VIG of Imperata cylindrica (Rehman et al. 2000).
Chlorophyll contents in faba leaves were also lowered as salinity was increased while was enhanced by calcium addition. Chlorophyll plays a crucial role in the absorption and transmission of light quanta and chlorophyll concentration is an indicator of a plant's capacity to utilize light for photosynthesis (Zhang et al. 2018; Danial et al. 2023). Under saline conditions the reduction of seedling shoot, and root lengths FW, and DW is a common phenomenon in many plants. The effects of salinity on root length of legume plants were more drastic than on shoot length, which might be due to the effect of NaCl being more inhibitory on root growth than on shoot growth (El-Beltagi et al. 2022; Alnefaie et al. 2023). Na+ accumulates more in roots than in shoots, e.g., in Sorghum plants (Tester and Davenport 2003). Similarly, faba plants responded. Roots are the first organs exposed to salinity and are in direct contact with the soil, absorbing water and salts from the soil and supplying it to the shoot (Asaadi 2009). However, the reduction in FW and DW may be due to the toxic effect of Na+ on photosynthesis (Kawasaki et al. 1983). In fact, salinity influences the germination process by several modes of action; namely, less water uptake, Na+ and Cl− ion toxicity, disturbed nutrient uptake, enzymatic and subsequent metabolic disturbances. First, salinity reduces the imbibition of water by seeds due to the lower water potential of germination media, i.e., osmotic or pseudo-drought stress. Outer osmotic stress inhibits water uptake and may further drain water from inside to the outer hypertonic medium. Delayed water absorption, thus, reduces germination (Farhoudi and Tafti 2011; Dehnavi et al. 2020; Misra and Gupta 2020). In this respect, NaCl inhibited seed germination due to the high osmotic potential, specific ion toxicity (Na+ and Cl−) and inhibited maintenance of nutrient levels essential for plant growth such as NO3− (Chien et al. 2009). In the present work, water content of faba shoots and roots was decreasing with the increase of NaCl level, which is in accordance with the above interpretations of salinity-inhibited water uptake (Zheng et al. 2022; Cen et al. 2023). On the other side, as NaCl ions passively accumulate inside cells, salinity causes disruption of enzymatic activities, which subsequently leads to major changes in plants during germination, such as altering the metabolism of nucleic acid and protein (Dantas et al. 2007), disturbing the hormonal balance (Ryu and Cho 2015), and reducing the use of seed reserves (Othman et al. 2006). Additionally, it seems that by inducing disturbance of the metabolic process, salinity increases phenolic compounds, which can reduce germination (Ayaz et al. 2000). Specifically, salinity reduces intercellular CO2 concentration and then photosynthesis rate by stomatal closure (Kaymakanova and Stoeva 2008). In addition, salinity exerts a negative effect on the ultrastructures of cells, tissues, and organs (Koyro 2002). Ultimately, salinity is limiting to root emergence and seedling growth (Krishnamurthy et al. 2007; Abari et al. 2011; Bilgili et al. 2011). Collectively, salinity inhibits seeds germination by multiple and diverse modes of action, which disturbs homeostasis of various nutrients and metabolic processes. Furthermore, various seeds internal factors, such as coat properties, age, polymorphism, dormancy, seedling vigor; and external factors, such as temperature, light, water, and gases, can affect seed germination under saline conditions (Wahid et al. 2011). Numerous studies reported that, under saline conditions, genotypes which maintain higher germination rates are salt tolerant and produce higher biomass and yield (Dehnavi et al. 2020). It has been argued that retention of Na+ ions occurred in roots of salt tolerant genotypes (Assaha et al. 2017). Otherwise, Chien et al. (2009) reported that tolerant genotypes have lower uptake of Na+ than sensitive genotypes do.
All biological nitrogen fixation parameters (BNF) were inhibited as the salinity level was increased and ameliorated upon addition of calcium; these parameters include nodule number, nodule weight, leghemoglobin, nitrogenase activity. A novel isolate of Rhizobium leguminosarum (OP715892) was supplemented to faba plants for nodulation; it has been inhibited by salinity and enhanced by calcium in terms of the assessed BNF parameters. Although this bacterial strain was inhibited by salinity, it can be inferred that it is a halotolerant one as it survived salinity and its BNF parameters continued and were supportive for the bean plants’ growth, although in defected rates. Plants are usually less tolerant to stress than the microsymbiont (Zahran 1991). The addition of a Ca2+ supplement can recover nodulation inhibited by salt (Etesami and Adl 2020). Moreover, salinity inhibits nitrogen fixation in nodules by the deficiency of important nutrients, which can be recovered by a balanced Ca2+ nutrition (El-Hamdaoui et al. 2003). The role of Ca2+ seems related to the protection of nitrogenase enzyme complex from oxygen (Sabra et al. 2000). Similar studies should establish the best Ca2+ concentration for each type of legume that ensures the success of symbiosis and plant development in salinity (El-Hamdaoui et al. 2003). Since early times, calcium is known as a requirement at the early stages of infection events (Munns 1970) to increase the number of nodules (Lowther and Loneragan 1968), and for symbiotic nitrogen fixation (Banath et al. 1966). Purified nodulation (Nod) factors elicit several physiological responses in susceptible root hair cells, such as calcium influx, membrane depolarization, and rhythmic Ca2+ oscillations (spiking) in and around the nucleus (Oldroyd and Downie 2004). Martins and Livina (2019) supported the idea of two symbiotic Ca2+ signals: a Ca2+ flux at the root hair tip and nuclear Ca2+ oscillations with different frequencies characteristic of different symbiotic phenomena. Ca2+ spiking is a key component in the activation of zone I nodulation via root hair curling (RHC) invasion (Capoen et al. 2009). Nitrogen fixers trigger faster Ca2+ oscillations than during RHC nodulation. Modulation of the ethylene or JA levels slowed down the Ca2+ spiking frequency and stimulated RHC invasion but was incompatible with nodule development. Bioimaging suggests that there are distinct Ca2+ signals predominantly associated with each region: a Ca2+ influx at the root hair tip region, which is thought to be involved in the formation of an infection thread, and nuclear Ca2+ oscillations, which are required for infection thread growth and nodule organogenesis. There are diverse patterns of spatiotemporal Ca2+ signals and dynamics, known as Ca2+ signatures (Miwa et al. 2006; McAinsh and Pittman 2009).
Following the elucidation of the salinity-induced alterations in Vicia faba germination, growth and metabolism, the possible antagonistic or protective effects of calcium against stress was pursued. Calcium has ubiquitous occurrence and interferences in structure and metabolism of plant cells: normal or stressed, in addition to improving soil properties. Accordingly, calcium could, at least partially, restore the physiological activity that has been impaired by the stress imposed (Tester and Davenport 2003). Similarly in this work Na+/Ca2+ ratio of 1/5, enhanced growth of faba shoots and roots by reversing the NaCl-induced impairments in membranes. Calcium usually interferes by counteracting the stress-induced injuries (e.g., to membranes), and thereby energy consumption for maintenance processes is saved. Membrane stability index and injury was also disordered by salinity, while well ordered by calcium. NaCl toxifies plants by the toxicity effect of Na+ and Cl− ions, e.g., via triggering membrane injury and subsequent leakage of metabolites (Jia et al. 2014). In this respect, Cramer et al. (1990) reported that high Ca2+ increased the osmolality for two maize cultivars; Na+ and Ca2+ were the principal solutes involved in osmotic adjustment with minor increases in K+ and soluble sugars. The addition of Ca to ameliorate the harmful effects of NaC1 has been highlighted by La Haye and Epstein (1971). Externally supplied Ca2+ reduces salt toxicity presumably by facilitating higher K+/Na+ selectivity (Cramer et al. 1985; Liu and Zhu 1997). Therefore, applying Na/Ca combinations might have improved soil characteristics, of which availability of nutrients to V. faba plants in this work. Ca2+ is warranted as a second messenger and function in signal transduction of many stimuli. An increase of cytosolic Ca2+ in response to salt potentiates stress signal transduction and leads to salt adaptation (Mendoza et al. 1994). Calcium triggered water influx into tobacco cells; the Ca2+ depleted cells preserved only about half (54%) relative to the control (Ca2+-supplemented) water content (Abdel-Basset and Matsumoto 2008). Changes in intracellular free calcium levels for signal transduction is the onset of response to stresses. The calcium-induced calcium release (CICR) is the calcium-mediated abiotic stress signaling in regulating primary root growth of plants (Wilkins et al. 2016; Zhang et al. 2020).
Respiration of Rhizobium was indicative for its survival at salinity. Leaves respiration followed, more or less, the same attitude, i.e., inhibition by salinity and enhancement by calcium. Increasing NaCl concentration clearly depressed respiration of both roots and shoots. Leaf respiration rates decreased under salinity in most species, but the decline was always smaller than that of photosynthesis, therefore resulting in decreased photosynthesis to respiration ratio (indicative of shoot carbon balance). The decline in respiration in response to salinity seems to be part of a systemic metabolic cascade, which occurs at conditions where salinity severely restricts CO2 availability inside leaf cells, therefore creating the risk of a secondary oxidative stress (Flexas et al. 2008). A response of respiration to salinity is primarily associated with the direct effects of salinity on enzyme function (Walker and Loneragan 1981; Seemann and Critchly 1985). High concentrations of salinity have often been reported to increase respiration: such increase is greater in salt sensitive than salt tolerant species (Semikhatova 1993). Salt inhibited O2 uptake of bacteroids isolated from nodules of faba bean; however, salt treatment of plants decreased respiratory capacity in faba-bean bacteroids. Inhibition of ARA under moderate saline stress may be related to the drop in bacteroid respiration (Delgado et al. 1994). Rates of respiration of Vicia faba were higher in mesophyll cell protoplasts than epidermal cell protoplasts (Long et al. 2015).
In this work, calcium induced ameliorative response of Vicia faba to salinity stress via improving membrane stability, which preserves cellular metabolites from leakage and, thus, served for enhancing growth (fresh and dry mass). Also, nitrogen fixation by Rhizobium was improved and supported growth by nitrogenous intermediates despite the presence of salinity.
Conclusions
Na+ toxicity and other inhibitory effects of NaCl on Vicia faba plants like water or osmotic and ion imbalance stresses can be overcome, at least partially, by substitution of Ca2+ for Na+. Ca2+ concentration can be optimized to antagonize Na+ toxicity. Particularly, Na+/Ca2+ ratio of 1/5 ameliorated the inhibitory action of NaCl and induced improvements in germination and growth of Vicia faba relative to the control or salinized plants. Rhizobium addition was also ameliorative via enhancing nodulation and nitrogen fixation.
Data availability
The data will be provided on request.
References
Abari AK, Nasr MH, Hojjati M, Bayat D (2011) Salt effects on seed germination and seedling emergence of two Acacia species. Afr J Plant Sci 5:52–56
Abdel-Basset R, Issa AA (1994) Membrane stabilization and survival of dehydrated Chlorella fusca cells induced by calcium. Biol Plant 36:389–395
Abdel-Basset R, Matsumoto H (2008) Aluminum toxicity and Ca depletion may enhance cell death of tobacco cells via similar syndrome. Plant Signal Behavior 3(5):290–295
Ahmed AM, Radi AF, Heikal MD, Abdel-Basset R (1989) Effect of Na-Ca combinations on photosynthesis and some related processes of Chlorella vulgaris. J Plant Physiol 135:175–178
Ali AYA, Ibrahim MEH, Zhou G, Nimir NEA, Jiao X, Zhu G, Elsiddig AMI, Suliman MSE, Elradi SBM, Yue W (2020) Exogenous jasmonic acid and humic acid increased salinity tolerance of sorghum. Agronomy 112:871–884
Alnefaie RM, EL-Sayed SA, Ramadan AA, Elmezien AI, El-Taher AM, Randhir TO, Bondok A, (2023) Physiological and anatomical responses of faba bean plants infected with chocolate spot disease to chemical inducers. Life 3(2):392. https://doi.org/10.3390/life13020392
Arvola L (1981) Spectrophotometric determination of chlorophyll a and phaeopigments in ethanol extractions. Ann Bot Fennici 18:221–227
Asaadi AM (2009) Investigation of salinity stress on seed germination of Trigonella foenum-graecum. Res J Biol Sci 4:1152–1155
Assaha DVM, Ueda A, Saneoka H, Al-Yahyai R, Yaish MW (2017) The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front Physiol 8:509. https://doi.org/10.3389/fphys.2017.00509
Ayaz FA, Kadioglu A, Turgut R (2000) Water stress effects on the content of low molecular weight carbohydrates and phenolic acids in Ctenanthe setosa (Rose.) Eichler. Can J Plant Sci 80:373–378
Banath CL, Greenwood EAN, Loneragan JF (1966) Effects of calcium deficiency on symbiotic nitrogen fixation. Plant Physiol 41(5):760–763. https://doi.org/10.1104/pp.41.5.760
Bilgili U, Carpici EB, Asik BB, Celik N (2011) Root and shoot response of common vetch (Vicia sativa L.), forage pea (Pisum sativum L.) and canola (Brassica napus L.) to salt stress during early seedling growth stages. Turk J Field Crop 16:33–38
Bojovic B, Delic G, Topuzovic M, Stankovic M (2010) Effects of NaCl on seed germination in some species from families Brassicaceae and Solanaceae. Krag J Sci 32:83–87
Bremner JM, Mulvaney CS (1982) Nitrogen-total. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, part 2. American Society of Agronomy, Soil Science Society of America, Madison, Wisconsin, Chemical and microbiological properties, pp 595–624
Brenner DJ, Krieg NR Staley JT (2005) The Proteobacteria. In: Garrity GM (ed) Bergey's manual of systematic bacteriology, Vol. 2, 2nd edn. Springer-Verlag, New York, 3 parts
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6(9): e04691. 10.1016/j
Capoen W, Herder JD, Sun J, Verplancke C, Keyser AD, Rycke R, Goormachtig S, Oldroyd G, Holsters M (2009) Calcium spiking patterns and the role of the calcium/calmodulin-dependent kinase CCaMK in lateral root base nodulation of Sesbania rostrata. Plant Cell 21:1526–1540
Cassman KG, Dobermann A (2022) Nitrogen and the future of agriculture: 20 years on. Ambio 51:17–24
Cen Z, Zheng Y, Guo Y, Yang S, Dong Y (2023) Nitrogen fertilization in a faba bean–wheat intercropping system can alleviate the autotoxic effects in faba bean. Plants 12: 1232. https:// doi.org/https://doi.org/10.3390/plants12061232
Chien SC, Liao J, Wang M, Mannepalli MR (2009) Effect of Cl−, SO2− 4, and fulvate anions on Cd2+ free ion concentrations in simulated rhizosphere soil solutions. J Hazard Mater 172:809–817
Cramer GR, Abdel-Basset R, Seeman JR (1990) Salinity-calcium interactions on root growth and osmotic adjustment of two corn cultivars differing in salt tolerance. J Plant Nutr 13:1453–1462
Cramer GR, Läuchli A, Polito VS (1985) Displacement of Ca2+ by Na+ from the plasmalemma of root cells. A primary response to salt stress? Plant Physiol 79(1): 207–211. https://doi.org/10.1104/pp.79.1.207
Cuartero J, Bolarín MC, Asíns MJ, Moreno V (2006) Increasing salt tolerance in the tomato. J Exp Bot 57(5):1045–1058. https://doi.org/10.1093/jxb/erj102
Dai X, Huo Z, Wang H (2011) Simulation for response of crop yield to soil moisture and salinity with artificial neural network. Field Crop Res 121:441–449
Danial AW, Abdel-Basset R, Abdel-Kader HAA (2023) Tuning photosynthetic oxygen for hydrogen evolution in synergistically integrated, sulfur deprived consortia of Coccomyxa chodatii and Rhodobium gokarnense at dim and high light. Photosynth Res 155(2):203–218
Dantas BF, De Sá RL, Aragão CA (2007) Germination, initial growth and cotyledon protein content of bean cultivars under salinity stress. Revista Brasileira Sementes 29:106–110
Dehnavi AR, Zahedi M, Ludwiczak A, Perez SC, Piernik A (2020) Effect of salinity on seed germination and seedling development of sorghum (Sorghum bicolor (L.) Moench) Genotypes. Agronomy 10: 859. https://doi.org/10.3390/agronomy10060859
Delgado MJ, Ligero F, Lluch C (1994) Effects of salt stress on growth and nitrogen fixation by pea, faba-bean, common bean and soybean plants. Soil Biol Biochem 26(3):371–376
Dogan M (2020) Effect of salt stress on in vitro organogenesis from nodal explant of Limnophila aromatic (Lamk.) Merr. and Bacopa monnieri (L.) Wettst. and their physio-morphological and biochemical responses. Physiol Mol Biol Plants 26(4): 803–816. https://doi.org/10.1007/s12298-020-00798-y
El-Beltagi HS, Eshak NS, Mohamed HI, Bendary ESA, Danial AW (2022) Physical characteristics, mineral content, and antioxidant and antibacterial activities of Punica granatum or Citrus sinensis peel extracts and their applications to improve cake quality. Plants 11(13):1740
El-Enany AE, Issa AA, Abdel-Basset R (2001) Calcium enhancement of shoot organogenesis in salinity-stressed tomato explants. Acta Agron Hung 49(1):35–42
El-Hamdaoui A, Redondo-Nieto M, Torralba B, Rivilla R, Bonilla I, Bolaños L (2003) Influence of boron and calcium on the tolerance to salinity of nitrogen-fixing pea plants. Plant Soil 251(1):93–103
El-Zohri M, Hifney AF, Ramadan T, Abdel-Basset R (2014) Use of sewage in agriculture and related activities. In: Pessarakli M (ed) Handbook of plant and crop physiology. 3rd edn. CRC Press, eBook ISBN: 978–1–4665–5329–3, pp 931–966
Etesami PH, Adl SM (2020) Can interaction between silicon and non–rhizobial bacteria help in improving nodulation and nitrogen fixation in salinity–stressed legumes? A Review Rhizosphere 15:100229
FAO (2021) Global network on integrated soil management for sustainable use of salt affected soils. FAO Land and Plant Nutrition Management Service: Rome, Italy
Farhoudi R, Tafti MM (2011) Effect of salt stress on seedlings growth and ions homeostasis of soybean (Glycine max) cultivars. Adv Environ Biol 5:2522–2526
Flexas JJ, Bota F, Loreto GC, Sharkey TD (2008) Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol 6:269–279
Gachande B, Khansole G (2011) Morphological, cultural and biochemical characteristics of Rhizobium japonicum syn and Bradyrhizobium japonicum of soybean. Biosci Discov 2(1):1–4
Geressu K, Gezaghegne M (2008) Response of some lowland growing sorghum (Sorghum bicolor L. Moench) accessions to salt stress during germination and seedling growth. Afr J Agric Res 3:044–048
Hakim MA, Juraimi AS, Begum M, Hanafi MM, Ismail MR, Selamat A (2010) Effect of salt stress on germination and early seedling growth of rice (Oryza sativa L.). Afr J Biotech 9(13): 1911–1918
Hussein H, Lambert LAA (2020) Rentier state under blockade: Qatar’s water-energy-food predicament from energy abundance and food insecurity to a silent water crisis. Water 12(4):1051. https://doi.org/10.3390/w12041051
Jia Y, Chen K, Lin P, Lieber G, Nishi M, Yan R, Wang Z, Yao Y, Li Y, Whitson BA, Duann P, Li H, Zhou X, Zhu H, Takeshima H, Hunter JC, McLeod RL, Weisleder N, Zeng C, Ma J (2014) Treatment of acute lung injury by targeting MG53-mediated cell membrane repair. Nat Commun 5:4387
Juan CA, la Lastra JMP, Plou FJ, Pérez-Lebeña E (2021) The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int J Mol Sci 22(9):4642. https://doi.org/10.3390/ijms22094642
Kawasaki T, Akiba T, Moritsugu M (1983) Effects of high concentrations of sodium chloride and polyethylene glycol on the growth and ion absorption in plants: I. Water culture experiments in a greenhouse. Plant Soil 75:75–85
Kaymakanova M, Stoeva N (2008) Physiological reaction of bean plants (Phaseolus vulgaris L.) to salt stress. Gen Appl Plant Physiol 34:177–188
Keshavarizi B, Mohammed H (2012) Studying the effects of different levels of salinity which caused by NaCl on early and germination of Lactuca sativa L. seedling. J Stress Physiol Biochem 8:203–208
Koyro HW (2002) Ultrastructural effects of salinity in higher plants. In: Läuchli A, Lüttge U (eds) Salinity: Environment—plants—molecules. Kluwer: Amsterdam, The Netherland, pp 139–157
Krishnamurthy L, Serraj R, Hash CT, Dakheel AJ, Reddy BVS (2007) Screening sorghum genotypes for salinity tolerant biomass production. Euphytica 156:15–24
Lahaye PA, Epstein E (1971) Calcium and salt toleration by bean plants. Physiol Plant 25(2):213–218. https://doi.org/10.1111/j.1399-3054.1971.tb01430.x
LaRue TA, Child, (1979) Sensitive fluorometric assay for leghemoglobin. Anal Biochem 92(1):11–15. https://doi.org/10.1016/0003-2697(79)90618-3
Liu J, Zhu JK (1997) Proline accumulation and salt-stress-induced gene expression in a salt-hypersensitive mutant of Arabidopsis. Plant Physiol 114(2):591–596. https://doi.org/10.1104/pp.114.2.591
Long BM, Bahar NHA, Atkin OK (2015) Contributions of photosynthetic and non-photosynthetic cell types to leaf respiration in Vicia faba L. and their responses to growth temperature. Plant Cell Environ 38(11): 2263–2276. doi: https://doi.org/10.1111/pce.12544
Lotfi R, Abbasi A, Kalaji H, Eskandari I, Sedghieh V, Khorsandi H, Sadeghian N, Yadav S, Rastogi A (2022) The role of potassium on drought resistance of winter wheat cultivars under cold dryland conditions: Probed by chlorophyll a fluorescence. Plant Physiol Biochem 182:45–54
Lowry OH, Rosebrough NJ, Fare AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Lowther W L, Loneragan JF (1968) Calcium and nodulation in subterranean clover (Trifolium subterraneum L.). Plant Physiol 43(9):1362–1366. https://doi.org/10.1104/pp.43.9.1362
Mahender A, Anandan A, Pradhan SK (2015) Early seedling vigour, an imperative trait for direct-seeded rice: an overview on physio-morphological parameters and molecular markers. Planta 241(5):1027–1050. https://doi.org/10.1007/s00425-015-2273-9
Martins TV, Livina VN (2019) What drives symbiotic calcium signalling in legumes? Insights and challenges of imaging. Int J Mol Sci 20(9):2245. https://doi.org/10.3390/ijms20092245
Mbinda W, Kimtai M (2019) Evaluation of morphological and biochemical characteristics of sorghum [Sorghum bicolor [L.] Moench] varieties in response salinity stress. Annu Res Rev Biol 33(1): 1–9. https://doi.org/10.1101/720789
McAinsh MR, Pittman JK (2009) Shaping the calcium signature. New Phytol 181:275–294
Mehlich A (1984) Mehlich 3 soil test extractant. A modification of the Mehlich 2 extractant. Comm Soil Sci Plant Anal 15:1409–1416. https://doi.org/10.1080/00103628409367568
Mendoza I, Rubio F, Rodriguez-Navarro A, Pardo JM (1994) The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. J Biol Chem 2691(1):8792–8796
Metzner H, Rau H, Senger H (1965) Untersuchungen zur Synchronisierbareit einzelner Pigment mangel Mutanten von Chlorella. Planta 65:186–194
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428. https://doi.org/10.1021/ac60147a030
Misra N, Gupta AK (2005) Effect of salt stress metabolism in two high yielding genotypes of green gram. Plant Sci 169:331–339
Miwa H, Sun J, Oldroyd GED, Downie JA (2006) Analysis of calcium spiking using a cameleon calcium sensor reveals that nodulation gene expression is regulated by calcium spike number and the developmental status of the cell. Plant J 48:883–894
Munns DN (1970) Nodulation of Medicago sativa in solution culture. V. Calcium and pH requirements during infection. Plant Soil 32:90–102
Nijabat A, Bolton A, Mahmood-ur-Rehman M, Shah AI, Hussain R, Naveed NH, Ali A, Simon P (2020) Cell membrane stability and relative cell injury in response to heat stress during early and late seedling stages of diverse carrot (Daucus carota L.) germplasm. HortSci 55(9): 1446–1452
Nimir A, Eltyb N, Lu S, Zhou G, Ma BL, Guo W, Wang Y (2014) Exogenous hormones alleviated salinity and temperature stresses on germination and early seedling growth of sweet sorghum. Agron J 106:2305–2315
Oldroyd GED, Downie JA (2004) Calcium, kinases and nodulation signalling in legumes. Nat Rev Mol Cell Biol 5(7):566–576. https://doi.org/10.1038/nrm1424
Othman Y, Al-Karaki G, Al-Tawaha AR, Al-Horani A (2006) Variation in germination and ion uptake in barley genotypes under salinity conditions. World J Agric Sci 2:11–15
Poovaiah BW, Reddy, (1987) Calcium messenger system in plants. Rev Plant Sci 6(1):47–103. https://doi.org/10.1080/07352688709382247
Rehman S, Harris PJC, Bourne WF, Wilkin J (2000) The relationship between ions, vigour and salinity tolerance of Acacia seeds. Plant Soil 220:229–233
Ruan S, Xue Q, Tylkowska K (2002) The influence of priming on germination of rice (Oryza sativa L.) seeds and seedling emergence and performance in flooded soil. Seed Sci Technol 30:61–67
Ryu H, Cho YG (2015) Plant hormones in salt stress tolerance. J Plant Biol 58:147–155
Sabra W, Zeng AP, Lünsdorf H, Deckwer WD (2000) Effect of oxygen on formation and structure of Azotobacter vinelandii alginate and its role in protecting nitrogenase. Appl Environ Microbiol 66(9):4037–4044. https://doi.org/10.1128/aem.66.9.4037-4044.2000
Seemann NJR, Critchley C (1985) Effect of salt stress on the growth, ion content, stomatal behaviour and photosynthetic capacity of a salt sensitive species, Phaseolus vulgaris L. Planta 164:151–162
Semikhatova O, Ivanova T, Yudina O (1993) Respiratory cost of plant growth under conditions of salinity. Russian Plant Physiol 40:558–566
Shrivastava P, Kumar R (2015) Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J Biol Sci 22:123–131. https://doi.org/10.1016/j.sjbs.2014.12.001
Singleton PW, Bohlool BB (1984) Effect of salinity on nodule formation by soybean. Plant Physiol 74(1):72–76. https://doi.org/10.1104/pp.74.1.72
Somasegaran P, Hoben HJ (1985) Methods in legume-rhizobium technology. NifTAL Project and MIRCEN. Department of Agronomy, 2nd Soil Science, Hawaii Institute Tropical Agriculture Human Research, University of Hawaii at Manoa, Honolulu, pp 1–52
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91(5):503–527. https://doi.org/10.1093/aob/mcg058
Vincent JM (1970) A manual for practical study of root nodule bacteria. IBP Handbook No. 15. Blackwell Scientific Publishers, Oxford
Wahid A, Farooq M, Basra SMA, Rasul E, Siddique KHM (2011) Germination of seeds and propagules under salt stress. In: Pessarakli M (ed) Handbook of plant and crop stress, 3rd edn. CRC Press: Boca Raton, FL, USA, pp 321–337
Walker CD, Loneragan JF (1981) Effects of copper deficiency on copper and nitrogen concentrations and enzyme activities in aerial parts of vegetative subterranean clover plants. Ann Bot 48(1):65–73
Walkley AJ, Black IA (1934) Estimation of soil organic carbon by the chromic acid titration method. Soil Sci 37:29–38
Wang LG, Ye CL, Chen J, Li JJ, Luo JJ (2021) Effects of oil flax/wheat intercropping and oil flax wheat rotation on soil physical and chemical properties and flax growth. China Agric Sci Technol Bull 23:161–171
Wilkins KA, Matthus E, Swarbreck SM, Davies JM (2016) Calcium-mediated abiotic stress signaling in roots. Front Plant Sci 7:1296. https://doi.org/10.3389/fpls.2016.01296
Zahran HH (1991) Conditions for successful Rhizobium-legume symbiosis in saline environments. Biol Fertil Soils 12:73–80
Zahran HH (1999) Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol Mol Biol Rev 63:968–989
Zhang XP, Ma CX, Sun LR, Hao FS (2020) Roles and mechanisms of Ca2+ in regulating primary root growth of plants. Plant Signal Behav 15(5):1748283. https://doi.org/10.1080/15592324.2020.1748283
Zhang Y, Guanter L, Joiner J, Song L, Guan K (2018) Spatially‐explicit monitoring of crop photosynthetic capacity through the use of space‐based chlorophyll fluorescence data. Remote Sensing Environ 210: 362–374. https://doi.org/10.1016/j.rse.2018.03.031
Zheng Y, Guo Y, Li Y, Yang W, Dong Y (2022) Intercropping of wheat alleviates the adverse effects of phenolic acids on faba bean. Front Plant Sci 13:997768
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This work was financially supported by the Assiut University fund (www.aun.edu.eg)
Author information
Authors and Affiliations
Contributions
The first author (A. W. D.) designed and implemented the experiments, data recording and analysis, statistical analysis, writing the manuscript, and followed up publication. The second author (R. A) shared in designing the experiments and writing the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Danial, A.W., Basset, R.A. Amelioration of NaCl stress on germination, growth, and nitrogen fixation of Vicia faba at isosmotic Na–Ca combinations and Rhizobium. Planta 259, 69 (2024). https://doi.org/10.1007/s00425-024-04343-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-024-04343-z