Abstract
Main conclusion
We advance Ulva ’s genetic tractability and highlight its value as a model organism by characterizing its APAF1_C/WD40 domain-encoding gene, which belongs to a family that bears homology to R genes.
Abstract
The multicellular chlorophyte alga Ulva mutabilis (Ulvophyceae, Ulvales) is native to coastal ecosystems worldwide and attracts both high socio-economic and scientific interest. To further understand the genetic mechanisms that guide its biology, we present a protocol, based on adapter ligation-mediated PCR, for retrieving flanking sequences in U. mutabilis vector-insertion mutants. In the created insertional library, we identified a null mutant with an insertion in an apoptotic protease activating factor 1 helical domain (APAF1_C)/WD40 repeat domain-encoding gene. Protein domain architecture analysis combined with phylogenetic analysis revealed that this gene is a member of a subfamily that arose early in the evolution of green plants (Viridiplantae) through the acquisition of a gene that also encoded N-terminal nucleotide-binding adaptor shared by APAF-1, certain R-gene products and CED-4 (NB-ARC) and winged helix-like (WH-like) DNA-binding domains. Although phenotypic analysis revealed no mutant phenotype, gene expression levels in control plants correlated to the presence of bacterial symbionts, which U. mutabilis requires for proper morphogenesis. In addition, our analysis led to the discovery of a putative Ulva nucleotide-binding site and leucine-rich repeat (NBS-LRR) Resistance protein (R-protein), and we discuss how the emergence of these R proteins in green plants may be linked to the evolution of the APAF1_C/WD40 protein subfamily.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chlorophyte and streptophyte algae (green algae) are diverse oxygenic photosynthetic eukaryotes that obtained their chloroplasts through an ancient endosymbiotic event with a cyanobacterium [McFadden 2001; and reviewed in Graham et al. (2009); Gawryluk et al. 2019]. Our understanding of their evolutionary relationships has greatly benefited from recent advances in phylogenomics, which retraced, for example, the trajectory by which land plants evolved from streptophyte algae (Turmel et al. 2006; Wodniok et al. 2011; Timme et al. 2012) and discerned the existence of three phyla in the clade of green plants (Viridiplantae) (Li et al. 2020).
Green algae are important to the global carbon cycle and local ecosystems as primary producers. Although some of them may also occupy terrestrial, subaerial niches, streptophyte algae are regarded as strict freshwater lineages, whereas chlorophyte algae have adapted to oceanic and coastal marine environments, as well as freshwater (Becker and Marin 2009). In addition to the various ecosystems in which chlorophyte algae have settled, the different lineages are also characterized by highly variable morphologies, ranging from motile single cells to sessile multicellular organisms with body plans consisting of multiple tissues (Leliaert et al. 2007). Because of the well-resolved green algal phylogeny, it has been established that the transition from unicellularity to multicellularity took place on multiple independent occasions in both streptophyte and chlorophyte algae (Niklas and Newman 2013; Umen 2014; Del Cortona et al. 2020). Similarly, it is likely that streptophyte algae conquered land multiple times during the course of evolution [reviewed in Delwiche and Cooper (2016) and Fürst-Jansen et al. (2020)], while in the chlorophyte lineage, freshwater to marine transitions (or vice versa) frequently occurred (Dittami et al. 2017).
To better understand the intriguing biology of chlorophyte algae several model organisms have been established; for example, the single-celled Chlamydomonas reinhardtii and the multicellular colonial alga Volvox carteri, which have been valuable for understanding photoreceptor biology and morphological complexity, respectively (Nagel et al. 2002; Matt and Umen 2016). Given the enormous diversity in green algal biology, it is remarkable, however, that the insight into the molecular mechanisms that guided chlorophyte algae evolution is still relatively limited in comparison to our understanding of streptophyte algae and land plants.
To empower further research on chlorophyte algae, several genome sequencing initiatives have been undertaken. Recently, also the genome of the sea lettuce Ulva mutabilis, a multicellular chlorophyte alga belonging to the class Ulvophyceae that is abundant in intertidal zones worldwide (Wichard et al. 2015), was published (De Clerck et al. 2018). The body of U. mutabilis consists of a flat distromatic blade connected via a stem-like structure to a holdfast that can attach to the stratum. Within the genus, tubular forms also exist, and Ulva species generally display a high degree of phenotypic plasticity, which has made the unification of its taxonomy challenging (Steinhagen et al. 2019). Ulva species are also characterized by their ability to proliferate quickly under high nutrient conditions and are therefore infamous for producing blooms, or green tides, in eutrophicated waters, which can be harmful to local economies and ecosystems (Valiela et al. 1997; Smetacek and Zingone 2013). Conversely, under normal conditions, Ulva species are important ecosystem organizers that contribute to the habitat of numerous coastal dwellers and thus sustain biodiversity (Mann 1973). In addition to their role in the intertidal zone, Ulva species, as well as other marine algae, are increasingly appreciated as a sustainable source for animal feed, plant fertilizer and various biomaterials, and in processes as integrated multi-trophic aquaculture and the bioremediation of wastewater (Bikker et al. 2016).
U. mutabilis was developed as a laboratory organism in the mid-1900s. Pioneering studies unraveled its life cycle, in which isomorphic haploid and diploid phases alternate, but also showed that unfertilized gametes could form parthenosporophytes via autodiploidization (Føyn 1958; Hoxmark 1975). Additionally, the fast-growing mutant “slender” was isolated, which can be maintained in the haploid phase, thereby simplifying genetic analysis (Føyn 1959). U. mutabilis has furthermore received considerable attention because of its dependence on specific bacterial symbionts, or the compounds they produce, to undergo proper morphogenesis and development (Spoerner et al. 2012), a trait it shares with the related alga Monostroma oxyspermum (Monostromataceae, Ulvales) (Matsuo et al. 2005). Central to both species is their reliance on the compound thallusin, which U. mutabilis acquires from the epiphytic bacterium Maribacter sp. MS6 (Alsufyani et al. 2020) and requires for rhizoid development and regular cell wall formation. In addition to Maribacter sp. (or thallusin), U. mutabilis morphogenesis also depends on the activity of Roseovarius sp. MS2, which releases compounds that promote cell division and blade formation. Only under the synergistic effect of the compounds produced by both Roseovarius sp. and Maribacter sp., U. mutabilis will develop properly (Spoerner et al. 2012; Grueneberg et al. 2016).
Recently, the genetic tractability of U. mutabilis (Oertel et al. 2015) has been boosted by the development of a molecular toolkit that improved and broadened the strategies for ectopic gene expression and the targeting of proteins to specific cellular compartments (Blomme et al. 2021). These transformation tools also allow for the generation of mutant libraries by integrating foreign DNA into the Ulva genome. One of the most critical steps after constructing a random insertion mutant library is the identification of the insertion sites, which often is laborious, but a variety of techniques has been developed for the efficient retrieval of the regions that flank insertion sites (Tam and Lefebvre 1993; Liu et al. 1995; O'Malley et al. 2007; Sun et al. 2019). Together, these methods have proven very successful for elucidating gene function in land plants and green algae (Alonso et al. 2003; Dent et al. 2005). Hence, functional gene studies based on mutant analysis in U. mutabilis could aid in harnessing its agronomic potential (Charrier et al. 2015) and help answer, for example, whether the genetic networks that evolved in complex chlorophyte algae resemble those from streptophyte and other archaeplastidal lineages.
To accommodate genetic analysis in U. mutabilis, we present in this study an adapter ligation-mediated PCR method that allowed us to efficiently map several flanking regions in a collection of insertional mutants. Moreover, in this library, we identified a null mutant in an APAF1_C/WD40 repeat domain-encoding gene. Further phylogenetic and protein domain architecture analysis was used to track the fate of this gene family, thereby also shedding light on the evolution of NB-ARC domain-containing proteins in the green lineage.
Materials and methods
Plant material and cultivation
For all experiments, the U. mutabilis laboratory strain “slender” was used, unless indicated otherwise. Plants were cultivated in Ulva culture medium (UCM) (Stratmann et al. 1996; Wichard and Oertel 2010) in a growth chamber with a long-day (17 h light/7 h dark) light regime (~ 30 µmol photons m−2 s−1) at 18 °C. For phenotyping of transgenic plants, axenic cultures were prepared according to Califano and Wichard (2018) and subsequently inoculated with Roseovarius sp. strain MS2 and/or Maribacter sp. strain MS6 (Spoerner et al. 2012).
Creation of a library of Ulva mutabilis transgenic lines
Polyethylene glycol-mediated transformation was performed according to the protocol described in Oertel et al. (2015) and subsequently genotyped according to Boesger et al. (2018). In brief, before transformation, gametes were obtained from induced Ulva gametophytes, purified by phototactic migration through Pasteur pipettes (Califano and Wichard 2018) and transformed using SspI-linearized vector pPIBT7 (GenBank EU176859.1), which carries a codon-optimized phleomycin-resistance gene expression cassette as a selection marker. Transformed gametes were selected on 50 µg ml−1 phleomycin in Ulva culture medium incubated for 4 days. After about 4 weeks of cultivation in UCM, selected surviving gametophytes were individually grown in tissue culture flasks. For genotyping, a tissue sample (up to 100 mg per plant) was used for genomic DNA extraction using the GenElute Plant Genomic DNA Miniprep Kit (Merck). Genotyping PCR was performed using Prime-STAR GXL DNA Polymerase (Takara) in the presence of 5% DMSO using a primer pair specific for the resistance cassette and as a control for the gene UM008_0183. Oligomers are listed in Suppl. Table S1.
Identification of insertion sites by adapter ligation-mediated PCR
To create a template suited for PCR, genomic DNA (gDNA) containing the pPIBT7 insert was digested with a restriction enzyme and adapters were ligated to the overhanging ends of the gDNA. A short strand and a long strand make up the adapter. When the two strands of the adapter are hybridized, the short strand has a 5′ overhang, which is complementary to the restriction half-site ‘sticky end’ of the gDNA and will anneal to it; subsequently, the fragments are fused by ligation (O'Malley et al. 2007). Approximately 100 ng of gDNA was double-digested by the restriction enzymes BamHI/BclI (New England Biolabs), producing a 5′ GATC overhang. The digested gDNA was subsequently purified with the Nucleospin Gel and PCR Clean-up Kit (Macherey–Nagel) and ligated to a compatible adapter, present in a calculated 20-fold excess of DNA ends, that was made by annealing oligomers p46 and p48. The adapters were largely based on those used by Thole et al. (2009) but sequence-optimized to reduce aspecific amplification during PCR. They were created by mixing 6.25 µl of each adapter oligo (100 µM) in a total volume of 50 µl 1 × restriction enzyme buffer solution to provide positively charged ions to accommodate annealing. Subsequently, the solution was heated for 2 min at 95 °C and cooled gradually over 45 min. To amplify flanking sequences, a nested PCR approach was performed using the non-proofreading enzyme TAKARA taq (Takara) according to the manufacturer's instructions, but in the presence of 5% DMSO. The first PCR consisted of 20 cycles using the oligos p39/30 on ~ 35 ng adapter-gDNA template. The second, nested PCR consisted of 32 cycles using oligo p47/34 on 1 µl of the reaction products from the first PCR. Alternatively, the primer combinations p39/52 and p47/43 can be used for the first and nested PCR. Products from the second PCR were loaded on a 2% agarose gel and stained using HD-Green Plus (Intas). Amplicons that appeared as distinct bands were cut out and gel purified to serve directly as templates for Sanger sequencing. The resulting sequence reads were screened for the presence of a vector-flanking region border, and the identified flanking regions were analyzed by blasting them against the annotated wild-type U. mutabilis genome sequence (Sterck et al. 2012; De Clerck et al. 2018) (see also https://bioinformatics.psb.ugent.be/orcae/). Oligomers are listed in Suppl. Table S1.
cDNA cloning and qPCR expression analysis
The cDNAs of wild-type UM033_0004 and UM005_0337 were cloned using the SMARTer RACE 5′3′ Kit (Takara). First, total RNA was extracted using the Spectrum plant total RNA kit, including the optional on-column DNase I digestion (Merck). Then, (nested) 5′RACE was used to determine the 5′UTR region, and subsequently, a forward primer in the 5′UTR was designed for 3′RACE of the full-length transcript, which was cloned in pJET1.2 (Thermo Fisher) and sequenced. Normalized relative expression of UM033_0004 and UM005_0337 was determined using real-time quantitative PCR (qPCR) using the reference genes UM008_0183 (Ubiquitin) and UM010_0003 (PP2A 65 kDa regulatory subunit A). For qPCR, 1 µg of total RNA was reverse transcribed using the SuperScript IV VILO Master Mix Reagents (Thermo Fisher). Subsequent qPCR was done using PowerUp SYBR green chemistry (Thermo Fisher) on a CFX96 Real-Time PCR Detection System (Bio-Rad). The Cq values and melting curves were analyzed with CFX Maestro qPCR Analysis Software that implements the Pfaffl method (Pfaffl 2001; Vandesompele et al. 2002). Amplification efficiencies were determined from the dilution series. Additionally, qPCR amplicons were analyzed on a 2.5% agarose gel, and direct sequencing of the amplicons verified specific cDNA amplification. RACE and qPCR primers are listed in Suppl. Table S1.
Sequence identification and protein domain analysis
Initial protein domain analysis of UM033_0004 was performed using the online portals SMART (Letunic et al. 2020) (http://smart.embl-heidelberg.de) and Interproscan (Blum et al. 2020) (https://www.ebi.ac.uk/interpro). Chlorophyte homologs of UM033_0004 were identified by performing a blastp query (Altschul et al. 1997; Camacho et al. 2009; Cock et al. 2015) against a BLAST database that was built from all published chlorophyte algae proteomes (filtered models) available on Phycocosm (Grigoriev et al. 2020). All hits (0.001 e-value cutoff) were selected and then filtered for the presence of the APAF1 helical domain (APAF1_C, PF17908/IPR041452) or its corresponding gene3D signature (G3DSA:1.25.40.370). To identify homologs from more distantly related taxa, hidden Markov model (HMM) searches were performed (Finn et al. 2011). A custom APAF1_C HMM profile was generated (hmmbuild) from an alignment (hmmalign) of all the chlorophyte UM033_0004 homologs identified, against the Pfam APAF1_C HMM profile. Subsequently, separate HMM searches were performed against the following Joint Genome Institute datasets: Streptophyta (including the embryophyte genomes from Marchantia polymorpha, Physcomitrella patens, Sphagnum fallax and Arabidopsis thaliana), the prasinodermophyte Prasinoderma coloniale, the glaucophyte Cyanophora paradoxa and Rhodophyta. Complete species lists and data sources are listed in Suppl. Table S2. Also, an additional HMM search against all the streptophyte algae included in the One Thousand Plant Transcriptomes Initiative project was performed (Carpenter et al. 2019). For the identification of non-archaeplastidal eukaryote and eubacterial sequences, we used the HMM search tool from the EBI (Potter et al. 2018) with our custom APAF1_C profile. The presence of APAF1_C, WD40 (IPR001680), WH-like (IPR036388), NB-ARC (IPR002182), and LRR (IPR032675) domains was investigated via Interproscan ( Zdobnov and Apweiler 2001; Quevillon et al. 2005; Hunter et al. 2008; Cock et al. 2015) based on the Pfam release 34.0, (Mistry et al. 2020). Tools from the Galaxy webserver were used for all analyses (Afgan et al. 2018) unless indicated otherwise. All identified sequences are presented in Supplementary Data File S1.
Phylogenetic analysis
We included UM033_0004 and all the chlorophyte and streptophyte homologs identified in the phylogenetic analysis, except those that encoded only the APAF1_C domain. Additionally, from the HMM searches against Rhodophyta, non-archaeplastidal eukaryotes and eubacteria, the top 2–4 sequences (from different species) with the lowest e-value were included, as well as Apoptotic human protease activating factor 1 (APAF1). To root the phylogenetic tree, we also aligned the WD40 repeat domain of the fungal protein HET-E, which contains an N-terminal NACHT-domain ATPase. An initial protein alignment was created against the custom APAF1_C HMM profile, whereas the C-terminal part, containing the WD40 repeats, was aligned using MUSCLE (Edgar 2004). Sequences N-terminal to the APAF1_C domain were not aligned. Multiple sequence alignment and maximum likelihood analysis was performed using the software package MEGA7 (Kumar et al. 2016), using the substitution model LG + G + F. All aligned positions were considered with a cutoff of 85% (678 positions). The protein alignment with annotated domains can be found in Supplementary Data File S2.
Results
Adapter ligation PCR retrieves four unique pPIBT7 insertion sites
To establish a method to retrieve vector-insertion flanking sequences from U. mutabilis, we first created a collection of insertional mutants via polyethene glycol-mediated vector transformation. After selection and genotyping, an adapter-ligation PCR approach (Fig. 1) (O'Malley et al. 2007; Thole et al. 2009) was adopted to recover the sequences flanking the vector-insertion sites. Notably, we needed to modify the adapter sequences established by Thole et al. (2009) because the corresponding primers generated high background amplification when tested on genomic DNA extracted from untransformed control plants. Additionally, we found that adapter-ligation PCR required a polymerase without 3′–5′ proofreading activity because this exonuclease activity seemed incompatible with the presence of a C7 amino modification at the 3′ end of the short adapter oligo. Ultimately, single flanking sequences were identified from each of four plants, whereas two plants both yielded two distinct sequences (Table 1). Notably, the insertion site could not be pinpointed in four cases because the BLAST search using the retrieved flanking sequence as a query returned multiple hits in the Ulva genome, indicating that these insertions were probably in repetitive sequences. Of the other sites, two were situated in intergenic regions and two in predicted introns. In summary, we identified eight insertion sites from six transgenic lines (Table 1).
Schematic overview of adapter-ligation PCR approach. a For insertional mutagenesis, pPIBT7 carrying the phleomycin-resistance marker RBCSr::ble:RBCSt was linearized using SspI and transformed into Ulva, thereby creating genomic insertion sites as schematically shown in b. c Digestion of genomic DNA (gDNA) using BamHI (and BclI, not shown) leaves 5'GATC overhangs to which a compatible asymmetric adapter is ligated. The adapter 3′ amino-terminal group prevents DNA polymerase extension of the adapter sequence; consequently, the annealing site (dotted line) for the reverse primer (r1, arrow) only becomes available after the successful extension of the pPIBT7 insert specific primer f1 (arrow). A nested PCR using primers f2 and r2 further ensures the specific amplification of vector-gDNA segments. Arrows indicate primers. Sequences are not drawn to scale. Photography of U. mutabilis was reprinted from Wichard and Oertel (2010) with permission from JohnWiley and Sons, Copyright© (2010) Wiley
Molecular characterization of the UM033_0004 and UM005_0337 insertional mutants
Next, we characterized the insertion sites in the predicted introns in more detail by amplifying and sequencing the vector-gDNA junctions using pairs of gene-specific primers, which verified their location in a WD40 repeat-encoding gene (UM033_0004, see Suppl. Fig S1) and in a membrane-bound protein-encoding gene (UM005_0337) that were also identified in the initial BLAST search (see Fig. 2a, b). Subsequently, the exon–intron structures of the loci were determined by identifying the 5′UTR using 5′RACE and subsequently cloning the cDNAs via 3′RACE. Sequence analysis showed that both genes followed the annotated exon–intron structure that corresponds to the coding sequence. Interestingly, in the case of UM033_0004 we additionally identified an intron in the 5′UTR and a 3′UTR of more than 1000 base pairs (Fig. 2a).
Molecular characterization of the Ulva UM033_0004 and Um005_0337 insertional mutants. a Exon–intron structure and position of the pPIBT7 insertion. Shaded boxes represent exons, white boxes represent UTRs and lines introns. Open triangles indicate the insertion sites. b Domain architecture of the proteins corresponding to loci UM033_0004 and UM005_0337. Closed triangles indicate the positions where the pPIBT7 insertions interrupt the corresponding transcripts. c Transcript abundance of the identified genes in the insertional mutant lines and untransformed Ulva “slender” control plants. Error bars show the standard error of the mean (n = 3). N.D., not detectable. **, Student’s t test P < 0.01
Real-time quantitative PCR (qPCR) revealed the effect of the intronic insertions on the accumulation of the gene transcripts. Using primer pairs that anneal downstream or on opposite sides of the insertions, no transcript of the WD40 repeat-encoding gene could be detected (Fig. 2c), and transcript levels of the membrane-bound protein-encoding gene were reduced approximately threefold (Fig. 2d). Therefore, although both insertions are located in introns, the insertion in the UM005_0337 locus seems to only partly downregulate the production of a mature mRNA, whereas the transcription or pre-mRNA processing of the WD40 repeat-encoding gene appears to have been disrupted, thereby creating a null mutant.
UM033_0004 has homology to NB-ARC domain-containing proteins
As a complete knock-out mutant was established for UM033_0004, we further investigated its potential function using protein domain analysis. Genome annotation and the SMART protein domain architecture tool indicated it contained 13–15 WD40 repeats. InterProScan additionally identified the presence of the APAF1_C domain located N-terminally to the WD40 repeat domain (see Fig. 2b). Interestingly, Pfam HMM profile analysis revealed that UM033_0004 is the only Ulva gene that encodes this domain, while there are a total of 156 genes that matched the WD40 profile. The specific domain architecture of UM033_0004 prompted us to investigate the distribution of any homologs among the group of Archaeplastida by systematically performing similarity searches in a total of 41 taxa, including 25 chlorophyte algae, 5 streptophyte algae, 4 embryophytes, 1 prasidermophyte, 1 glaucophyte and 6 rhodophyte species, which were accessed via the Phycocosm and Phytozome genome repositories. Within the phylum of chlorophyte algae, proteins containing this module were detected in all investigated classes of core chlorophytes (Ulvophyceae, Chlorophyceae, Chlorodendrophyceae and Trebouxiophyceae) (Fig. 3). In Dunaliella salina (Chlorophyceae), we identified two proteins that only contained the APAF1_C helical domain. In contrast, two out of four Tetraselmis striata (Chlorodendrophyceae) homologs possessed both an additional N-terminal NB-ARC and a domain resembling the WH-like DNA-binding domain superfamily. In one out of three investigated Chlorella species, the N-terminal WH-like domain was detected, too, and in one case, accessory C-terminal transcription factor 25 (TCF25) Ribosome quality control complex subunit 1 in yeast) and the actin-binding domain Ysc84 domains were also present. Among the other major groups of Viridiplantae, searches using the HMM profile did not detect the APAF1_C domain in the investigated Embryophyte, Prasinodermophyte or Glaucophyte proteomes However, it was present in the Mesostigmatophyceae (Streptophyta) and Rhodophyta sequences. The retrieved Mesostigma viride CCAC 1140 and the rhodophyte proteins displayed N-terminal NB-ARC and WH-like domains, resembling the domain architecture observed in two of the T. striata proteins. However, the M. viride CCAC 1140 protein had an additional C-terminal ankyrin domain repeat, whereas the M. viride NIES-296 sequence only contained the WH-like and APAF1_C domains. A similar HMM search against the streptophyte algal proteomes included in the One Thousand Plant Transcriptomes Initiative did not deliver any further homologs. The HMM hits with the lowest e-value from non-archaeplastidal eukaryotes belonged to basal groups of metazoans, whereas the most significant bacterial hits were cyanobacterial. Interestingly, in these proteins, the APAF1_C/WD40 module was linked to NB-ARC and WH-like domains as in T. striata and M. viride CCAC 1140, and in the metazoan homologs, it was additionally linked to an N-terminal caspase activation and recruitment (CARD) domain, which is typical for APAF1-like proteins (Hofmann and Bucher 1997).
Cladogram showing the domain architectures of the retrieved APAF1_C/WD40 domain-encoding genes and the phylogenetic relationship of the lineages in which they were identified. The relationship among Archaeplastida lineages (A) follows Li et al. (2020). The position of Picocystophyceae basal to Chloropicophyceae is according to Turmel et al. (2019). For Streptophyta (S) (except Embryophytes), Chlorophyta (C), Prasinodermaphyta (P) and Glaucophyta (G), the taxonomic class and order of the studied species are according to AlgaeBase (Guiry and Guiry 2021). The nomenclature of C. melkonianii follows the recent revision of the Chlorokyboceae by Irisarri et al. (2021). Numbers in brackets are fractions of the total number of species (some species include multiple accessions, see Supplementary Table S2) in which the depicted APAF1_C/WD40 module was positively identified, whereas red crosses indicate that no homologs were identified in the corresponding lineage. Protein domain abbreviations: ANK (Ankyrin repeat-containing domain), APAF1_C (apoptotic protease activating factor 1 helical domain), CARD (caspase activation and recruitment domain), NB-ARC (nucleotide-binding adaptor shared by APAF1, certain R-gene products and CED-4), TCF25 (transcription factor 25), WD40 (WD40 domain-containing repeat), WH (winged helix-like DNA-binding domain superfamily). Protein domain lengths are not to scale
Phylogenetic analysis reveals that UM033_0004 is a member of a green algal APAF1_C/WD40 domain repeat-encoding gene subfamily
To study the evolutionary relationship between the identified proteins, we created a multiple sequence alignment of their APAF1_C and WD40 domains. We added to the alignment the human homolog APAF1, and the WD40 repeat domain from the fungal protein HET-E, which was later used to root the phylogenetic tree. Maximum-likelihood analysis gave rise to highly supported clades that corresponded to groups of metazoa, cyanobacteria and chlorophyte/streptophyte algae, whereas the red algal sequences did not form a single clade (Fig. 4). Additionally, all Chlorophycea sequences clustered together, with proteins from the orders Chlamydomonadales and Sphaeropleales forming subclades within this group, and the sequences from the orders Chlorellales and Trebouxiales formed distinct subgroups. However, the interrelationships between these groups and the representatives from the orders Chlorodendrales, Bryopsidales and Ulvales were not entirely resolved. Although other routes cannot be excluded, the clear separation of the green algal proteins from the other groups suggests a common evolutionary origin of all the chlorophyte and streptophyte APAF1_C/WD40 domain-containing proteins.
Maximum-likelihood phylogenetic tree using the LG + G + F substitution model based on the alignment of UM033_0004 and 37 identified homologs. Only the aligned APAF1_C/WD40 domain-containing regions were included (678 positions) with a gap cutoff of 85% (see Suppl. Data File 2). Bootstrap values (500 resamplings) > 80% are shown. The scale bar indicates the number of amino acid substitutions per site. Sequences are represented by the name of the organism from which they originated and the sequence identifier (see also Suppl. Data File S1). The NB-ARC domain-containing T. striata proteins have the identifiers TSEL_012356.t1 and TSEL_035785.t1
Furthermore, the occurrence and distribution of the N-terminal NB-ARC and WH-like domains among representatives of both algal lineages aligns with a parsimonious scenario in which these domains were inherited from an NB-ARC/WH-like/APAF1_C/WD40 domain-containing protein present in the ancestor of these lineages. Interestingly, the identification of the cyanobacterial sequences (Fig. 3), which mirrored such a domain architecture and the architecture of the rhodophyte sequences, hinted at a vertical inheritance of a NB-ARC/WH-like/APAF1_C/WD domain-encoding gene via the ancestral cyanobacterial endosymbiont. However, a close relationship between the proteins from cyanobacterial, rhodophyte, and chlorophyte/streptophyte sequences was neither supported nor rejected by the phylogenetic analysis (Fig. 4). Notably, in the cyanobacterium Gloeomargarita lithophora, the closest extant relative of plastids (Ponce-Toledo et al. 2017), we could not detect any sequences that matched either our custom chlorophyte or the Pfam APAF1_C HMM profile. Therefore, as is being considered for the metazoan APAF1 homologs (Urbach and Ausubel 2017), the putative ancestral chlorophyte/streptophyte NB-ARC/WH-like/APAF1_C/WD40 repeat domain-encoding gene might also have been acquired through lateral gene transfer.
Survey of NB-ARC domain-containing proteins in Ulva
The evolutionary loss of the N-terminal NB-ARC domain of the potentially ancestral NB-ARC/WH-like/APAF1_C/WD40 protein in the lineage to Ulva and other chlorophyte algae, could likely have drastic effects on the function of this protein. Therefore, we considered that the Ulva APAF1_C/WD40 protein might form a functional complex with an NB-ARC domain encoded on a separate locus. However, a survey of the Ulva proteome did not identify any solitary NB-ARC domains, which we envisaged as candidates for such a role. Interestingly, the survey did identify an NB-ARC domain associated with a tetratricopeptide repeat (UM014_0006.1) and an NB-ARC domain-containing protein (UM003_0200.1) with C-terminal LRR-domains (Suppl. Fig. S2). Therefore, the latter protein is likely a representative of the NBS-LRR protein family that is involved in immunity (van der Biezen and Jones 1998).
Assessment of the role of Ulva’s APAF1_C/WD40 protein
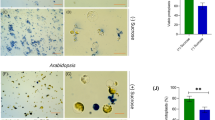
To examine the effect of the insertions in UM033_0004 (APAF1_C/WD40 domain-encoding) and UM005_0337 (membrane-bound protein-encoding) on the phenotype of the mutant plants, we propagated the primary transformants and grew new generations of gametophytes from unfertilized gametes. Microscopic inspection of germlings 12 days after gamete release did not show apparent phenotypic alterations compared with untransformed control plants (Fig. 5a–c). In the case of UM005_0337, this could suggest that the degree of downregulation achieved by the insertion (Fig. 2d) is insufficient to evoke a phenotypic change, although it may also be redundant with other genes. For the UM033_0004 null mutant (Fig. 2c), redundancy is unlikely because protein domain analysis indicate it is a single-copy gene. Therefore, UM033_0004 might be required only under specific external conditions. Because Ulva development largely depends on the presence of two bacterial symbionts that have complementing and synergetic effects (Spoerner et al. 2012; Grueneberg et al. 2016), we also investigated the phenotypes of UM033_0004 mutant and control plants under different microbial regimes. These bioassays corroborated the specific deficiencies when one or both of the symbionts (Roseovarius sp. strain MS2 and Maribacter sp. strain MS6) are missing, as previously observed (Spoerner et al. 2012), but it did not expose a difference between the control (Fig. 5d–g) and mutant plants (Fig. 5h–k). However, an examination of transcript abundance in control plants that were grown under identical conditions showed that UM033_0004 transcript levels were reduced when one or both of its symbionts were absent (Fig. 5l). Transcript reduction was equally large in the absence of Roseovarius sp. MS2 alone or in the absence of both symbionts, whereas in the absence of Maribacter sp. MS6 only, transcript levels were reduced to intermediate levels.
Phenotypic analysis of insertional mutants and transcript abundance of UM033_0004 under different bacterial regimes. Phenotypes of representative individuals of untransformed “slender” (a), insertional line UM033_0004 (b) and UM005_0337 (c) 12 days after gamete release and grown in tissue culture flask in the presence of their bacterial symbionts. Phenotypes of untransformed “slender” under axenic conditions (d) or in the presence of Roseovarius sp. MS2 only (e), Maribacter sp. MS6 only (f), or in the presence of both Roseovarius sp. MS2 and Maribacter sp. MS6 (g) after 18 days of growth in a 96-well plate. Phenotypes of insertional mutant UM033_0004 under axenic conditions (h) or in the presence of Roseovarius sp. only (i), Maribacter sp. only (j), or in the presence of both Roseovarius sp. and Maribacter sp. (k) after 18 days of growth in a 96-well plate. l Relative transcript abundance of UM033_0004 in Ulva “slender” under different symbiont regimes. Statistical significance was inferred using a one-way ANOVA. Treatments not sharing the same letter are significantly different (Tukey HSD test, P < 0.01). Error bars show the standard error of the mean (n = 3). Arrows highlight bubble-like protrusions that occur in the absence of Maribacter sp., triangles indicate degenerate cells. Size bar is 200 µm
Discussion
The recent increase in the availability of chlorophyte and streptophyte algae genome sequences has broadened the scope of plant comparative and functional genomics (Blaby-Haas and Merchant 2019). As a result, there is an incessant demand for molecular tools tailored to each prospective model organism to explore the role of individual genes experimentally. In the recently sequenced multicellular seaweed U. mutabilis, a transformation method that provides the means to create mutants through vector insertion is already available (Oertel et al. 2015), but had not yet resulted in the production of verified gain- or loss-of-function mutants. In the present study, we build on this technology by showing that it is possible to pinpoint the location of genomic vector-insertion events upon vector transformation by adopting an adapter-ligation PCR approach. Moreover, among the insertional mutants that were characterized, a null mutant was identified. This demonstrates the feasibility of future forward genetic screens in Ulva to successfully identify the casual insertional mutation, which promises to be more cost- and time-efficient than positional cloning or sequencing-based strategies.
Identifying the UM033_0004 null mutant in our insertional library encouraged us to investigate further the potential role of this gene and its evolutionary history. Protein domain architecture combined with phylogenetic analysis indicated that the Ulva UM033_0004 gene is a member of an APAF1_C/WD40 repeat-encoding gene family that was probably founded early in the evolution of the chlorophyte/streptophyte lineage by the acquisition of a gene that also encoded N-terminal NB-ARC and WH-like domains. This scenario implies that members of this family have been independently lost in several individual species of chlorophyte algae and the class of Mamiellophycean algae, as well as after the clade of Mesostigmata/Spirotaenia/Chlorokyboceae (from which we analyzed the genome sequence of one C. melkonianii and two M. viride accessions) split from the other streptophytes. The latter event also explains the absence of this gene family in the investigated land plants. The resulting scattered distribution indicates that the cooption of the genes was highly selective rather than that they played a role common to green algae.
A comprehensive study of signal transduction NTPases with numerous domains proteins by Urbach and Ausubel (2017) showed a close relationship between NB-ARC domain-containing plant R proteins, which have C-terminal LRR repeats and are involved in immunity (van der Biezen and Jones 1998), and the metazoan pro-apoptosis factor APAF1-like proteins that possess C-terminal WD40 domain repeats (Cecconi 1999). The functioning of both classes of proteins depends on NB-ARC domains that can oligomerize and build geometric protein complexes, while the repeat domains perform a sensory role (Reubold et al. 2011; Wang et al. 2019). The loss of the likely ancestral N-terminal NB-ARC- and WH-like domains in many chlorophyte lineages, including Ulva, suggests that this gene family acquired a restricted or specialized role in these lineages during evolution that solely depends on the APAF1_C/WD40 domain repeat. Because the WD40 repeats fold into beta-propellers that promote protein–protein interactions (Mishra et al. 2014), its ability to serve as a scaffold or interact otherwise probably dictates the function of the APAF1_C/WD40 protein in Ulva and other chlorophyte algae. Interestingly, structural studies of the metazoan APAF1 protein, which is an essential pro-apoptosis factor, have shown that the APAF1_C subdomain [described as helical domain 2 (HD2)] (Riedl et al. 2005; Dorstyn et al. 2018) serves as a rigid arm that positions the WD40 beta-propeller relative to the NB-ARC and WH-like domains. On the other hand, the WH-like domain can make various inter-and intramolecular contacts with the NB-ARC domain (Dorstyn et al. 2018). Therefore, the conserved association of the APAF1_C domain, and in some cases the WH-like domain, to the WD40 repeat suggests that they may assist in configuring the spatial orientation of any interacting molecules. Further identification of the interaction partners will be the next step to understanding the molecular function of the Ulva APAF1_C/WD40 protein.
Because of the homology of the chlorophyte APAF1_C/WD40 protein with APAF1-like proteins, and because chlorophyte algae also possess regulated cell death mechanisms (Moharikar et al. 2007; Bidle 2016), it is conceivable that the NB-ARC/WD40 gene acquired by an ancestral chlorophyte was integrated into a similar pathway as the metazoan APAF1-like proteins. However, phenotypic analysis of the UM033_0004 knock-out under normal conditions or in the absence of one or both of its symbionts did not reveal a mutant phenotype. Interestingly, transcript abundance levels showed a pattern that reflects the synergistic nature of the growth-promoting role of Ulva's symbionts. Further functional studies of UM033_0004 may thus aid in understanding the genetic networks underlying bacteria-dependent morphogenesis in Ulva.
In addition to these results, our research also revealed the existence of a plant-type NBS-LRR R-protein-encoding gene in the Ulva genome. Only recently the existence of such proteins, which in land plants are important in host–pathogen interactions, has been determined in streptophyte algae (Urbach and Ausubel 2017; Gao et al. 2018) as well as the chlorophyte algae Chromochloris zofingiensis (class Chlorophyceae) and two Botryococcus braunii accessions (class Trebouxiophyceae) (Shao et al. 2019). The discovery of an additional R-protein in Ulva (class Ulvophyceae) therefore considerably strengthens Shao et al.’s conclusion (2019) that a protein with an NBS-LRR architecture was already present in the common ancestor of the entire green lineage. However, the exact origin and molecular mechanisms that led to the emergence of this ancestral plant R-protein are still under debate. It has been suggested that NBS-LRR proteins evolved through an NB-ARC/WD40 domain-containing intermediate (Urbach and Ausubel 2017), but the closest relative of the R-protein family was not determined, nor was a protein family encoding an NB-ARC/WD40 module identified in the green lineage. Our analysis has now shown that a gene family which encoded these domains was likely present early in the evolution of green plants. Therefore, if NB-ARC/WH-like/APAF1_C/WD40 proteins predate R proteins, R proteins might not have gained their NB-ARC domain from a foreign source (Andolfo et al. 2019). Instead, R proteins could have emerged in an ancestral green alga through a genetic recombination event between endogenously available NB-ARC- and LRR-encoding genes. Finally, it will be fascinating to discover which effector molecules interact with algal R proteins, from which (microbial) organism they originate, and if these molecules are the result of harmful or symbiotic interactions. In addition to effector-triggered immunity, pattern-triggered immunity involving receptor-like kinases (Gong and Han 2021) is also an important mediator of plant immunity. Further investigations of both modes of action, which evolved in parallel (de Vries et al. 2018; Gong and Han 2021) will likely deepen our understanding of the nature of early plant–microbe interactions.
Conclusions
In summary, our research offers a technical advance in the genetic tractability of Ulva mutabilis. We provide a methodology for obtaining flanking sequences from U. mutabilis vector-insertion mutants using adapter ligation-mediated PCR. A null mutant was identified in an APAF1 C/WD40 repeat domain-encoding gene. Although no mutant phenotype was observed, gene expression levels in control plants were linked with the presence of bacterial symbionts, which U. mutabilis requires for its morphogenesis. Moreover, in addition to defining a green algal family of APAF1_C/WD40-containing proteins, our research revealed a likely plant-type NBS-LRR R-protein-encoding gene that provided insight into the evolution of plant R proteins, thereby underlining the value of Ulva as a reference model organism.
Author contribution statement
TW and MK conceived the study and interpreted the results. MK performed the genotyping and all following experiments, analyzed the data and wrote the first draft of the manuscript. TW and MK contributed to the final version of the manuscript and act both as corresponding authors.
Data availability
The data for this study have been deposited in the European Nucleotide Archive (ENA) at EMBL-EBI under accession number PRJEB46783 (https://www.ebi.ac.uk/ena/browser/view/PRJEB46783).
Abbreviations
- APAF1:
-
Apoptotic protease activating factor 1
- APAF1_C:
-
Apoptotic protease activating factor 1 helical domain
- HMM:
-
Hidden Markov model
- LRR:
-
Leucine rich repeat
- NB-ARC:
-
Nucleotide-binding adaptor shared by APAF-1, certain R-gene products and CED-4
- NBS-LRR:
-
Nucleotide-binding site and leucine-rich repeat
- WH-like:
-
Winged helix-like
References
Afgan E, Baker D, Batut B, Bouvier D, Čech M, Chilton J, Clements D, Coraor N, Grüning BA, Guerler A, Hillman-Jackson J, Hiltemann S, Jalili V, Rasche H, Soranzo N, Goecks J, Taylor J, Nekrutenko A, Blankenberg D (2018) The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res 46:W537–W544
Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, Gadrinab C, Heller C, Jeske A, Koesema E, Meyers CC, Parker H, Prednis L, Ansari Y, Choy N, Deen H, Geralt M, Hazari N, Hom E, Karnes M, Mulholland C, Ndubaku R, Schmidt I, Guzman P, Aguilar-Henonin L, Schmid M, Weigel D, Carter DE, Marchand T, Risseeuw E, Brogden D, Zeko A, Crosby WL, Berry CC, Ecker JR (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:653–657
Alsufyani T, Califano G, Deicke M, Grueneberg J, Weiss A, Engelen AH, Kwantes M, Mohr JF, Ulrich JF, Wichard T (2020) Macroalgal–bacterial interactions: identification and role of thallusin in morphogenesis of the seaweed Ulva (Chlorophyta). J Exp Bot 71:3340–3349
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Andolfo G, Di Donato A, Chiaiese P, De Natale A, Pollio A, Jones JDG, Frusciante L, Ercolano MR (2019) Alien domains shaped the modular structure of plant NLR proteins. Genome Biol Evol 11:3466–3477
Becker B, Marin B (2009) Streptophyte algae and the origin of embryophytes. Ann Bot 103:999–1004
Bidle KD (2016) Programmed cell death in unicellular phytoplankton. Curr Biol 26:R594–R607
Bikker P, van Krimpen MM, van Wikselaar P, Houweling-Tan B, Scaccia N, van Hal JW, Huijgen WJ, Cone JW, Lopez-Contreras AM (2016) Biorefinery of the green seaweed Ulva lactuca to produce animal feed, chemicals and biofuels. J Appl Phycol 28:3511–3525
Blaby-Haas CE, Merchant SS (2019) Comparative and functional algal genomics. Annu Rev Plant Biol 70:605–638
Blomme J, Liu X, Jacobs TB, De Clerck O (2021) A molecular toolkit for the green seaweed Ulva mutabilis. Plant Physiol 186:1442–1454
Blum M, Chang H-Y, Chuguransky S, Grego T, Kandasaamy S, Mitchell A, Nuka G, Paysan-Lafosse T, Qureshi M, Raj S, Richardson L, Salazar GA, Williams L, Bork P, Bridge A, Gough J, Haft DH, Letunic I, Marchler-Bauer A, Mi H, Natale DA, Necci M, Orengo CA, Pandurangan AP, Rivoire C, Sigrist CJA, Sillitoe I, Thanki N, Thomas PD, Tosatto SCE, Wu CH, Bateman A, Finn RD (2020) The InterPro protein families and domain s database: 20 years on. Nucleic Acids Res 49:D344–D354
Boesger J, Kwantes M, Wichard T (2018) Polyethylene glycol (PEG)-mediated transformation in the green macroalgae Ulva mutabilis (Chlorophyta): a forward genetic approach. In: Charrier B, Wichard T, Reddy CRK (eds) Protocols for macroalgae research, chapter 31. CRC Press, Francis & Taylor Group, Boca Raton
Califano G, Wichard T (2018) Preparation of axenic cultures in Ulva (Chlorophyta). In: Charrier B, Wichard T, Reddy CRK (eds) Protocols for macroalgae research, chaper 9. CRC Press, Francis & Taylor Group, Boca Raton
Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL (2009) BLAST+: architecture and applications. BMC Bioinform 10:421
Carpenter EJ, Matasci N, Ayyampalayam S, Wu S, Sun J, Yu J, Jimenez Vieira FR, Bowler C, Dorrell RG, Gitzendanner MA, Li L, Du W, Ullrich K, Wickett NJ, Barkmann TJ, Barker MS, Leebens-Mack JH, Wong GK-S (2019) Access to RNA-sequencing data from 1,173 plant species: The 1000 Plant transcriptomes initiative (1KP). GigaScience 8(10):[giz126]
Charrier B, Rolland E, Gupta V, Reddy CRK (2015) Production of genetically and developmentally modified seaweeds: exploiting the potential of artificial selection techniques. Front Plant Sci 6:127
Cock PJA, Chilton JM, Grüning B, Johnson JE, Soranzo N (2015) NCBI BLAST+ integrated into Galaxy. GigaScience 4:39
De Clerck O, Kao SM, Bogaert KA, Blomme J, Foflonker F, Kwantes M, Vancaester E, Vanderstraeten L, Aydogdu E, Boesger J, Califano G, Charrier B, Clewes R, Del Cortona A, D’Hondt S, Fernandez-Pozo N, Gachon CM, Hanikenne M, Lattermann L, Leliaert F, Liu X, Maggs CA, Popper ZA, Raven JA, Van Bel M, Wilhelmsson PKI, Bhattacharya D, Coates JC, Rensing SA, Van Der Straeten D, Vardi A, Sterck L, Vandepoele K, Van de Peer Y, Wichard T, Bothwell JH (2018) Insights into the evolution of multicellularity from the sea lettuce genome. Curr Biol 28:2921–2933
de Vries S, de Vries J, von Dahlen JK, Gould SB, Archibald JM, Rose LE, Slamovits CH (2018) On plant defense signaling networks and early land plant evolution. Commun Integr Biol 11:1–14
Del Cortona A, Jackson CJ, Bucchini F, Van Bel M, D’Hondt S, Skaloud P, Delwiche CF, Knoll AH, Raven JA, Verbruggen H, Vandepoele K, De Clerck O, Leliaert F (2020) Neoproterozoic origin and multiple transitions to macroscopic growth in green seaweeds. Proc Natl Acad Sci USA 117:2551–2559
Delwiche CF, Cooper ED (2016) The evolutionary origin of a terrestrial flora. Curr Biol 25:899–910
Dent RM, Haglund CM, Chin BL, Kobayashi MC, Niyogi KK (2005) Functional genomics of eukaryotic photosynthesis using insertional mutagenesis of Chlamydomonas reinhardtii. Plant Physiol 137:545–556
Dittami SM, Heesch S, Olsen JL, Collen J (2017) Transitions between marine and freshwater environments provide new clues about the origins of multicellular plants and algae. J Phycol 53:731–745
Dorstyn L, Akey CW, Kumar S (2018) New insights into apoptosome structure and function. Cell Death Differ 25:1194–1208
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Finn RD, Clements J, Eddy SR (2011) HMMER web server: interactive sequence similarity searching. Nucleic Acids Res 39:W29–W37
Føyn B (1958) Über die Sexualität und den Generationswechsel von Ulva mutabilis. Arch Protistenk 102:473–480
Føyn B (1959) Geschlechtskontrollierte Vererbung bei der marinen Grünalge Ulva mutabilis. Arch Protistenk 104:236–253
Fürst-Jansen JMR, de Vries S, de Vries J (2020) Evo-physio: on stress responses and the earliest land plants. J Exp Bot 71:3254–3269
Gao Y, Wang W, Zhang T, Gong Z, Zhao H, Han GZ (2018) Out of water: The origin and early diversification of plant R-genes. Plant Physiol 177:82–89
Gawryluk RMR, Tikhonenkov DV, Hehenberger E, Husnik F, Mylnikov AP, Keeling PJ (2019) Non-photosynthetic predators are sister to red algae. Nature 572:240–243
Gong Z, Han G-Z (2021) Flourishing in water: the early evolution and diversification of plant receptor-like kinases. Plant J 106:174–184
Graham L, Graham J, Wilcox L (2009) Algae. Benjamin Cummings (Pearson), San Francisco
Grigoriev IV, Hayes RD, Calhoun S, Kamel B, Wang A, Ahrendt S, Dusheyko S, Nikitin R, Mondo Stephen J, Salamov A, Shabalov I, Kuo A (2020) PhycoCosm, a comparative algal genomics resource. Nucleic Acids Res 49:D1004–D1011
Grueneberg J, Engelen AH, Costa R, Wichard T (2016) Macroalgal morphogenesis induced by waterborne compounds and bacteria in coastal seawater. PLoS ONE 11:e0146307
Guiry MD, Guiry GM (2021) AlgaeBase. World-wide electronic Publication, National University of Ireland, Galway
Hofmann K, Bucher P (1997) The CARD domain: a new apoptotic signalling motif. Trends Biochem Sci 22:155–156
Hoxmark RC (1975) Experimental analysis of life-cycle of Ulva mutabilis. Bot Mar 18:123–129
Hunter S, Apweiler R, Attwood TK, Bairoch A, Bateman A, Binns D, Bork P, Das U, Daugherty L, Duquenne L, Finn RD, Gough J, Haft D, Hulo N, Kahn D, Kelly E, Laugraud A, Letunic I, Lonsdale D, Lopez R, Madera M, Maslen J, McAnulla C, McDowall J, Mistry J, Mitchell A, Mulder N, Natale D, Orengo C, Quinn AF, Selengut JD, Sigrist CJA, Thimma M, Thomas PD, Valentin F, Wilson D, Wu CH, Yeats C (2008) InterPro: the integrative protein signature database. Nucleic Acids Res 37:D211–D215
Irisarri I, Darienko T, Pröschold T et al (2021) Unexpected cryptic species among streptophyte algae most distant to land plants. Proc R Soc B 288:20212168
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Leliaert F, De Clerck O, Verbruggen H, Boedeker C, Coppejans E (2007) Molecular phylogeny of the Siphonocladales (Chlorophyta: Cladophorophyceae). Mol Phylogenet Evol 44:1237–1256
Letunic I, Khedkar S, Bork P (2020) SMART: recent updates, new developments and status in 2020. Nucleic Acids Res 49:D458–D460
Li L, Wang S, Wang H, Sahu SK, Marin B, Li H, Xu Y, Liang H, Li Z, Cheng S, Reder T, Cebi Z, Wittek S, Petersen M, Melkonian B, Du H, Yang H, Wang J, Wong GK, Xu X, Liu X, Van de Peer Y, Melkonian M, Liu H (2020) The genome of Prasinoderma coloniale unveils the existence of a third phylum within green plants. Nat Ecol Evol 4:1220–1231
Liu YG, Mitsukawa N, Oosumi T, Whittier RF (1995) Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J 8:457–463
Mann KH (1973) Seaweeds—their productivity and strategy for growth. Science 182:975–981
Matsuo Y, Imagawa H, Nishizawa M, Shizuri Y (2005) Isolation of an algal morphogenesis inducer from a marine bacterium. Science 307:1598
Matt G, Umen J (2016) Volvox: a simple algal model for embryogenesis, morphogenesis and cellular differentiation. Dev Biol 419:99–113
McFadden GI (2001) Primary and secondary endosymbiosis and the origin of plastids. J Phycol 37:951–959
Mishra AK, Muthamilarasan M, Khan Y, Parida SK, Prasad M (2014) Genome-wide investigation and expression analyses of WD40 protein family in the model plant foxtail millet (Setaria italica L.). PLoS ONE 9:e86852
Mistry J, Chuguransky S, Williams L, Qureshi M, Salazar Gustavo A, Sonnhammer ELL, Tosatto SCE, Paladin L, Raj S, Richardson LJ, Finn RD, Bateman A (2020) Pfam: the protein families database in 2021. Nucleic Acids Res 49:D412–D419
Moharikar S, D’Souza JS, Rao BJ (2007) A homologue of the defender against the apoptotic death gene (dad1) in UV-exposed Chlamydomonas cells is downregulated with the onset of programmed cell death. J Biosci 32:261–270
Nagel G, Ollig D, Fuhrmann M, Kateriya S, Mustl AM, Bamberg E, Hegemann P (2002) Channelrhodopsin-1: a light-gated proton channel in green algae. Science 296:2395–2398
Niklas KJ, Newman SA (2013) The origins of multicellular organisms. Evol Dev 15:41–52
Oertel W, Wichard T, Weissgerber A (2015) Transformation of Ulva mutabilis (Chlorophyta) by vector plasmids integrating into the genome. J Phycol 51:963–979
O’Malley RC, Alonso JM, Kim CJ, Leisse TJ, Ecker JR (2007) An adapter ligation-mediated PCR method for high-throughput mapping of T-DNA inserts in the Arabidopsis genome. Nat Protoc 2:2910–2917
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Ponce-Toledo RI, Deschamps P, Lopez-Garcia P, Zivanovic Y, Benzerara K, Moreira D (2017) An early branching freshwater cyanobacterium at the origin of plastids. Curr Biol 27:386–391
Potter SC, Luciani A, Eddy SR, Park Y, Lopez R, Finn RD (2018) HMMER web server: 2018 update. Nucleic Acids Res 46:W200–W204
Quevillon E, Silventoinen V, Pillai S, Harte N, Mulder N, Apweiler R, Lopez R (2005) InterProScan: protein domains identifier. Nucleic Acids Res 33:W116–W120
Reubold TF, Wohlgemuth S, Eschenburg S (2011) Crystal structure of full-length Apaf-1: how the death signal is relayed in the mitochondrial pathway of apoptosis. Structure 19:1074–1083
Riedl SJ, Li WY, Chao Y, Schwarzenbacher R, Shi YG (2005) Structure of the apoptotic protease-activating factor 1 bound to ADP. Nature 434:926–933
Shao ZQ, Xue JY, Wang Q, Wang B, Chen JQ (2019) Revisiting the origin of plant NBS-LRR genes. Trends Plant Sci 24:9–12
Smetacek V, Zingone A (2013) Green and golden seaweed tides on the rise. Nature 504:84–88
Spoerner M, Wichard T, Bachhuber T, Stratmann J, Oertel W (2012) Growth and thallus morphogenesis of Ulva mutabilis (Chlorophyta) depends on a combination of two bacterial species excreting regulatory factors. J Phycol 48:1433–1447
Steinhagen S, Barco A, Wichard T, Weinberger F (2019) Conspecificity of the model organism Ulva mutabilis and Ulva compressa (Ulvophyceae, Chlorophyta). J Phycol 55:25–36
Sterck L, Billiau K, Abeel T, Rouze P, Van de Peer Y (2012) ORCAE: online resource for community annotation of eukaryotes. Nat Methods 9:1041
Stratmann J, Paputsoglu G, Oertel W (1996) Differentiation of Ulva mutabilis (Chlorophyta) gametangia and gamete release are controlled by extracellular inhibitors. J Phycol 32:1009–1021
Sun L, Ge YB, Sparks JA, Robinson ZT, Cheng X, Wen JQ, Blancaflor EB (2019) TDNAscan: A Software to identify complete and truncated T-DNA insertions. Front Genet 10:685
Tam LW, Lefebvre PA (1993) Cloning of flagellar genes in Chlamydomonas reinhardtii by DNA insertional mutagenesis. Genetics 135:375–384
Thole V, Alves SC, Worland B, Bevan MW, Vain P (2009) A protocol for efficiently retrieving and characterizing flanking sequence tags (FSTs) in Brachypodium distachyon T-DNA insertional mutants. Nat Protoc 4:650–661
Timme RE, Bachvaroff TR, Delwiche CF (2012) Broad phylogenomic sampling and the sister lineage of land plants. PLoS ONE 7:e29696
Turmel M, Otis C, Lemieux C (2006) The chloroplast genome sequence of Chara vulgaris sheds new light into the closest green algal relatives of land plants. Mol Biol Evol 23:1324–1338
Umen JG (2014) Green algae and the origins of multicellularity in the plant kingdom. Cold Spring Harb Perspect Biol 6:a016170
Urbach JM, Ausubel FM (2017) The NBS-LRR architectures of plant R-proteins and metazoan NLRs evolved in independent events. Proc Nat Acad Sci USA 114:1063–1068
Valiela I, McClelland J, Hauxwell J, Behr PJ, Hersh D, Foreman K (1997) Macroalgal blooms in shallow estuaries: controls and ecophysiological and ecosystem consequences. Limnol Oceanogr 42:1105–1118
van der Biezen EA, Jones JD (1998) The NB-ARC domain: a novel signalling motif shared by plant resistance gene products and regulators of cell death in animals. Curr Biol 8:R226–R227
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034
Wang J, Hu M, Wang J, Qi J, Han Z, Wang G, Qi Y, Wang H-W, Zhou J-M, Chai J (2019) Reconstitution and structure of a plant NLR resistosome conferring immunity. Science 364:eaav5870
Wichard T, Oertel W (2010) Gametogenesis and gamete release of Ulva mutabilis and Ulva lactuca (Chlorophyta): regulatory effects and chemical characterization of the “swarming inhibitor.” J Phycol 46:248–259
Wichard T, Charrier B, Mineur F, Bothwell JH, Clerck OD, Coates JC (2015) The green seaweed Ulva: a model system to study morphogenesis. Front Plant Sci 6:72
Wodniok S, Brinkmann H, Glockner G, Heidel AJ, Philippe H, Melkonian M, Becker B (2011) Origin of land plants: do conjugating green algae hold the key? BMC Evol Biol 11:104
Zdobnov EM, Apweiler R (2001) InterProScan—an integration platform for the signature-recognition methods in InterPro. Bioinformatics 17:847–848
Acknowledgements
We gratefully acknowledge the financial support of the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—through Grant no. SFB 1127/2 ChemBioSys–239748522 (M.K., T.W.). The Galaxy server that was used for some calculations is in part funded by Collaborative Research Centre 992 Medical Epigenetics (DFG Grant SFB 992/1 2012) and German Federal Ministry of Education and Research [BMBF Grants 031 A538A/A538C RBC, 031L0101B/031L0101C de.NBI-epi, 031L0106 de.STAIR (de.NBI)]. We thank Georg Pohnert (University Jena, Germany) for his outstanding support throughout this project and Jens Bösger, who contributed to this project in an early stage.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kwantes, M., Wichard, T. The APAF1_C/WD40 repeat domain-encoding gene from the sea lettuce Ulva mutabilis sheds light on the evolution of NB-ARC domain-containing proteins in green plants. Planta 255, 76 (2022). https://doi.org/10.1007/s00425-022-03851-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-022-03851-0