Abstract
Main conclusion
Integrated root phenotypes and transcriptome analysis have revealed key candidate genes responsible for maize root growth and development in potassium deficiency.
Abstract
Potassium (K) is a vital macronutrient for plant growth, but our understanding of its regulatory mechanisms in maize root system architecture (RSA) and K+ uptake remains limited. To address this, we conducted hydroponic and field trials at different growth stages. K+ deficiency significantly inhibited maize root growth, with metrics like total root length, primary root length, width and maximum root number reduced by 50% to 80% during early seedling stages. In the field, RSA traits exhibited maximum values at the silking stage but continued to decline thereafter. Furthermore, K deprivation had a pronounced negative impact on root morphology and RSA growth and grain yield. RNA-Seq analysis identified 5972 differentially expressed genes (DEGs), including 17 associated with K+ signaling, transcription factors, and transporters. Weighted gene co-expression network analysis revealed 23 co-expressed modules, with enrichment of transcription factors at different developmental stages under K deficiency. Several DEGs and transcription factors were predicted as potential candidate genes responsible for maize root growth and development. Interestingly, some of these genes exhibited homology to well-known regulators of root architecture or development in Arabidopsis, such as Zm00001d014467 (AtRCI3), Zm00001d011237 (AtWRKY9), and Zm00001d030862 (AtAP2/ERF). Identifying these key genes helps to provide a deeper understanding of the molecular mechanisms governing maize root growth and development under nutrient deficient conditions offering potential benefits for enhancing maize production and improving stress resistance through targeted manipulation of RSA traits in modern breeding efforts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potassium (K) is an essential macronutrient for plant growth and development, and its role in various critical physiological processes in plants cannot be overstated. K plays a fundamental role in plant growth and development by enhancing photosynthetic assimilation (Tränkner et al. 2018), improving nutrient uptake (Sustr et al. 2019; Xu et al. 2020), maintaining adequate leaf inclination (Carroll et al. 1994), controlling stomata-opening (Andrés et al. 2014), facilitating chloroplast integrity and light-absorption efficiency, regulating rubisco biosynthesis and activity (Jia et al. 2008; Jákli et al. 2017). It is well-established in regulating plant water balance and ion homeostasis (Schachtman and Shin 2007). Overall, K+ plays a crucial part in regulating transport of water, metabolites, and nutrients throughout different plant organs and tissues, as well as in protecting plants from oxidative stress while maintaining osmotic balance.
Although grain crop production has significantly improved over the past few decades, the challenge of meeting global food demands remains significant, particularly due to the persistent problem of inadequate access to energy and protein in the diets of more than 10% of the world’s population (Ray et al. 2013; Grote et al. 2021). Maize is an important cereal crop cultivated extensively for food and feed production (Ranum et al. 2014). It is a critical food crop that plays a significant role in global food security, providing 20% of the world's caloric and 15% of the protein intake (Bhatnagar et al. 2004). For optimal growth and productivity, maize requires high levels of K (Kogbe and Adediran 2003). Nevertheless, K deficiency is a pervasive issue in several maize-growing regions, leading to a significant reduction in crop yield and quality (Wu et al. 2014; Ul-Allah et al. 2020). Hence, comprehending the mechanisms of K uptake, transport, and utilization in maize is crucial for enhancing crop performance and sustainability.
Despite the importance of K in maize nutrition, relatively little is known about the underlying physiological and molecular mechanisms that control K uptake and allocation in different tissues and developmental stages. In this study, we aimed to investigate the performance of maize cultivar CD30 under low K conditions and to identify the key genes and pathways involved in response to K deficiency. Specifically, we addressed two main research questions: (i) How does the root system architecture of maize respond to K deprivation at different growth stages? (ii) What are the transcriptomic changes in maize roots under low K stress, and what are the hub genes and pathways involved in post-transcriptional modulation of the response to K stress? K+ plays a critical role in various levels of root system growth and development, including protein synthesis, enzyme activity, cell expansion, root maturation, and phloem transport (Zhao et al. 2016; Zhang et al. 2020; Feng et al. 2021). Root system architecture and root hair coverage can adapt to enhance K+ uptake under K limiting conditions, but the molecular mechanism governing this phenomenon in maize remains elusive. Recently, the transcriptome profiles of plant roots under K deficiency have been analyzed in different crops, including rice, wheat, soybean, cotton, and tomato (Ruan et al. 2015; Zhao et al. 2018; Ma et al. 2020; Yang et al. 2021). These analyses have highlighted the crucial role of metabolic pathways and regulatory genes in maintaining plant growth under low K conditions. Additionally, the significance of the root as the primary site for mineral nutrient uptake was emphasized, with a particular emphasis on its role in K uptake regulation. Regulators such as high-affinity K+ transporters regulate of root K uptake in response to deficiency, further underscoring the root's importance (Sustr et al. 2019). Recently, a study explored the growth, transcriptional, and metabolic responses of maize shoots to long-term potassium deficiency. They found that under K insufficiency conditions, the biomass yield of silage maize decreased, and several genes were differentially regulated, particularly many stress-induced genes (Xiong et al. 2022). Given the importance of K deficiency in several aspects of plant growth, it is essential to understand how root architecture is modulated under inadequate K nutrition.
To answer these questions, we conducted a series of experiments to evaluate the effects of K deficiency on maize growth, biomass accumulation, root system architecture (RSA), and grain yield, as well as the changes in gene expression and regulation at five key developmental stages. We used a hydroponic pouch system to simulate K deprivation in early seedling stages and conducted field trials to assess the phenotypic traits and nutrient concentrations at different growth stages. We also performed transcriptomic analysis using RNA sequencing (RNA-seq) to identify differentially expressed genes (DEGs) and co-expression modules in response to K stress and to identify the hub genes and pathways involved in post-transcriptional modulation of the response to K stress.
Materials and methods
Plant material
We used Zea mays cv. Chengdan 30 (CD30), a high-yielding spring maize cultivar developed by the Sichuan Academy of Agricultural Sciences in 2004 and being currently dominant in the hilly region of central Sichuan (Guo et al. 2022).
Assessment of root system architecture (RSA) in hydroponics
Maize seeds were surface-sterilized with 8% (v/v) sodium hypochlorite for 20 min. The sodium hypochlorite was removed by washing the seeds with distilled water three times. The seeds were then germinated in moistened filter paper. Four days after germination, seedlings with similar growth vigor were transferred to 100 L polypropylene tubs and grown hydroponically using a specially designed and constructed plant growth pouch system, which holds the seedling and root system on the surface of filter paper, as described by Gladman et al. (2022). The full strength nutrient solution contains: 1 mM Ca(NO3)2·4H2O, 1 mM NH4NO3, 1 mM KCl, 0.85 mM MgSO4·7H2O, 0.25 mM NH4H2PO4, 77 μM Fe-EDTA, 25 μM H3BO3, 0.8 μM Na2MoO4·2H2O, 0.6 μM CuSO4·5H2O, 9 μM MnCl2·4H2O, 2 μM ZnSO4·7H2O. For a nutrient solution with low-K (LK) treatment, the potassium concentration was adjusted to 10 μM and 1000 μM as sufficient K conditions (SK), respectively. The pH of the solution was adjusted to 5.7 ± 0.1 with NaOH or HCl as required and was continuously aerated and replaced every 3 days. The seedlings were cultured in a growth chamber (16 h of light at 27 °C, 8 h dark at 22 °C, a photosynthetic photon-flux density of ~ 350 µmol m−2 s−1 at canopy height, and 60% relative humidity). Each treatment had six replicates.
After 14 days of growth in hydroponics, individual pouches with one maize plant were removed from the hydroponic tub. The plant on the pouch system was carefully placed horizontally in a glass tray containing water, and the root system was submerged in the water to collect root system images using Plant Root Imaging and Data Acquisition (PRIDA), a Python-based image acquisition and data management software (http://www.plantmineralnutrition.net/software/prida/index.html). The images were extracted as TIFF files for further processing and computation of root traits. WinRHIZO software (Regents Instruments Inc, Quebec, Canada) was used to quantify root growth and topology traits, while GiA Roots (Galkovskyi et al. 2012) was used to quantify 2D root architecture traits. The investigated root architecture traits included: total root length (cm), root surface area (cm2), primary root length (cm), root width (cm), average root diameter (cm), convex area (cm2), maximum root number, and root volume (cm3). After imaging, the whole maize seedlings were harvested, and the shoots and roots were dissected. The samples were heated at 105 ℃ for 30 min and then oven-dried at 65 ℃ for 72 h. The shoot dry weight, root dry weight, and root-to-shoot ratio (R/S) were determined by weighing the samples.
Field experiment, sample collection and phenotype investigation
A field experiment was conducted at Sichuan Agricultural Research Institute Modern Experimental Station, Deyang City, Sichuan Province. The experimental design was a spit-plot with potassium (K) fertilizer treatments in the main plots and the cultivar in the subplots. The variable between two application rates of K fertilizer, namely 150 kg K ha−1 (SK) and no K supplied (LK). No K fertilizer has been applied since 2012. The plots were fertilized with 300 kg ha−1 N and 90 kg ha−1 P2O5. Phosphorus and K fertilizers were applied before sowing, 50% of N fertilizer was applied as a base dressing, and the remaining 50% was applied at the stem jointing stage. Soil physical and chemical characteristics were evaluated at the beginning of the experiment for SK and LK treatments by analyzing three soil samples, respectively (Suppl. Table S1). The topsoil layer (0–30 cm) in SK treatment contained organic matter 11.4 g kg−1, total N 0.83 g kg−1, alkali-hydrolyzable N 63 mg kg−1, available phosphorus (Olsen-P) 5.9 mg kg−1, ammonium acetate extractable potassium 133 mg kg−1 and pH 8.60 (1:1.25 g/v). In LK treatment, chemical characteristics were organic matter 9.9 g kg−1, total N 0.76 g kg−1, alkali-hydrolyzable N 55 mg kg−1, available phosphorus (Olsen-P) 5.7 mg kg−1, ammonium acetate extractable potassium 93 mg kg−1 and pH 8.61. The subplot area was 20 m2 (5-m-long × 4-m-width). Maize was seeded on 1st April and harvested on 11th August 2021.
During specific developmental stages, namely the 10th leaf stage (V10), tasseling stage (VT), silking stage (R1), milk-ripe stage (R3), and physiological maturity (R6), root samples were obtained for RNA sequencing. We selected three uniform phenotype plants for each stage, carefully extracting two layers of roots from beneath the soil surface. These root samples were subsequently cleaned using running water and combined into a single representative sample. To ensure robust analysis, we collected three replicates for each developmental stage, promptly freezing them in liquid nitrogen, and then storing them at − 80 °C for subsequent RNA isolation.
Total RNA was isolated from root tissues using a Trizol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. RNA integrity was confirmed using the 2100 Bioanalyzer system (Agilent Technologies, Santa Clara, CA, USA). Reverse transcription was performed to synthesize first-strand cDNAs as templates for preparing mRNA-seq libraries. All RNA-seq libraries were constructed using a Ribo-Zero rRNA Removal kit (Illumina Inc., San Diego, CA, USA) followed by a TruSeq Stranded Total RNA Library Prep Plant and then sequenced on an Illumina HiSeq2500 Platform (NovoMagic, Beijing, China).
Root system architecture (RSA) was evaluated at each developmental stage. Three uniform phenotype plants were selected from the central rows of each subplot, then the soil volume with 33 cm in width, 50 cm in length and 30 cm in depth surrounding the plant was excavated, and the whole seedlings were dissected into shoot and root. The roots were cleaned with running water. Root segments were floated in water in an acrylic tray on the scanner (ESPON Perfection V850). The scanned images were analyzed with WinRHIZO (Regents Instruments Inc.) commercial software to obtain total root length, surface area, volume and average root diameter. After imaging, the shoot and root were heated at 105 ℃ for 30 min and then oven dried at 65 °C for 72 h to obtain shoot and root dry weight. The grain yield was determined and then standardized to 14% moisture. Total N concentration in grain and plant shoot was determined by the semi-micro Kjeldahl procedure (Bremner 1996), total P concentration by spectrophotometer (Chapman and Pratt 1962) and total K concentration by flame photometer (Chapman and Pratt 1962), respectively.
Alignment and identification of differentially expressed genes
The RNA-seq reads generated by the Illumina Genome Analyzer were initially preprocessed to remove low-quality reads and adaptor sequences. After filtering, the clean reads of each sample were used to align the reads to the maize reference genome (B73 RefGen_V4, http://ensemblgramene.org/Zea_mays/Info/Index) using Hisat2 (Kim et al. 2015). Differentially expressed genes (DEG) were identified by the DESeq2 package in R (Love et al. 2014) with the absolute value of the Log2 fold change ≥ 1.0, P value < 0.05 (Luo et al. 2021; Gao et al. 2022) between LK and SK conditions at each growth stage. The fragments per kilobase of transcript per million reads (FPKM) of each gene were calculated for further use.
Functional enrichment analysis
To determine which DEGs were significantly enriched in Gene Ontology (GO) terms at a Bonferroni-corrected P value < 0.05 compared to the whole-transcriptome background, we conducted GO enrichment analysis. The GO functional enrichment analysis was performed using agriGO v2.0 (http://systemsbiology.cau.edu.cn/agriGOv2/index.php) with singular enrichment analysis (Du et al. 2010). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway annotation and enrichment analysis of the DEGs was performed using the website https://www.genome.jp/kegg/, with a corrected P value of 0.05 as the threshold for significantly enriched KEGG.
Weighted gene co-expression network analysis (WGCNA)
The FPKM value of each gene from the five comparisons (i.e., V10, VT, R1, R3, and R6) was used for co-expression analysis using the WGCNA (Langfelder and Horvath 2008). The soft threshold was determined whereby more than 85% of the models would fit to scale-free topology and low mean connectivity. The minimum gene number assigned to one module was set as 30. The top 20 connected hub genes in each module were used to construct the module network graphs using Cytoscape software (version 3.7.1).
Statistical analysis
We performed all statistical analyses using the R software package (Team 2013). To test for significant differences between treatments, we used a one-way analysis of variance (ANOVA), which was conducted using the “Anova” function as implemented in the “car” package (Fox et al. 2012). The data were visualized using the “ggplot2” package (Wickham 2016).
Results
Performance of root system architecture response to low K
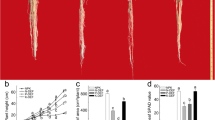
We evaluated maize cultivar CD30 performance of the above-ground and below-ground parts at early seedling and late developmental stages in response to potassium (K) deficit (Figs. 1 and 2). In the hydroponic pouch system, K deprivation reduced shoot dry weight by 67.7% (Fig. 1b). Significant differences were also observed in RSA between sufficient K (SK) and low K (LK) treatments (Fig. 1a). Total root length, primary root length, root width, and maximum root number were reduced under K starvation by 82.4%, 51.9%, 52.2%, and 62.7%, respectively. A similar trend was observed for root surface area, convex area, and root volume. The average root diameter increased by 11.5% under LK conditions (Fig. 1c–j).
Root system architecture of maize cultivar grown under sufficient K (SK) and low K (LK) conditions. Plants were grown in a hydroponic pouch system and harvested at 14 days after transplanting. a Representative root images of CD30 under SK and LK conditions, b Shoot dry weight, c total root length, d root surface area, e primary root length, f root width, g average root diameter, h convex area, i maximum root number, and j root volume under SK and LK conditions. Mean values ± SE, n = 6. Asterisks indicate the significance of differences between treatments, as determined by Student’s t test: *Significant at P < 0.05; **Significant at P < 0.01; ***Significant at P < 0.001
Phenotypic identification of maize cultivar in the field. a Shoot dry weight, b shoot N concentration, c shoot P root dry weight concentration, d shoot K concentration, e grain yield, f seed N concentration, g seed P concentration, and h seed K concentration at five different developmental stages under sufficient K (SK) and low K (LK) conditions. Mean values ± SE, n = 3. Asterisks indicate the significance of differences between treatments, as determined by Student’s t test: *Significant at P < 0.05; **Significant at P < 0.01; ***Significant at P < 0.001. V10: the 10th leaf stage, VT tasseling stage, R1 silking stage, R3 milk-ripe stage, R6 physiological maturity
In the field, phenotypic identification of RSA and biomass-related traits was carried out at V10, VT, R1, R3, and R6 stages, respectively. Grain yield and N, P, and K concentrations in seeds were also investigated at the mature stage. Shoot dry weight in SK is slightly higher than in LK condition at different developmental stages. Only a significant difference was observed at VT (Fig. 2a). However, K concentration in the shoot was reduced significantly in response to LK starvation at all tested stages. K deficit may not affect N and P concentration in the shoot part (Fig. 2b–d). K starvation led to a 16.8% (P < 0.05) decrease in grain yield, a 2.2% (P < 0.05) decrease in seed K concentration, and a 6.8% (P < 0.01) in seed P concentration, respectively, but not altered seed N concentration (Fig. 2e–h). Root dry weight was increased from V10 to VT in both conditions, reached the maximum values at R1 stages and then continued decline. Significant differences in root dry weight were observed at VT and R3 stages between SK and LK conditions (Fig. 3a). A similar pattern was observed in RSA traits, where SK showed an average larger total root length (16.3%), root surface area (10.3%), and root volume (14.7%) than in LK conditions (Fig. 3b–d).
Root system characterization in field conditions. a Root dry weight, b total root length, c average root diameter, d root surface area, and e root volume at five different developmental stages under sufficient K (SK) and low K (LK) conditions. Mean values ± SE, n = 3. Asterisks indicate the significance of differences between treatments, as determined by Student’s t test: *Significant at P < 0.05; **Significant at P < 0.01; ***Significant at P < 0.001. V10 the 10th leaf stage, VT tasseling stage, R1 silking stage, R3 milk-ripe stage, R6 physiological maturity
Transcriptomic of maize root response to low K stress
We performed transcriptomic analysis of maize roots by RNA-seq at five key developmental stages (V10, VT, R1, R3, and R6). To mitigate background influences, the roots from three plants were pooled as one sample. Thirty RNA-seq libraries were constructed and sequenced from maize roots at different developmental stages with three biological replicated under SK and LK conditions. Totally, 142 million raw reads were generated for all the samples, and 138 million clean reads (97.27%) were obtained after performing quality control with a Q20 value of 97.68%. The average GC content is 54.83% (Suppl. Table S2). Then, the clean reads were aligned against the maize reference genome (B73 RefGen_V4), and an average of 82.43% clean reads were mapped to the B73 reference genome (Suppl. Table S3). A 77.72% of known genes (30,565 known genes vs. 39,324 reference genes) were covered, and an average of 4,811 new genes were detected among the five developmental stages. The detected known gene number of each stage were 31,276 (LK) and 31,063 (SK) for V10, 30,673 (LK) and 31,137 (SK) for VT, 30,783 (LK) and 30,221 (SK) for R1, 30,071 (LK) and 30,056 (SK) for R3, 30,124 (LK) and 30,294 (SK) for R6 (Suppl. Table S4) The Pearson correlation analysis plots showed strong correlation coefficient among the replicates under SK and LK conditions, respectively, indicating the reliability of our RNA-seq data (Suppl. Fig. S1).
We performed a pairwise comparison between SK and LK conditions at five developmental stages. Totally, 5,972 genes were referred to as DEGs and used for the subsequent analysis. The number of DEGs at each stage was as follows: 2,316 (V10), 740 (VT), 1,090 (R1), 1,306 (R3), and 520 (R6), respectively. Of those DEGs, 4,315 specific genes were identified at all stages, 678 genes were shared by two development stages, 93 genes by three, two genes by four, and one gene by five stages, respectively (Fig. 4a–c). To better understand the dynamic changes of gene expression in maize roots with all the five developmental stages, further analyses of the DEGs were performed, especially of those genes in which up- or down-regulated follow root development. Within the identified DEGs, 1,003, 193, 615, 824, and 170 DEGs were up-regulated in stages V10, VT, R1, R3 and R6. Likewise, 1,313 (V10), 547 (VT), 475 (R1), 482 (R3), and 350 (R6) DEGs were down-regulated at each developmental stage (Fig. 4d).
Differentially expressed genes (DEGs) analysis in maize cultivar CD30 in response to K stress. a Venn diagram of DEGs, b DEGs number in each developmental stage, c Shared DEGs number among the five different stages, d Summary of up-regulated and down-regulated DEGs number in each developmental stage. V10: the 10th leaf stage, VT tasseling stage, R1 silking stage, R3 milk-ripe stage, R6 physiological maturity
Additionally, many genes related to the K+ signaling pathway, transcription factors and transporters were identified (Table 1). For instance, a type III peroxidase family, Rare Cold Inducible gene 3 (RCI3), was a component of the low K signal transduction pathway in Arabidopsis roots. It was reported that RCI3 was induced upon K deprivation and participated in the induction of the high-affinity K+ uptake transporter HAK5, which ultimately facilitated K acquisition under K starvation (Kim et al. 2010). In maize roots, Zm00001d014467, the homolog gene of RCI3, was up-regulated in response to LK condition in V10 and R1 stages. Several other K signaling genes such as SYNTAXIN OF PLANTS 121 (Zm00001d013114), CALCINEURIN B-LIKE INTERACTING PROTEIN KINASE 23 (Zm00001d018799), CALCINEURIN B-LIKE PROTEIN 10 (Zm00001d044285) were also observed up-regulated in specific developmental stage under LK conditions. Moreover, important transcription factors (TFs) related to K metabolism were found on the list. Maize WRKY TRANSCRIPTION FACTOR 9 (Zm00001d011237, Zm00001d018203) was significantly induced by LK in VT, R3, and R6 stages (Table 1). In Arabidopsis, RAP2.11 is an AP2/ERF transcription factor, identified as a component in response to low K affecting root hair development (Kim et al. 2012). The homolog gene in maize, Zm00001d030862, was observed down-regulated in root tissue at the earlier developmental stage. Remarkably, the homolog gene of high-affinity K+ uptake transporter HAK5 in maize, Zm00001d033067, down-regulated in root tissue at the V10 stage and up-regulated upon K deprivation at later developmental stages. The transporter POTASSIUM CHANNEL PROTEIN (Zm00001d016160) was notably induced in response to K starvation at V10, VT, and R1 stages, respectively (Table 1).
Co-expression network analysis
A gene regulation network was built using the WGCNA method to obtain a comprehensive understanding of the transcriptional network in the successive developmental stages and to clarify that it controls the adaptation of LK condition in maize roots. Totally, 24,043 expressed genes identified in 30 samples were divided into 23 co-expressed modules (CMs), color-coded for reference, with the CM grey set containing the genes that could not be assigned to other CMs (Fig. 5a). The 23 CMs correlated with phenotypic traits are shown in Fig. 5b. A positive correlation was observed in modules of magenta and green with root biomass and RSA traits. There was a positive correlation of magenta (P < 0.01), green (P < 0.01), and red (P < 0.001) CMs with shoot dry weight and a positive correlation of the pink (P < 0.001) module with shoot K concentration. A negative correlation with shoot dry weight was observed in light yellow, midnight blue, and pink CMs.
Weighted gene co-expression network analysis (WGCNA). a The significantly differentially expressed genes were assigned to different co-expression modules (CMs). CMs were identified via the Dynamic Tree Cut method; the merged dynamic indicates modules divided according to the similarity of the module (represented by assigned module colors). The subsequent analysis was conducted considering the merged modules. In the tree diagram, the vertical distance indicates the separation between two nodes, corresponding to the genetic distance between the genes. b The correlative relationships of the assigned CMs and root system architecture. *Significant at P < 0.05; **Significant at P < 0.01; ***Significant at P < 0.001
We further analyzed the magenta, green, red, light green, light yellow, midnight blue, and pink modules among all K deficit responding modules. Gene ontology enrichment analysis of each selected module highlighted key biological processes by a set of co-expressed genes (Fig. 6a and Suppl. Table S5). The CM magenta (799 genes), associated with shoot dry weight, root dry weight, total root length, root surface area, and root volume traits, showed enrichment of GO terms related to nucleic acid metabolic process, nucleobase-containing compound metabolic process, and regulation of gene expression. A total of 1372 genes grouped into the CM green, and RNA modification, RNA metabolic process, and nitrogen compound metabolic process were enriched in this module. The red module consisted of 1216 genes and showed enrichment of GO terms related to RNA processing, nitrogen compound metabolic process and nucleic acid metabolic process. The CM light yellow identified 157 genes and was mainly involved in the cellular response to starvation and extracellular stimulus. A total of 194, 286, and 971 genes grouped into the light green, midnight blue, and pink modules, respectively. Likewise, the CMs do not show a significant enrichment of GO terms in biological processes (Fig. 6a and Suppl. Table S5).
The DEGs identified in the CM green were found to be significantly enriched in several KEGG pathways, including circadian rhythm—plant (zma04712), spliceosome (zma03040), RNA transport (zma03013), purine metabolism (zma00230), ubiquitin-mediated proteolysis (zma04120), and aminoacyl-tRNA biosynthesis (zma00970). In CM light green, a noteworthy KEGG pathway enriched was zeatin biosynthesis (zma00908). In contrast, two significant KEGG pathways, glycerophospholipid metabolism (zma00564) and glycerolipid metabolism (zma00565), were observed in the CM light yellow. In the CM midnight blue, the DEGs were notably enriched in four KEGG pathways: Galactose metabolism (zma00052), plant hormone signal transduction (zma04075), MAPK signaling pathway—plant (zma04016), and carotenoid biosynthesis (zma00906). In the case of CM magenta and red, the DEGs were significantly enriched in the RNA polymerase (zma03020) pathway (Fig. 6b and Suppl. Table S6).
Hub genes involved in post-transcriptional modulation are essential regulators in response to K stress
Hub genes showed the most connections in the network, as indicated by their high Maximal Clique Centrality (MCC) score (Chin et al. 2014). The top twenty genes with the highest MCC values in each of the specific modules are shown in Fig. 7 and Suppl. Table S7. Among the identified hub genes, many TFs were highly enriched in different developmental stages (Fig. 8). In the green CM, 10 genes encode TFs, including three DEAD-box ATP-dependent RNA helicase, a plant UBX domain-containing protein, a nucleolin-like family protein, a pentatricopeptide repeat-containing protein, two ATP-dependent DNA helicase, an MYB family TF, and a serine/threonine-protein kinase TOR. In light green CM, 8 genes encode TFs, including three ethylene-responsive TFs, an auxin response factor, an armadillo/beta-catenin-like repeat family protein, a receptor-like protein kinase, a sorbitol dehydrogenase, and a protein phosphatase 2C. The light yellow CM hub genes were mainly down-regulated at V10, VT, R3, and R6 stages. Three MYB family TFs, a UDP-sulfoquinovose synthase chloroplastic TF, and a calcineurin-like metallophosphoesterase superfamily protein were identified in the light yellow module. The hub genes in Midnight blue and magenta CMs were more highly up-regulated expressed in the R1 stage. Six and eleven TFs were identified in these two modules, respectively. However, highly down-regulated expression of the hub genes was observed in pink CM at the R1 stage. Nine of 20 hub genes encode TFs, including a BHLH TF, leucine-rich repeat receptor-like tyrosine-protein kinase PXC3, and leucoanthocyanidin reductase. In the red CM, hub genes were mostly up-regulated expression at different developmental stages, and 11 out of those hub genes encode TFs (Fig. 8).
Discussion
Impacts of K deficiency on RSA and grain yield in maize
The root, the primary organ responsible for sensing and absorbing nutrients, exhibits a remarkable level of plasticity in response to changes in nutrient availability. The extent of this plasticity varies depending on factors such as plant species, genotypes, the severity and duration of the deficiency, as well as the developmental stage of the plant. Previous studies have demonstrated that low-K stress-induced root growth inhibition is a common phenomenon shared by most plants (Hermans et al. 2006; Jordan-Meille et al. 2018). For example, K+ deficiency was found to inhibit the elongation of the main roots and decrease the number and length of the lateral roots in Arabidopsis (Kellermeier et al. 2014). Similar results have been observed in various crops, including rice, cotton, and maize (Zhang et al. 2009; Ruan et al. 2015). In the present study, we also found K+ deficiency significantly inhibited the root growth of maize. Most root traits, including the total root length, primary root length, root width, and maximum root number, were reduced by more than 50% to 80% (Fig. 1). The inhibition of root growth induced by K+ deficiency may be attributed to the reduction in carbohydrate transport from the shoot to the root, which is crucial for supporting root growth, as K+ is necessary for stimulating H+-ATPase, which is required for the proper functioning of sucrose transporters (Sustr et al. 2019).
It is known that sufficient K supply is required for crop production. In a long-term experiment conducted to determine the responses of wheat and rice yield to different potassium levels, the results revealed that without the application of K fertilizer over an extended period, the wheat and rice yields were reduced by 47% and 15%, respectively (Song et al. 2018). A similar trend of severe wheat grain yield reduction was reported following a long-term K deficiency (Zörb et al. 2014). In our study, K deficiency led to a 16.8% (P < 0.05) reduction in grain yield (Fig. 2). It has been demonstrated that K supply significant effects crop yield by regulating the photosynthesis rate and driving assimilate transport into grains. Potassium impacts photosynthesis through influences on stomatal functioning. It activates enzymes involved in photosynthesis and helps regulate stomata movement, which is controlled mostly by K ion concentration (Srivastava et al. 2020). There is also evidence that plants experiencing inadequate potassium levels can exhibit an elevated production of reactive oxygen species (ROS) within their photosynthetic tissue, consequently leading to photooxidative damage when exposed to higher light intensities (Cakmak 2005).
Unveiling the influence of K deficiency on nitrogen and phosphorus uptake in maize
Nutrient interactions are common in higher plants, with one element's deficiency or excess affecting the absorption, distribution or function of another. For instance, K deficiency could inhibit N absorption in cotton and significantly reduce leaf nitrate content (Hu et al. 2017). K deficiency also hindered phosphates and magnesium uptake in plants. In our study, K starvation significantly reduced P levels and had a minor effect on N in maize seeds (Fig. 2). K deficiency can inhibit N accumulation in plants by downregulating root nitrate transporters (Daliparthy et al. 1994). Additionally, the role of potassium in regulating water uptake and transport in plants is crucial. This function may aid in the absorption and utilization of other essential nutrients, including nitrogen and phosphorous (Malvi 2011). Maintaining a balanced supply of nutrients is important for optimal plant growth and crop production. Further molecular studies are needed to better understand how K influences other essential elements' homeostasis.
Gene expression profiling of maize roots reveals stage-specific responses to K deficiency
The plant response to K deficiency involves the regulation of a large number of genes (Ma et al. 2020). To gain a deeper understanding of the complex molecular regulatory mechanisms controlling maize root response to K deficiency, we used a comparative RNA-Seq-based approach to compare gene expression profiles of maize roots under K deficiency at five-time points during plant development. In this study, we identified multiple differentially expressed genes (DEGs) at various developmental stages (Fig. 4). The varying numbers of genes responding to potassium deficiency at different developmental stages suggest that the specific genes involved in the plant root response to potassium deficiency vary across developmental stages. The V10 stage exhibits the highest number of DEGs, whereas the R6 stage shows the lowest number, indicating that maize roots are more sensitive to potassium deficiency during early developmental stages. In other words, maize may require more potassium during the early developmental stage compared to the later stages, which aligns with our K accumulation data, demonstrating a declining trend throughout plant development.
The role of K+ channels and transporters in plant response to K deficiency
Plants have a wide variety of transport systems that play crucial roles in K+ uptake, redistribution, and homeostasis. Among them, K+ absorption and transport are facilitated by various channels and transporters, with K+ channels primarily responsible for mediating low-affinity K+ uptake, while K+ transporters predominantly facilitate high-affinity K+ uptake (Hafsi et al. 2014). It was well documented that many high-affinity K+ transporter genes from different plant species are induced by K deficiency, such as AtHAK5, HvHAK1, and OsHAK1 (Gierth et al. 2005; Fulgenzi et al. 2008; Okada et al. 2018). Moreover, a transcriptome analysis of maize roots response to K+ starvation revealed that several K+ channel genes, including potassium channel KAT1 and inward rectifying potassium channel ZMK1were markedly up-regulated (Zhou et al. 2022). In the present study, consistent with previous reports, a POTASSIUM CHANNEL PROTEIN (Zm00001d016160) was notably induced in response to K starvation at various developmental stages, and HAK5 (Zm00001d033067) was up-regulated upon K deprivation at later developmental stages (Table 1). The findings presented above show that increase in expression levels of genes encoding K+ transporters and channels may be a rapid and common regulatory strategy shared by different plant species to increase K+ uptake and overcome K+ deficiency.
Transcription factors as key players in maize root response to K deficiency
Transcription factors (TFs) are important regulatory nodes in the regulatory network of plant K deficiency stress response. ARF2 (Auxin Response Factor 2) is well-characterized TF responsible for the negative regulation of AtHAK5 expression. Under K+ sufficient conditions, AtARF2 binds to the AtHAK5 promoter and represses its transcription. However, in response to low K+ stress, AtARF2 undergoes rapid phosphorylation, leading to its dissociation from the AtHAK5 promoter and subsequent relief of repression on HAK5 transcription (Zhao et al. 2016). An AP2/ERF transcription factor RAP2.11 was identified as a component in response to low potassium through regulation of the high-affinity K+ uptake transporter AtHAK5 and other components of the low-potassium signal transduction pathway (Kim et al. 2012).
Other TFs, including AtbHLH121, AtMYB59, and OsMYBc, have been demonstrated to bind to the promoters of high-affinity K+ transporters and activate gene expression under K+ limiting conditions (Hong et al. 2013; Wang et al. 2015). In this study, ARF3 (Zm00001d012731) was identified as one of the hub genes, exhibiting significant downregulation across all developmental stages. Interestingly, the maize homolog of RAP2.11, Zm00001d030862, displayed downregulation in the root tissue during the early developmental stage (Fig. 8). These findings indicate that while maize roots share some common regulatory strategies with other plants, they exhibit distinct differences in the adaption of K starvation, highlighting the complexity of gene regulation in maize root response to K deficiency. Numerous transcriptome studies have recently reported the differential expression of dozens to hundreds of TFs in plants in response to K deficiency. The MYB, ERF, and WRKY families have emerged as the most frequently identified TFs (Zhao et al. 2018; Yang et al. 2020, 2021).
Consistently, we also found many transcription factors, including MYB, bHLH, ERF, and WRKY families, were highly enriched in different developmental stages response to K starvation (Fig. 8). These results suggest that in addition to the well-established TFs, a multitude of TFs within the MYB, ERF, and WRKY families are implicated in the adaptation to external K+ deprivation. However, additional molecular studies are required to validate and further elucidate their specific functions.
Insights into K+ sensing and regulatory mechanisms in maize root response to potassium deficiency
At the cellular level, K+ deficiency has been demonstrated to elevate cytoplasmic Ca2+ levels that activate downstream signaling through a well-explored CBL (calcineurin B-like protein)-CIPK module (Wang and Wu 2010). Within this module, CBL1/CBL9 interacts with CIPK23 and targets it to the plasma membrane of root cells to phosphorylate a voltage-gated high-affinity K+ channel, AKT1 (Arabidopsis K+ transporter 1). The phosphorylation of AKT1 enhances its K+ uptake activity (Xu et al. 2006). In our study, CALCINEURIN B-LIKE INTERACTING PROTEIN KINASE 23 (Zm00001d018799), CALCINEURIN B-LIKE PROTEIN 10 (Zm00001d044285) were observed up-regulated in specific developmental stage under LK conditions, suggesting that K+ sensing and signaling pathway was activated upon maize roots subject to K deficiency (Table 1).
A member of the type III peroxidase family, Rare Cold Inducible gene 3 (RCI3), was identified as a component of the low K signal transduction pathway in Arabidopsis roots. RCI3 is known to be induced in response to K deprivation and is involved in activating of the high-affinity K+ uptake transporter HAK5, thereby facilitating K acquisition during K starvation (Kim et al. 2010). Similarly, in our study, the homologous gene of RCI3, Zm00001d014467, was found to be up-regulated in V10 and R1 stages in response to low K conditions, indicating a possible involvement of RCI3 in the early response of maize root to K starvation.
Phosphorylation and dephosphorylation may be important regulatory mechanisms in the plant responses to K deficiency. In the present study, several kinase genes were transcriptionally regulated by K deficiency. Among them, an important serine/threonine-protein kinase TOR (Target of rapamycin) was in the hub gene list (Fig. 8). TOR proteins are members of the phosphatidylinositol kinase-related kinase (PIKK) family, which are highly conserved among yeasts, plants, and mammals. In plants, TOR signaling is a central hub that integrates diverse signals, including nutrients, energy levels, hormones, and environmental cues (Fu et al. 2020). Increasing evidence demonstrated that TOR is involved in plant nutrient deficiency response, such as N, P and S (Dobrenel et al. 2021; Liu et al. 2021; Dong et al. 2022). The identification of TOR as one of the hub genes within the maize root response to K deficiency in our study suggests its potential involvement in the plant's response to K deficiency. However, further research is required to elucidate the specific functions of TOR in this process.
Conclusion
In summary, our comprehensive analysis of root phenotypes and transcriptome data from five key developmental stages has successfully identified important root system architectures and gene regulatory networks that are implicated in both root development and the response to potassium deficiency in maize. Additionally, we have identified a diverse set of TFs and potential hub genes that are closely associated with the response of roots to potassium deficiency. Interestingly, some of these genes exhibited homology to well-known regulators of root architecture or development in Arabidopsis, such as Zm00001d014467 (AtRCI3), Zm00001d011237 (AtWRKY9), and Zm00001d030862 (AtAP2/ERF). These findings provide a valuable resource for future functional experiments, enriching our comprehension of how root system architecture influences nutrient uptake efficiency and contributes to the continued enhancement of maize grain yield under limiting nutrient availability environments.
Data availability
All data generated or analyzed during this study are available from the corresponding author upon request.
Abbreviations
- CMs:
-
Co-expression modules
- DEG:
-
Differentially expressed gene
- GO:
-
Gene ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- LK:
-
Low K condition
- RSA:
-
Root system architecture
- SK:
-
Sufficient K condition
- TF:
-
Transcription factor
- WGCNA:
-
Weighted correlation network analysis
References
Ahn SJ, Shin R, Schachtman DP (2004) Expression of KT/KUP genes in Arabidopsis and the role of root hairs in K+ uptake. Plant Physiol 134(3):1135–1145. https://doi.org/10.1104/pp.103.034660
Andrés Z, Pérez-Hormaeche J, Leidi EO, Schlücking K, Steinhorst L, McLachlan DH, Schumacher K, Hetherington AM, Kudla J, Cubero B, Pardo JM (2014) Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. P Natl Acad Sci USA 111(17):E1806–E1814. https://doi.org/10.1073/pnas.1320421111
Bhatnagar S, Betran F, Rooney L (2004) Combining abilities of quality protein maize inbreds. Crop Sci 44(6):1997–2005. https://doi.org/10.2135/cropsci2004.1997
Bremner JM (1996) Nitrogen-Total. In: Sparks DL, Page AL, Helmke PA et al. (eds) Methods of soil analysis, part 3: Chemical methods. Wiley, pp 1085–1121. https://doi.org/10.2136/sssabookser5.3.c37
Cakmak I (2005) The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J Plant Nutr Soil Sc 168(4):521–530. https://doi.org/10.1002/jpln.200420485
Carroll MJ, Slaughter LH, Krouse JM (1994) Turgor potential and osmotic constituents of Kentucky bluegrass leaves supplied with four levels of potassium. Agron J 86(6):1079–1083. https://doi.org/10.2134/agronj1994.00021962008600060028x
Chapman HD, Pratt PF (1962) Methods of analysis for soils, plants and waters. Soil Sci 93(1):68
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT, Lin CY (2014) cytoHubba: identifying hub objects and sub-networks from complex interactome. BMC Syst Biol 8(4):S11. https://doi.org/10.1186/1752-0509-8-S4-S11
Corratge-Faillie C, Ronzier E, Sanchez F, Prado K, Kim JH, Lanciano S, Leonhardt N, Lacombe B, Xiong TC (2017) The Aabidopsis guard cell outward potassium channel GORK is regulated by CPK33. FEBS Lett 591(13):1982–1992. https://doi.org/10.1002/1873-3468.12687
Daliparthy J, Barker AV, Mondal SS (1994) Potassium fractions with other nutrients in crops: a review focusing on the tropics. J Plant Nutr 17(11):1859–1886. https://doi.org/10.1080/01904169409364852
Dobrenel T, Kushwah S, Mubeen U, Jansen W, Delhomme N, Caldana C, Hanson J (2021) The phosphate starvation response recruits the TOR pathway to regulate growth in Arabidopsis cell cultures. bioRxiv:2021.2003. 2026. 437164
Dong YH, Aref R, Forieri I, Schiel D, Leemhuis W, Meyer C, Hell R, Wirtz M (2022) The plant TOR kinase tunes autophagy and meristem activity for nutrient stress-induced developmental plasticity. Plant Cell 34(10):3814–3829. https://doi.org/10.1093/plcell/koac201
Du Z, Zhou X, Ling Y, Zhang Z, Su Z (2010) agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res 38 (Web Server issue):W64–70. https://doi.org/10.1093/nar/gkq310
Feng CZ, Luo YX, Wang PD, Gilliham M, Long Y (2021) MYB77 regulates high-affinity potassium uptake by promoting expression of HAK5. New Phytol 232(1):176–189. https://doi.org/10.1111/nph.17589
Fox J, Weisberg S, Adler D, Bates D, Baud-Bovy G, Ellison S, Firth D, Friendly M, Gorjanc G, Graves S (2012) Package ‘car’. R Foundation for Statistical Computing 16, Vienna
Fu L, Wang PC, Xiong Y (2020) Target of rapamycin signaling in plant stress responses. Plant Physiol 182(4):1613–1623. https://doi.org/10.1104/pp.19.01214
Fulgenzi FR, Peralta ML, Mangano S, Danna CH, Vallejo AJ, Puigdomenech P, Santa-María GE (2008) The ionic environment controls the contribution of the barley HvHAK1 transporter to potassium acquisition. Plant Physiol 147(1):252–262. https://doi.org/10.1104/pp.107.114546
Gajdanowicz P, Michard E, Sandmann M, Rocha M, Corrêa LG, Ramírez-Aguilar SJ, Gomez-Porras JL, González W, Thibaud JB, van Dongen JT, Dreyer I (2011) Potassium (K+) gradients serve as a mobile energy source in plant vascular tissues. P Natl Acad Sci USA 108(2):864–869. https://doi.org/10.1073/pnas.1009777108
Galkovskyi T, Mileyko Y, Bucksch A, Moore B, Symonova O, Price CA, Topp CN, Iyer-Pascuzzi AS, Zurek PR, Fang S (2012) GiA Roots: software for the high throughput analysis of plant root system architecture. BMC Plant Biol 12(1):126. https://doi.org/10.1186/1471-2229-12-116
Gao J, Wang MJ, Wang JJ, Lu HP, Liu JX (2022) bZIP17 regulates heat stress tolerance at reproductive stage in Arabidopsis. aBIOTECH 3 (1):1 –11. https://doi.org/10.1007/s42994-021-00062-1
Gierth M, Mäser P, Schroeder JI (2005) The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol 137(3):1105–1114. https://doi.org/10.1104/pp.104.057216
Gladman N, Hufnagel B, Regulski M, Liu Z, Wang X, Chougule K, Kochian L, Magalhães J, Ware D (2022) Sorghum root epigenetic landscape during limiting phosphorus conditions. Plant Direct 6(5):e393. https://doi.org/10.1002/pld3.393
Grote U, Fasse A, Nguyen TT, Erenstein O (2021) Food security and the dynamics of wheat and maize value chains in Africa and Asia. Front Sustain Food S 4:617009. https://doi.org/10.3389/fsufs.2020.617009
Guo S, Liu ZG, Zhou ZJ, Lu TQ, Chen SH, He MJ, Zeng XZ, Chen K, Yu H, Shangguan YX, Dong YJ, Chen FJ, Liu YH, Qin YS (2022) Root system architecture differences of maize cultivars affect yield and nitrogen accumulation in Southwest China. Agriculture-Basel 12(2):209. https://doi.org/10.3390/agriculture12020209
Hafsi C, Debez A, Abdelly C (2014) Potassium deficiency in plants: effects and signaling cascades. Acta Physiol Plant 36(5):1055–1070. https://doi.org/10.1007/s11738-014-1491-2
Hermans C, Hammond JP, White PJ, Verbruggen N (2006) How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci 11(12):610–617. https://doi.org/10.1016/j.tplants.2006.10.007
Hong JP, Takeshi Y, Kondou Y, Schachtman DP, Matsui M, Shin R (2013) Identification and characterization of transcription factors regulating Arabidopsis HAK5. Plant Cell Physiol 54(9):1478–1490. https://doi.org/10.1093/pcp/pct094
Hu W, Coomer TD, Loka DA, Oosterhuis DM, Zhou Z (2017) Potassium deficiency affects the carbon-nitrogen balance in cotton leaves. Plant Physiol Biochem 115:408–417. https://doi.org/10.1016/j.plaphy.2017.04.005
Jákli B, Tavakol E, Tränkner M, Senbayram M, Dittert K (2017) Quantitative limitations to photosynthesis in K deficient sunflower and their implications on water-use efficiency. J Plant Physiol 209:20–30. https://doi.org/10.1016/j.jplph.2016.11.010
Jia YB, Yang XE, Feng Y, Jilani G (2008) Differential response of root morphology to potassium deficient stress among rice genotypes varying in potassium efficiency. J Zhejiang Univ-Sc B 9(5):427. https://doi.org/10.1631/jzus.B0710636
Jordan-Meille L, Martineau E, Bornot Y, Lavres J, Abreu-Junior CH, Domec J-C (2018) How does water-stressed corn respond to potassium nutrition? A shoot-root scale approach study under controlled conditions. Agriculture 8(11):180. https://doi.org/10.3390/agriculture8110180
Kellermeier F, Armengaud P, Seditas TJ, Danku J, Salt DE, Amtmann A (2014) Analysis of the root system architecture of Arabidopsis provides a quantitative readout of crosstalk between nutritional signals. Plant Cell 26(4):1480–1496. https://doi.org/10.1105/tpc.113.122101
Kim MJ, Ciani S, Schachtman DP (2010) A peroxidase contributes to ROS production during Arabidopsis root response to potassium deficiency. Mol Plant 3(2):420–427. https://doi.org/10.1093/mp/ssp121
Kim MJ, Ruzicka D, Shin R, Schachtman DP (2012) The Arabidopsis AP2./ERF transcription factor RAP211 modulates plant response to low-potassium conditions. Mol Plant 5(5):1042–1057. https://doi.org/10.1093/mp/sss003
Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12(4):357–360. https://doi.org/10.1038/nmeth.3317
Kogbe J, Adediran J (2003) Influence of nitrogen, phosphorus and potassium application on the yield of maize in the savanna zone of Nigeria. Afr J Biotechnol 2(10):345–349
Langfelder P, Horvath S (2008) WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9(1):559. https://doi.org/10.1186/1471-2105-9-559
Liu YL, Duan XL, Zhao XD, Ding WL, Wang YW, Xiong Y (2021) Diverse nitrogen signals activate convergent ROP2-TOR signaling in Arabidopsis. Dev Cell 56 (9): 1283–1295. e1285. https://doi.org/10.1016/j.devcel.2021.03.022
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15(12):550. https://doi.org/10.1186/s13059-014-0550-8
Luo JH, Wang M, Jia GF, He Y (2021) Transcriptome-wide analysis of epitranscriptome and translational efficiency associated with heterosis in maize. J Exp Bot 72(8):2933–2946. https://doi.org/10.1093/jxb/erab074
Ma NN, Dong LN, Lü W, Lü J, Meng QW, Liu P (2020) Transcriptome analysis of maize seedling roots in response to nitrogen-, phosphorus-, and potassium deficiency. Plant Soil 447:637–658. https://doi.org/10.1007/s11104-019-04385-3
Malvi UR (2011) Interaction of micronutrients with major nutrients with special reference to potassium. Karnataka J Agr Sci 24(1):106–109
Okada T, Yamane S, Yamaguchi M, Kato K, Shinmyo A, Tsunemitsu Y, Iwasaki K, Ueno D, Demura T (2018) Characterization of rice KT/HAK/KUP potassium transporters and K+ uptake by HAK1 from Oryza sativa. Plant Biotechnol (tokyo) 35(2):101–111. https://doi.org/10.5511/plantbiotechnology.18.0308a
Pilot G, Pratelli R, Gaymard F, Meyer Y, Sentenac H (2003) Five-group distribution of the Shaker-like K+ channel family in higher plants. J Mol Evol 56(4):418–434. https://doi.org/10.1007/s00239-002-2413-2
Ragel P, Rodenas R, Garcia-Martin E, Andres Z, Villalta I, Nieves-Cordones M, Rivero RM, Martinez V, Pardo JM, Quintero FJ, Rubio F (2015) The CBL-interacting protein kinase CIPK23 regulates HAK5-mediated high-affinity K+ uptake in Arabidopsis roots. Plant Physiol 169(4):2863–2873. https://doi.org/10.1104/pp.15.01401
Ranum P, Peña-Rosas JP, Garcia-Casal MN (2014) Global maize production, utilization, and consumption. Ann NY Acad Sci 1312(1):105–112. https://doi.org/10.1111/nyas.12396
Ray DK, Mueller ND, West PC, Foley JA (2013) Yield trends are insufficient to double global crop production by 2050. PLoS ONE 8(6):e66428. https://doi.org/10.1371/journal.pone.0066428
Ren XL, Qi GN, Feng HQ, Zhao S, Zhao SS, Wang Y, Wu WH (2013) Calcineurin B-like protein CBL10 directly interacts with AKT1 and modulates K+ homeostasis in Arabidopsis. Plant J 74(2):258–266. https://doi.org/10.1111/tpj.12123
Ruan L, Zhang JB, Xin XL, Zhang C, Ma DH, Chen L, Zhao BZ (2015) Comparative analysis of potassium deficiency-responsive transcriptomes in low potassium susceptible and tolerant wheat (Triticum aestivum L.). Sci Rep 5 (1): 10090. https://doi.org/10.1038/srep10090
Schachtman DP, Shin R (2007) Nutrient sensing and signaling: NPKS. Annu Rev Plant Biol 58:47–69. https://doi.org/10.1146/annurev.arplant.58.032806.103750
Schachtman DP, Schroeder JI, Lucas WJ, Anderson JA, Gaber RF (1992) Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science 258(5088):1654–1658. https://doi.org/10.1126/science.8966547
Shin R, Schachtman DP (2004) Hydrogen peroxide mediates plant root cell response to nutrient deprivation. P Natl Acad Sci USA 101(23):8827–8832. https://doi.org/10.1073/pnas.0401707101
Song WJ, Xue R, Song Y, Bi Y, Liang ZH, Meng L, Dong CX, Wang CD, Liu GL, Dong JX (2018) Differential response of first-order lateral root elongation to low potassium involves nitric oxide in two tobacco cultivars. J Plant Growth Regul 37:114–127. https://doi.org/10.1007/s00344-017-9711-9
Srivastava AK, Shankar A, Nalini Chandran AK, Sharma M, Jung K-H, Suprasanna P, Pandey GK (2020) Emerging concepts of potassium homeostasis in plants. J Exp Bot 71(2):608–619. https://doi.org/10.1093/jxb/erz458
Sustr M, Soukup A, Tylova E (2019) Potassium in root growth and development. Plants-Basel 8(10):435. https://doi.org/10.3390/plants8100435
Team RC , (2013) R: A language and environment for statistical computing.
Tian H, Baxter IR, Lahner B, Reinders A, Salt DE, Ward JM (2010) Arabidopsis NPCC6/NaKR1 is a phloem mobile metal binding protein necessary for phloem function and root meristem maintenance. Plant Cell 22(12):3963–3979. https://doi.org/10.1105/tpc.110.080010
Tränkner M, Tavakol E, Jákli B (2018) Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol Plant 163(3):414–431. https://doi.org/10.1111/ppl.12747
Ul-Allah S, Ijaz M, Nawaz A, Sattar A, Sher A, Naeem M, Shahzad U, Farooq U, Nawaz F, Mahmood K (2020) Potassium application improves grain yield and alleviates drought susceptibility in diverse maize hybrids. Plants-Basel 9(1):75. https://doi.org/10.3390/plants9010075
Wang Y, Wu W-H (2010) Plant sensing and signaling in response to K+-deficiency. Mol Plant 3(2):280–287. https://doi.org/10.1093/mp/ssq006
Wang R, Jing W, Xiao L, Jin Y, Shen L, Zhang W (2015) The rice high-affinity potassium transporter1;1 is involved in salt tolerance and regulated by an MYB-type transcription factor. Plant Physiol 168(3):1076–1090. https://doi.org/10.1104/pp.15.00298
Wickham H (2016) Data analysis. In: ggplot2. Springer, pp 189–201
Wu L, Cui Z, Chen X, Zhao R, Si D, Sun Y, Yue S (2014) High-yield maize production in relation to potassium uptake requirements in China. Agron J 106(4):1153–1158. https://doi.org/10.2134/agronj13.0538
Xiong W, Wang Y, Guo Y, Tang W, Zhao Y, Yang G, Pei Y, Chen J, Song X, Sun J (2022) Transcriptional and metabolic responses of maize shoots to long-term potassium deficiency. Front Plant Sci 13:922581. https://doi.org/10.3389/fpls.2022.922581
Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH (2006) A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125(7):1347–1360. https://doi.org/10.1111/tpj.12123
Xu X, Du X, Wang F, Sha J, Chen Q, Tian G, Zhu Z, Ge S, Jiang Y (2020) Effects of potassium levels on plant growth, accumulation and distribution of carbon, and nitrate metabolism in apple dwarf rootstock seedlings. Front Plant Sci 11:904. https://doi.org/10.3389/fpls.2020.00904
Yang H, Li Y, Jin YM, Kan LP, Shen CW, Malladi A, Nambeesan S, Xu YC, Dong CX (2020) Transcriptome analysis of Pyrus betulaefolia seedling root responses to short-term potassium deficiency. Int J Mol Sci 21(22):8857. https://doi.org/10.3390/ijms21228857
Yang DD, Li FJ, Yi F, Eneji AE, Tian XL, Li ZH (2021) Transcriptome analysis unravels key factors involved in response to potassium deficiency and feedback regulation of K+ uptake in cotton roots. Int J Mol Sci 22(6):3133. https://doi.org/10.3390/ijms22063133
Zhang ZY, Wang QL, Li ZH, Duan LS, Tian XL (2009) Effects of potassium deficiency on root growth of cotton seedlings and its physiological mechanisms. Acta Agron Sin 35(4):718–723. https://doi.org/10.1016/S1875-2780(08)60079-6
Zhang ML, Huang PP, Ji Y, Wang S, Wang SS, Li Z, Guo Y, Ding Z, Wu WH, Wang Y (2020) KUP 9 maintains root meristem activity by regulating K+ and auxin homeostasis in response to low K. EMBO Rep 21(6):e50164. https://doi.org/10.15252/embr.202050164
Zhao S, Zhang ML, Ma TL, Wang Y (2016) Phosphorylation of ARF2 relieves its repression of transcription of the K+ transporter gene HAK5 in response to low potassium stress. Plant Cell 28(12):3005–3019. https://doi.org/10.1105/tpc.16.00684
Zhao XM, Liu Y, Liu X, Jiang J (2018) Comparative transcriptome profiling of two tomato genotypes in response to potassium-deficiency stress. Int J Mol Sci 19(8):2402. https://doi.org/10.3390/ijms19082402
Zhou DY, Wang K, Zhang H, Du Q, Liu YY, Wang J, Wang XG, Yu H, Zhao XH (2022) The potassium-dependent transcriptome analysis of maize provides novel insights into the rescue role of auxin in responses to potassium Ddeficiency. Agronomy-Basel 12(6):1318. https://doi.org/10.3390/agronomy12061318
Zörb C, Senbayram M, Peiter E (2014) Potassium in agriculture–status and perspectives. J Plant Physiol 171(9):656–669. https://doi.org/10.1016/j.jplph.2013.08.008
Acknowledgements
This research was supported by the Sichuan Science and Technology Program (2020YFH0171) to GS, the National Natural Science Foundation of China (41907080) to GS, and the Sichuan innovation team of national modern agricultural industry technology system to QYS. The research was also supported by a Canada Excellence Research Chair in Global Food Security and funding from the Global Institute for Food Security, University of Saskatchewan, to LK.
Author information
Authors and Affiliations
Contributions
Conceptualization, SG, ZL, and YQ; methodology, ZL, YL, and LK; validation, SG, ZL, ZZ, and MH; data analysis, ZL, SH, OT, and MW; writing—original draft preparation, SG and ZL; writing—review and editing, YQ and L.K.; project administration, SG; funding acquisition, LK, SG, and YQ All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, S., Liu, Z., Sheng, H. et al. Dynamic transcriptome analysis unravels key regulatory genes of maize root growth and development in response to potassium deficiency. Planta 258, 99 (2023). https://doi.org/10.1007/s00425-023-04260-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-023-04260-7