Abstract
Main conclusion
Both mutant ert-c.1 and ert-d.7 carry T2-T3 translocations in the Ert-c gene. Principal coordinate analyses revealed the translocation types and translocation breakpoints. Mutant ert-d.7 is an Ert-c Ert-d double mutant.
Abstract
Mutations in the Ert-c and Ert-d loci are among the most common barley mutations affecting plant architecture. The mutants have various degrees of erect and compact spikes, often accompanied with short and stiff culms. In the current study, complementation tests, linkage mapping, principal coordinate analyses and fine mapping were conducted. We conclude that the original ert-d.7 mutant does not only carry an ert-d mutation but also an ert-c mutation. Combined, mutations in Ert-c and Ert-d cause a pyramid-dense spike phenotype, whereas mutations in only Ert-c or Ert-d give a pyramid and dense phenotype, respectively. Associations between the Ert-c gene and T2-T3 translocations were detected in both mutant ert-c.1 and ert-d.7. Different genetic association patterns indicate different translocation breakpoints in these two mutants. Principal coordinate analysis based on genetic distance and screening of recombinants from all four ends of polymorphic regions was an efficient way to narrow down the region of interest in translocation-involved populations. The Ert-c gene was mapped to the marker interval of 2_0801to1_0224 on 3HL near the centromere. The results illuminate a complex connection between two single genes having additive effects on barley spike architecture and will facilitate the identification of the Ert-c and Ert-d genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant architecture has fundamental impact on crop performance. For example, the pronounced increase in crop yield due to application of fertilizers required the simultaneous introduction of short-culm alleles in cereal elite cultivars during the “green revolution”; the Rht-D1b and Rht-B1b DELLA alleles in wheat and sd1 alleles in rice (Hedden 2003). Also, in barley breeding, short stature became a desired trait. Loss-of-function allele of the barley dwarfing gene Sdw1/Denso, most likely orthologous to rice Sd1 (Jia et al. 2009; Xu et al. 2017), has been extensively used in breeding programs in North America and Europe (Mickelson and Rasmusson 1994).
Before selection of the sdw1/denso “green revolution” alleles, the plant architecture of wheat and barley had already been shaped by millennia-long selection during the process of domestication and adaptation in the Neolithic revolution and human migration. Compared to wild types, both modern wheat and barley cultivars tend to have shorter rachis internodes in the spike. The mutation in the Q gene was suggested first to occur in tetraploid wheat during domestication (Sormacheva et al. 2015). It encodes an APETALA2 (AP2) transcription factor which regulates many domestication-related traits. Increased expression of Q led to higher density of spikes, and normal or reduced plant height (Faris et al. 2005; Simons et al. 2006). In barley, HvAP2 alleles, Zeo2 and Zeo3, were also found in 2-row spring UK barley as natural occurrences (Houston et al. 2013). Both alleles cause pleiotropic effects including cleistogamy (Nair et al. 2010; Houston et al. 2013), denser spike, and a haplotype-dependent change in plant height (www.nordgen.org/bgs; Franckowiak and Lundqvist 2012).
With the rise of mutagenesis techniques in cereal breeding programs, mutants induced by chemicals and radiation were collected since the early 20th century and are valuable resources for both crop improvement and biological studies. Using such collections, new mutations were, for example, found at the Zeocriton (Zeo) locus, such as Ert-r.52, Ert-r.67, Ert-r.329, Ert-r.453, dsp.av, Pyr1 and Pyr3 (Houston et al. 2013). In addition, numerous other barley genes have been described to regulate plant architecture by affecting plant stature and spike morphology (www.nordgen.org/bgs; Druka et al. 2011; Franckowiak and Lundqvist 2012). In East Asia (Japan, Korea and China), nearly 80% of 147 genotyped dwarf accessions carry the uzu1.a allele, derived from a selected spontaneous mutagenesis event in the brassinosteroid receptor-encoding gene BRI1 (Zhang 1998; Jing 2000). Additionally, several independent breeding programs isolated induced dwarfing mutants representing multiple alleles in three brassinosteroid biosynthesis genes, BRD, CPD and DIM (Dockter et al. 2014). Using Bowman near-isogenic lines and breviaristatum-e (ari-e) original mutants, HvDep1, was identified as the dwarfing gene used in Scotland, affecting plant height, awn length and grain size (Wendt et al. 2016). In the Scandinavian countries, barley cultivar ’Pallas‘ and its descendants, carrying the dwarfing allele erectoides-k.32 (ert-k.32), gained wide acceptance in the 1960s (Gustafsson et al. 1971; Lundqvist 2014). This allele showed effect on spike density and plant height and improved lodging resistance (Skov Kristensen et al. 2016).
The Swedish mutant collection at the Nordic Genetic Resource Centre (NordGen) maintains barley germplasm altered in two other loci, Ert-c and Ert-d, with multiple alleles affecting plant height and spike density. One allele of each, ert-c.1 and ert-d.7, was introduced by recurrent backcrossing into a Bowman genetic background generating near-isogenic lines BW305 and BW306, respectively (Druka et al. 2011). Both lines were shown to have similar, rather large, introgression regions on chromosomes 2H and 3H and identical spike phenotypes (Druka et al. 2011), which contrasts with earlier results where ert-d.7 was mapped to a 7H pericentromeric region and caused a much denser spike phenotype (Persson and Hagberg 1969). Due to these contrasting findings, we set the following objectives: (i) substantiate the identities of ert-c.1, ert-d.7 and their corresponding near-isogenic lines BW305 and BW306, (ii) characterize the structure of chromosome 2H and 3H in the mutants, and (iii) explore the association between ert-d.7 and other extreme dense ert-d mutants.
Materials and methods
Plant materials
The ert-c.1 and ert-d.7 mutants were both induced by X-rays in barley cultivar ‘Gull’ (Gustafsson 1947). Compared to Gull, the two mutants have semi-compact and compact spikes, respectively, caused by a reduction in rachis internode length (www.nordgen.org/bgs; Franckowiak and Lundqvist 2012). Both ert-c.1 and ert-d.7 are recessive mutations.
The complementation tests were conducted at Aarhus University, Flakkebjerg, Denmark. Crosses were made (Table 1) in 2014 and the F2 plants were phenotyped in 2015 in a semi-field area (covered outdoor area with irrigated pots). With respect to flowering time, mutants with Gull background are 2 or 3 weeks later than Bowman and Bowman near-isogenic lines. To obtain parental plants at synchronized flowering time, the ert-c.1 and ert-d.7 mutants as fathers were planted once a week continuously for 4 weeks. Then from the third week, Bowman near-isogenic mothers were planted continuously for 3 weeks. From each cross, F1 seeds from two or three spikes were grown in the greenhouse in the winter 2014–2015. Three spikes from each cross were harvested from F1 plants. F2 seeds were planted in pots in the semi-field area in summer 2015. The phenotyping was conducted at maturity after the spikes were dried, so that mis-scoring due to insufficient grain-filling was minimized. Another F2 population between Bowman and the original ert-d.7 mutant was made for segregation test. F1 seeds were planted in the greenhouse in winter 2014–2015. A total of 160 F2 plants together with controls were planted and phenotyped in the semi-field area in summer 2015.
Additionally, four F2 populations for both BW305 and BW306 were developed for genetic mapping (Table 2). BW305 populations and their respective original lines were planted and phenotyped in the greenhouse at Lund University, Sweden. BW306 populations and their respective original lines were planted and phenotyped at Lund University, and in the semi-field area at Aarhus University, Flakkebjerg, Denmark.
Crosses were also performed between ert-c.1 and ert-d.7 used as fathers and ert-d.33, ert-d.43, ert-d.60, ert-d.89, ert-d.158, ert-d.307, ert-d.372, ert-d.375, ert-d.404 and ert-d.420 used as mothers. The original mutants along with the F2 plants were grown in field conditions in Lund, Sweden, during 2020. Twenty or 40 seeds were planted of each line. Phenotyping was conducted at maturity. The spike density was measured according to Persson and Hagberg (1969) as the total length of ten rachis internodes between kernel number 5 and 15.
Genotyping
Around 0.5 cm long leaf segments were sampled at seedling stage directly into 96-well plates. DNA was extracted with Extract-N-Amp™ (Sigma-Aldrich Co. LLC) according to manufacturer’s instructions. A ten-time dilution was used as DNA template in subsequent PCR analyses.
The introgression regions in BW305 and BW306 were previously defined primarily on 2H and 3H with a single-nucleotide polymorphism (SNP) array (Druka et al. 2011). Accordingly, SNPs in these regions are polymorphic in BW305/BW306 × Bowman populations. Primers for genotyping were designed based on the sequences of these SNPs (Close et al. 2009). SNPs used for the Bowman × ert-d.7 population were chosen based on Bowman near-isogenic lines with Gull introgression regions (Druka et al. 2011). SNPs used in complementation tests were also obtained this way. All the markers used in this study are listed in Suppl. Table S1 and S2.
Markers 1_1530 and 1_1283 were run with the KASP system (LGC Genomics) on ABI Viia7 Real-Time PCR System (Thermo Fisher) according to manufacturer’s guide. The KASP program was modified due to low template DNA concentration: 94 °C activation 15 min, 10 cycles of 94 °C denaturation 20 s, and 61–55 °C annealing/elongation 60 s with decreasing 0.6 °C each cycle, followed by 32 cycles of 94 °C denaturation 20 s and 55 °C annealing/elongation 60 s. If the lines were not clustered well after the first run, five additional cycles of 94 °C denaturation 20 s and 57 °C annealing/elongation 60 s were added. Other markers were run with the high-resolution melting (HRM) module on the Viia 7 instrument. HRM primers were designed with Primer 3 (http://bioinfo.ut.ee/primer3-0.4.0/) with product size 60–120 bp and annealing temperature around 57 °C. If SNPs were discovered from ESTs, the EST sequences were blasted against IPK database (http://webblast.ipk-gatersleben.de/barley/) for the corresponding contig. If introns occurred, the corresponding Bowman contig was used for primer design. The reaction mix was prepared with a reduced volume of 10 µl containing 5 µl MeltDoctor HRM Master Mix (Applied Biosystems), 0.3 µl forward primer (10 µM), 0.3 µl reverse primer (10 µM), 3.4 µl water and 1 µl DNA template. The PCR program was 95 °C enzyme activation 10 min, 45 cycles of 95 °C denaturation 15 s, and 63 °C annealing/extension 45 s, followed by an HRM program: 95 °C denaturation 10 s, 60 °C annealing 1 min, high-resolution melting at a rate of 0.025 °C/s–95 °C and hold for 15 s, 60 °C annealing 15 s at the end.
Genetic and bioinformatics analyses
The linkage map was calculated with R/qtl (Broman et al. 2003) and the map was drawn with Mapchart (Voorrips 2002). Pair-wise genetic distances were calculated with R/qtl (Broman et al. 2003), PCo was conducted in R (R Core Team 2019) and the three principal components (PCs) were visualized in 3D plots with R/rgl (Adler et al. 2016).
The primary sequences of the markers were obtained from Plant Genome and Systems Biology (PGSB) http://pgsb.helmholtz-muenchen.de/plant/barley/fpc/searchjsp/index.jsp. Physical positions of the markers were further obtained based on the blastn hit against Morex V2 reference at IPK barley blast server https://webblast.ipk-gatersleben.de/barley_ibsc/.
Results
Genetic identity of BW305, BW306 and the original ert-c.1 mutant
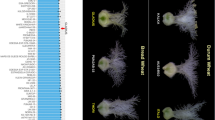
The ert-c.1 and ert-d.7 mutants were both isolated after X-ray treatment of the barley cultivar Gull in 1937 and 1941, respectively (Gustafsson 1947). Compared to Gull, the ert-c.1 mutant is denser at the bottom of the spike generating a pyramid-shaped phenotype, whereas the ert-d.7 mutant is a more compact version of ert-c.1, i.e. pyramid-dense (Fig. 1). The compact structures are caused by reduction in rachis internode length. In contrast, the near-isogenic lines BW305 and BW306, generated from recurrent backcrosses between cultivar Bowman and ert-c.1 and ert-d.7, respectively, showed similar spike phenotypes to each other (Fig. 1). According to SNP array results, BW305 and BW306 also have similar introgression regions (Druka et al. 2011). Therefore, we performed several crosses to substantiate the identities of the two near-isogenic lines and the original mutants (Table 1). Successful crosses were confirmed by testing F1 lines with polymorphic SNP markers (Suppl. Table S2) except for the ert-d.7 × ert-c.1 cross for which there is no polymorphic marker available. Here, segregating F2 plants were phenotyped.
Spike phenotypes of Bowman, near-isogenic lines BW305 and BW306, Gull and original mutants ert-c.1 and ert-d.7. The two ert mutants were induced by X-rays in the cultivar Gull in 1937 and 1941, respectively (www.nordgen.org/bgs). The near-isogenic lines BW305 and BW306, were generated from recurrent backcrosses between cultivar Bowman and ert-c.1 and ert-d.7, respectively (Druka et al. 2011). Scale bar: 5 cm
F2 spikes from the cross BW305 (ert-c.1) × ert-c.1 were all pyramid-shaped like their parents. That is, they were compact at the bottom of the spike (Fig. 2, Table 3). This phenotype is characteristic of the ert-c mutants (www.nordgen.org/bgs; Franckowiak and Lundqvist 2012). A BW305 × Bowman F2 population segregated into 102 wild type and 32 pyramid-shaped spikes fitting a one gene inheritance with P value 0.76 (Table 3). We concluded that BW305 is a near-isogenic line carrying the expected ert-c mutation (ert-c.1). Furthermore, only pyramid-shaped spikes were observed in F2 populations of BW305 × BW306 and of BW306 × ert-c.1 (Fig. 2, Table 3). Therefore, BW306 carries an ert-c mutation, which is allelic to ert-c.1. The segregation in a BW306 × Bowman population fitted a single-gene inheritance (Table 3). We conclude that BW305, BW306 and the original ert-c.1 mutant are single ert-c mutants.
The original ert-d.7 line is a double mutant
Three crosses were made with the original ert-d.7 mutant line for complementation tests; two as father crossed with BW305 and BW306, and one as mother crossed with ert-c.1 (Table 1). In each F2 population, there were two different types of compact spikes. One resembled the ert-c.1 mutant phenotype, being only compact at the bottom of the spike, i.e. pyramid-shaped (Fig. 2). The other type was pyramid-shaped but also dense along the entire spike, which resembled the phenotype of the original ert-d.7 mutant, i.e. pyramid-dense (Fig. 1). The results suggested segregation of an ert-d.7 mutation in an ert-c genetic background. Accordingly, the original ert-d.7 mutant line appears to be a double mutant carrying an ert-c mutation in addition to the ert-d.7 mutation. This was confirmed in a Bowman × ert-d.7 F2 population in which wild type spikes and three different compact spikes were observed, denoted pyramid-dense, pyramid and dense (Fig. 3, Table 3). The dense spike is suggested to represent the ert-d.7 phenotype in a Bowman genetic background, while the pyramid spike is the ert-c phenotype and the pyramid-dense is the ert-c ert-d.7 double mutant phenotype. The segregation ratio gave a Pearson’s chi-square χ2 = 4.74 (P value = 0.19). Thus, the hypothesis was accepted that the two genes are independent (Table 3). The observation of a double mutant phenotype in the original ert-d.7 mutant and in the segregating Bowman × ert-d.7 F2 population demonstrated an additive effect of the two genes on plant architecture.
Mapping of Ert-c in relation to T2-T3 translocations
Genetic mapping of Ert-c was initially conducted in F2 populations of BW305 × Bowman with 154 plants, BW306 × Bowman with 176 plants, and Bowman × ert-d.7 with 160 plants. The linkage map indicated that both of the two ert-c alleles are associated with markers on both chromosome 2H and 3H, which suggested translocation events. To investigate this, principal coordinate analyses were conducted as suggested by Farre et al. (2011). However, pair-wise genetic distance was used instead of similarity. In the next step, the candidate region for the Ert-c gene was refined by screening all segregating lines with flanking markers. Finally, the recombinants were further screened with all associated markers on 2H and 3H.
In linkage mapping, Ert-c was mapped between the markers 1_0380–1_0225 on chromosome 3H near the centromere using both F2 populations of BW305 × Bowman and of BW306 × Bowman (Fig. 4b, c). In the Bowman × ert-d.7 F2 population, Ert-c was co-segregating with chromosome 3H markers 1_1283 and 2_1381 and also the 2H markers 1_1302 and 2_0585 (Fig. 4d). Although Ert-c did not co-segregate with 2H markers in mapping populations of BW305 and BW306, it was still closely linked to them. This indicated that T2-T3 translocations are involved in BW305, BW306 and the ert-d.7 mutant. In addition, by adding few markers at a time to the chromosome, the map order varied constantly especially in BW306 × Bowman and Bowman × ert-d.7 populations where polymorphic markers span bigger regions and were distributed unevenly along the two chromosomes. This also indicated problematic mapping order. In conventional genetic mapping, pair-wise recombination frequencies of all markers are first estimated, then linkage groups are formed and markers from the same linkage group are placed into linear order. This will naturally cause a problem when a translocation is involved since markers from both chromosomes will be placed in one linkage group. Therefore, we converted pair-wise recombination frequencies into pair-wise genetic distances illustrated in 3D plots (Fig. 4e–g). By doing so, we not only showed associations between markers around the translocation point but also avoided placing all 2H and 3H markers into one linear linkage group. Despite a different marker order in the conventional linkage maps, the 3D maps showed similar patterns in the BW306 × Bowman (Fig. 4f) and Bowman × ert-d.7 (Fig. 4g) populations. The 3H marker 1_1283 was close to 2H marker 1_1302 based on pair-wise genetic distance, while the rest of 2H markers and 3H markers towards the telomere were gradually diverging. The closest points between 3 and 2H in the BW305 × Bowman population were 1_1314 and 1_0997 (Fig. 4e).
Genetic maps of the Ert-c locus. a The introgression regions detected by Druka et al. (2011) between BW305/BW306 and Bowman. The black bars indicate the location of markers defining the introgression regions in both BW305 and BW306. The gray bars indicate markers only found in BW306. b-g The linkage map and the 3D plot of pair-wise genetic distance among all the linked markers (principal coordinate analysis) in a BW305 × Bowman F2 population (b, e), in a BW306 × Bowman F2 population (c, f) and in a Bowman × ert-d.7 F2 population (d, g). In the 3D plots, the markers are connected with lines according to the chromosome order in the consensus map (Druka et al. 2011) and the proportion of variance captured is given as a percentage for each PCo dimension
To refine the candidate region, we performed an initial screening of available F2 plants from segregating populations (Table 2) with markers flanking the Ert-c locus to identify a small set of recombinant plants, which were further screened with all associated markers on 2H and 3H (Suppl. Table S1). The markers 1_0225 and 1_0380 were identified above as closely linked to Ert-c (Fig. 4b, c). However, 1_0225 was only polymorphic in BW305/BW306 × Bowman populations, while most of the other markers in the 3H interval were polymorphic in BW305/BW306 × Bowman and BW305/BW306 × Barke populations (Suppl. Table S1). Therefore, the closely linked 1_1314 marker was used instead of 1_0225 together with 1_0380 for the initial screening. In addition, since there were co-segregating markers on 2H in the Bowman × ert-d.7 population (Fig. 4d), 2H markers 1_1302 and 2_1166 were used as well in search for recombinant lines. In the Bowman × ert-d.7 population, all markers in the candidate region, and some markers on 2H co-segregated with Ert-c which did not provide more information. Therefore, this population was excluded from fine mapping. The Quench populations were also excluded from further screening due to low polymorphism in the region (Suppl. Table S1). The majority of markers in the 2H region are polymorphic in Morex populations rather than in the Barke populations and most 3H markers are polymorphic in Barke populations rather than Morex populations (Suppl. Table S1). So the recombinants from 1_0380 and 1_1314 screening in Barke populations were only used for 3H marker analyses, while the recombinants from 1_1302 and 2_1166 screening in Morex populations were only used for 2H marker analyses. The recombinants from Bowman populations were used for the markers from both chromosomes. In total, 1262 out of 1681 F2 plants from segregating populations were analyzed (Tables 2 and 4). The remaining plants had either missing or unreliable phenotypes and hence were excluded from further analyses. According to 3D plots in the principal coordinate analyses (Fig. 4e, f), the ert-c mutations from BW305 and BW306 are most likely from different mutation events. To avoid any unexpected consequences from different chromosome rearrangement, fine mapping was conducted separately in BW305 and BW306 populations. In BW305 populations, the candidate region was narrowed down to the interval of 2_0801 to 1_0224 on 3H and eight markers were co-segregating with Ert-c (Table 4). All 2H markers showed recombinations. Thus, the 2H markers were not closer to Ert-c compared to the co-segregating 3H markers. In BW306 populations, the candidate region was in the interval 1_0380 and 1_0225. Also in the BW306 population, all 2H markers showed recombinations. We conclude that the interval between 2_0801 and 1_0224 is the narrowest for Ert-c. Marker 2_0801 is located at bp 348,705,578 and marker 1_0224 at bp 390,615,716 according to the Morex V2 assembly (Monat et al. 2019). Thus, Ert-c is located in a 42 Mbp interval on chromosome 3H containing 376 genes (160 high-confidence genes).
Double mutations in other ert-d mutant lines
There is a considerable phenotypic variation among the available 27 ert-d mutants. The large phenotypic variation was also noted by Persson and Hagberg (1969), who identified two distinct groups; one group consisting of extremely compact mutants and one consisting of compact mutants. Combining plant height and spike density, the ert-d mutants also cluster in two groups (Suppl. Fig. S1). To evaluate if this variation is due to the presence of an additional mutation in Ert-c like in the case of mutant ert-d.7, a selection of ten ert-d mutants were further analyzed (Fig. 5). The mutants were selected from both groups identified by Persson and Hagberg. The extremely compact group consisted of mutants ert-d.33, ert-d.158, ert-d.307, ert-d.372 and ert-d.420 (Table 5). Their spike length, spike density and culm length were in the range of 49.6–58.4 mm, 15.6–17.6 mm and 53.9–55.5 cm, respectively. Concerning spike length and culm length, these measures are approximately 60% compared to the cultivar Bonus, while spike density is 51%. The compact group consisted of mutants ert-d.43, ert-d.60, ert-d.89, ert-d.375 and ert-d.404 (Table 5). Their spike length, spike density and culm length were in the range of 65.1–76.5 mm, 22.9–26.7 mm and 64.6–65.7 cm, respectively. Concerning spike length and spike density, these measures are approximately 75% compared to the cultivar Bonus, while culm length is 91% (Table 5). Mutant ert-d.7 appeared to be intermediate concerning spike length (62.1 mm) and spike density (19.8 mm) but was taller than Bonus (culm length 82.4 cm) (Table 5). Bonus, ert-d.7, ert-d.33, ert-d.43, ert-d.60, ert-d.89, ert-d.158 and ert-d.307 were also studied by Persson and Hagberg (1969). There is a good match between our measures and theirs except for ert-d.158, which belonged to the compact group in their study and to the extremely compact group in our study. The difference indicates that we studied a different accession of ert-d.158.
To investigate the possibility of ert-c ert-d double mutations in the ten ert-d mutant lines, crosses were performed between ert-c.1 (father in the crosses) and ert-d.33, ert-d.43, ert-d.60, ert-d.89, ert-d.158, ert-d.307, ert-d.372, ert-d.375, ert-d.404 and ert-d.420 (mothers). Mutants carrying an additional mutation in Ert-c were expected to display a segregation pattern similar to ert-d.7 in the F2 generation when crossed to ert-c.1, i.e. pyramid and pyramid-dense spikes in a 3:1 ratio. In contrast, ert-d mutants not carrying an additional ert-c mutation, were expected to show spikes with a wild type phenotype mixed with dense, pyramid and pyramid-dense spikes in a 9:3:3:1 ratio. Among the ten ert-d lines, only ert-d.372 is an ert-c ert-d double mutant. All other crosses generated spikes with a wild type phenotype in the F2 generation demonstrating that they are not carrying an additional ert-c mutation (Fig. 6, Table 6).
Spike phenotypes in the segregating F2 generation from a cross between ert-c.1 and ert-d.372, and ert-c.1 and ert-d.404. The presence of wild type spikes in the ert-c.1 × ert-d.404 population demonstrates that ert-d.404 does not carry an ert-c mutation. This is in contrast to the ert-c.1 × ert-d.372 population where no wild type spikes were seen, which strongly suggests that ert-d.372 is a double ert-c ert-d.372 mutant. Scale bars: 10 cm
Discussion
Phenotypical assessment and the identities of the lines
The ert-c.1 and ert-d.7 mutants were isolated from the barley cultivar Gull after X-rays treatment (www.nordgen.org/bgs). Their spikes have a compact appearance caused by a reduction in rachis internode length. They have historically been mapped to chromosomes 3HL and 7HS, respectively (www.nordgen.org/bgs), but a later study using near-isogenic lines BW305 (ert-c.1) and BW306 (made from the original ert-d.7 mutant) suggested that both mutations are located on chromosomes 3H or 2H (Druka et al. 2011), which can be interpreted as they would be allelic. In the present study we analyzed different populations derived from crosses of the ert-c and ert-d mutants to understand their nature. Segregation analysis of the original ert-d.7 mutant in an ert-c.1 genetic background demonstrated that ert-d.7 is an ert-c ert-d double mutant. Only the ert-c mutation remains in BW306. Moreover, a T2-T3 translocation associated with Ert-c was detected in the ert-d.7 mutant, as well as in the ert-c.1 mutant. The translocation in the ert-c.1 mutant was initially detected by karyotype analysis (Hagberg and Tjio 1951). After six generations of backcrosses, the introgression regions of BW305 and BW306 are still split on chromosomes 2H and 3H (Druka et al. 2011), which further indicate T2-T3 translocations in the ert-c alleles of BW305 and BW306. Using polymorphic markers in the region, we confirmed the translocations by linkage mapping and principal coordinate analyses through the fact that markers from both 2H and 3H are associated with Ert-c. According to the principal coordinate analyses, BW306 and the original ert-d.7 mutant have similar diverging pattern, which strongly support an identical reciprocal translocation that is different from that in BW305 and ert-c.1. In addition, the data also indicates different translocation breakpoints. In BW306 × Bowman and Bowman × ert-d.7 populations, 1_1283 and 1_1302 are the closest SNP markers joining chromosomes 3H and 2H. In the BW305 × Bowman population, the joining SNP markers are 1_1314 and 1_0997. The difference between BW305 and the ert-d.7 mutant/BW306 indicates that the ert-c mutation in BW305 and ert-c.1 is a different mutation event from that in BW306 and the ert-d.7 mutant. Analysis of ten additional ert-d mutants, demonstrated that only ert-d.372 is an ert-c ert-d double mutant like ert-d.7. Future analyses will show whether ert-d.372 is carrying a T2-T3 translocation.
Mapping in translocation-involved populations
Conventional linkage mapping generates only one-dimensional maps, which cause pseudo-linkage when translocations are involved. Livingstone et al. (2000) reported that the variance in the genetic distance between any two markers on the same segment was on average tenfold higher than that of markers on different segments and they were able to separate the markers distal to the translocation breakpoints by comparing these variances. Durrant et al. (2006) introduced QuadMap with modifications of this method. However, both methods are based on simulated data with all the markers spanning whole chromosomes at a relatively larger and more even distance. The polymorphic regions in the current study involve relatively small parts of the chromosome which would probably influence the variance estimation between markers from different chromosomes, especially in the BW305 × Bowman population. Farre et al. (2011) suggested to perform principal coordinate analyses and then divide the studied double-haploid population into normal type and translocated type according to the origin of the alleles at the translocation breakpoints. In the current study, this is not an option. In our F2 populations, there are also heterozygous plants with one translocation gamete from alternative segregation and one normal gamete. These heterozygous plants, which constitute about half of the F2 population would have to be excluded if applying the method of Farre et al. (2011). Therefore, we first applied principal coordinate analyses according to Farre et al. (2011) to investigate the possible translocation types and translocation breakpoints. Then, we screened 3H recombinants with 2H markers to see if they were closer and vice versa. This approach is independent from the genetic map and the translocation type. In the principal coordinate analyses, however, genetic distance was used instead of similarity which also includes the information of heterozygous genotypes. In principal coordinate 3D plots, the location of the Ert-c gene is not close to either of the chromosomes due to its dominant nature as a marker. Compared to co-dominant markers, dominant markers cannot distinguish heterozygous dominance from homozygous dominance, which largely influenced the estimation of genetic distance.
Genetic mapping and candidate gene selection
Many barley genes have been identified through fine mapping (Hicks et al. 2001; Chono et al. 2003; Akagi et al. 2004; Zakhrabekova et al. 2012; Schneider et al. 2016; Matyszczak et al. 2020). In the last decade with fast development of sequencing technologies, many plant genomes have been sequenced including barley (Mascher et al. 2017). This resource facilitates positional cloning since genetic mapping data can be directly translated into the physical map revealing all genes located between flanking markers (Hansson et al. 2018). The barley spike density gene Zeo was cloned in this way. The authors first mapped the locus in a region containing 29 barley gene models, where one possible candidate gene immediately drew attention due to its function in cereal spike development (Houston et al. 2013). Another successful example is the cloning of the plant architecture gene Ert-m (Zakhrabekova et al. 2015). In that study, one candidate gene in the mapped interval was orthologous to ERECTA in Arabidopsis which had already been cloned. The cloning of early-maturity gene Mat-a was performed in a similar way (Zakhrabekova et al. 2012). All these successful examples have mostly relied on the synteny to genes of known functions in other species.
In the current study, the Ert-c gene was mapped to a segment on the long arm of chromosome 3H near the centromere. Here, recombination frequencies are low in centromeric as well as in translocation regions, which make the cloning of Ert-c difficult by marker assisted methods. Although 1262 plants were screened, many markers still remained co-segregating with Ert-c in the physical position ranging from 349 to 391 Mbp on chromosome 3H. We took a closer look for candidate genes at this 42 Mbp interval comprising 376 genes of which 160 are high-confidence genes. Through BLAST searches in NCBI, there is no apparent indication that any of them are involved in development of spike architecture. We are currently trying to identify Ert-c by whole genome sequencing, which is independent of gene order and location relative to regions with reduced recombination.
Conclusion
In the present study, we found an association between the Ert-c gene and T2-T3 translocations, i.e. translocations between chromosomes 2H and 3H, in both ert-c.1 and ert-d.7 mutants. Our results demonstrate that the original ert-d.7 mutant carries mutations in two loci, both ert-d and ert-c. The ert-c locus was mapped into an interval of 42 Mbp comprising 376 genes, which will facilitate the further analysis towards identification of the mutated gene. The additive effect of the two genes on spike architecture suggests that they are involved in two different molecular pathways or are active at different timepoints during the development of the emerging spike. The Ert-c gene regulates the distance between the rachis nodes at the bottom of the spike, whereas Ert-d regulates the distances along the entire spike. This might be explored in future plant breeding where an extensive knowledge of genes and gene functions, combined with large sets of genetic markers, will allow detailed design of spike architecture.
Author contribution statement
QL, NS, SZ and MH performed crosses. QL performed genotyping. QL and MH prepared figures. QL, CD, NS, SZ, UL, PLG and MH performed phenotyping, wrote the article, and contributed to the discussion.
References
Adler D, Murdoch D, Nenadic O, Urbanek S, Chen M, Gebhardt A, Bolker B, Csardi G, Strzelecki A, Senger A (2016) rgl: 3D visualization using OpenGL. R package version 0.96. 0. https://CRAN.R-project.org/package=rgl
Akagi H, Nakamura A, Yokozeki-Misono Y, Inagaki A, Takahashi H, Mori K, Fujimura T (2004) Positional cloning of the rice Rf-1 gene, a restorer of BT-type cytoplasmic male sterility that encodes a mitochondria-targeting PPR protein. Theor Appl Genetics 108:1449–1457. https://doi.org/10.1007/s00122-004-1591-2
Broman KW, Wu H, Sen S, Churchill GA (2003) R/qtl: QTL mapping in experimental crosses. Bioinformatics 19:889–890. https://doi.org/10.1093/bioinformatics/btg112
Chono M, Honda I, Zeniya H, Yoneyama K, Saisho D, Takeda K, Takatsuto S, Hoshino T, Watanabe Y (2003) A semidwarf phenotype of barley uzu results from a nucleotide substitution in the gene encoding a putative brassinosteroid receptor. Plant Physiol 133:1209–1219. https://doi.org/10.1104/pp.103.026195
Close TJ, Bhat PR, Lonardi S, Wu Y, Rostoks N, Ramsay L, Druka A, Stein N, Svensson JT, Wanamaker S, Bozdag S, Roose ML, Moscou MJ, Chao S, Varshney RK, Szucs P, Sato K, Hayes PM, Matthews DE, Kleinhofs A, Muehlbauer GJ, DeYoung J, Marshall DF, Madishetty K, Fenton RD, Condamine P, Graner A, Waugh R (2009) Development and implementation of high-throughput SNP genotyping in barley. BMC Genomics 10:582. https://doi.org/10.1186/1471-2164-10-582
Dockter C, Gruszka D, Braumann I, Druka A, Druka I, Franckowiak J, Gough SP, Janeczko A, Kurowska M, Lundqvist J, Lundqvist U, Marzec M, Matyszczak I, Müller AH, Oklestkova J, Schulz B, Zakhrabekova S, Hansson M (2014) Induced variations in brassinosteroid genes define barley height and sturdiness, and expand the “Green Revolution” genetic toolkit. Plant Physiol 166:1912–1927. https://doi.org/10.1104/pp.114.250738
Druka A, Franckowiak J, Lundqvist U, Bonar N, Alexander J, Houston K, Radovic S, Shahinnia F, Vendramin V, Morgante M, Stein N, Waugh R (2011) Genetic dissection of barley morphology and development. Plant Physiol 155:617–627. https://doi.org/10.1104/pp.110.166249
Durrant JD, Gardunia BW, Livingstone KD, Stevens MR, Jellen EN (2006) An algorithm for analyzing linkages affected by heterozygous translocations: QuadMap. J Hered 97:62–66. https://doi.org/10.1093/jhered/esj002
Faris JD, Simons KJ, Zhang Z, Gill BS (2005) The wheat super domestication gene Q. Front Wheat Biosci 100:129–148
Farre A, Benito IL, Cistue L, de Jong JH, Romagosa I, Jansen J (2011) Linkage map construction involving a reciprocal translocation. Theor Appl Genet 122:1029–1037. https://doi.org/10.1007/s00122-010-1507-2
Franckowiak JD, Lundqvist U (2012) Descriptions of barley genetic stocks for 2012. Barley Genet Newsl 42:36–792
Gustafsson Å (1947) Mutations in agricultural plants. Hereditas 33:1–100
Gustafsson Å, Hagberg A, Persson G, Wiklund K (1971) Induced mutations and barley improvement. Theor Appl Genetics 41:239–248. https://doi.org/10.1007/BF00277792
Hagberg A, Tjio JH (1951) Cytological studies on some X-ray mutants of barley. Estación Experimental De Aula Dei 2:149–167
Hansson M, Komatsuda T, Stein N, Muehlbauer GJ (2018) Molecular mapping and cloning of genes and QTLs. In: Stein N, Muehlbauer GJ (eds) The barley genome. Springer Nature Switzerland AG, Cham, Switzerland, pp 139–154. https://doi.org/10.1007/978-3-319-92528-8_10
Hedden P (2003) The genes of the green revolution. Trends Genetics 19:5–9. https://doi.org/10.1016/S0168-9525(02)00009-4
Hicks KA, Albertson TM, Wagner DR (2001) EARLY FLOWERING3 encodes a novel protein that regulates circadian clock function and flowering in Arabidopsis. Plant Cell 13:1281–1292. https://doi.org/10.1105/tpc.13.6.1281
Houston K, McKim SM, Comadran J, Bonar N, Druka I, Uzrek N, Cirillo E, Guzy-Wrobelska J, Collins NC, Halpin C, Hansson M, Dockter C, Druka A, Waugh R (2013) Variation in the interaction between alleles of HvAPETALA2 and microRNA172 determines the density of grains on the barley inflorescence. Proc Natl Acad Sci 110:16675–16680. https://doi.org/10.1073/pnas.1311681110
Jia QJ, Zhang JJ, Westcott S, Zhang XQ, Bellgard M, Lance R, Li CD (2009) GA-20 oxidase as a candidate for the semidwarf gene sdw1/denso in barley. Funct Integr Genomic 9:255–262. https://doi.org/10.1007/s10142-009-0120-4
Jing Z (2000) Inheritance of agronomic traits from the Chinese barley dwarfing gene donors ‘Xiaoshan Lixiahuang’ and ‘Cangzhou Luodama.’ Plant Breed 119:523–524. https://doi.org/10.1046/j.1439-0523.2000.00543.x
Livingstone KD, Churchill G, Jahn MK (2000) Linkage mapping in populations with karyotypic rearrangements. J Hered 91:423–428. https://doi.org/10.1093/jhered/91.6.423
Lundqvist U (2014) Scandinavian mutation research in barley—a historical review. Hereditas 151:123–131. https://doi.org/10.1111/Hrd2.00077
Mascher M, Gundlach H, Himmelbach A, Beier S, Twardziok SO, Wicker T, Radchuk V et al (2017) A chromosome conformation capture ordered sequence of the barley genome. Nature 544:427–433. https://doi.org/10.1038/nature22043
Matyszczak I, Tominska M, Zakhrabekova S, Dockter C, Hansson M (2020) Analysis of early-flowering genes at barley chromosome 2H expands the repertoire of mutant alleles at the Mat-c locus. Plant Cell Rep 39:47–61. https://doi.org/10.1007/s00299-019-02472-4
Mickelson HR, Rasmusson DC (1994) Genes for short stature in barley. Crop Sci 34:1180–1183
Monat C, Padmarasu S, Lux T, Wicker T, Gundlach H, Himmelbach A, Ens J, Li C, Muehlbauer GJ, Schulman AH, Waugh R, Braumann I, Pozniak C, Scholz U, Mayer KFX, Spannagl M, Stein N, Mascher M (2019) TRITEX: chromosome-scale sequence assembly of Triticeae genomes with open-source tools. Genome Biol 20(1):284. https://doi.org/10.1186/s13059-019-1899-5
Nair SK, Wang N, Turuspekov Y, Pourkheirandish M, Sinsuwongwat S, Chen G, Sameri M, Tagiri A, Honda I, Watanabe Y, Kanamori H, Wicker T, Stein N, Nagamura Y, Matsumoto T, Komatsuda T (2010) Cleistogamous flowering in barley arises from the suppression of microRNA-guided HvAP2 mRNA cleavage. Proc Natl Acad Sci 107:490–495. https://doi.org/10.1073/pnas.0909097107
Persson G, Hagberg A (1969) Induced variation in a quantitative character in barley. Morphology and cytogenetics of erectoides mutants. Hereditas 61:115–178. https://doi.org/10.1111/j.1601-5223.1969.tb01836.x
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Schneider LM, Adamski NM, Christensen CE, Stuart DB, Vautrin S, Hansson M, Uauy C, von Wettstein-Knowles P (2016) The Cer-cqu gene cluster determines three key players in a beta-diketone synthase polyketide pathway synthesizing aliphatics in epicuticular waxes. J Exp Bot 67:2715–2730. https://doi.org/10.1093/jxb/erw105
Simons KJ, Fellers JP, Trick HN, Zhang Z, Tai Y-S, Gill BS, Faris JD (2006) Molecular characterization of the major wheat domestication gene Q. Genetics 172:547–555. https://doi.org/10.1534/genetics.105.044727
Skov Kristensen P, Dockter C, Lundqvist U, Lu Q, Gregersen PL, Thordal-Christensen H, Hansson M (2016) Genetic mapping of the barley lodging resistance locus Erectoides-k. Plant Breed 135:420–428. https://doi.org/10.1111/pbr.12377
Sormacheva I, Golovnina K, Vavilova V, Kosuge K, Watanabe N, Blinov A, Goncharov NP (2015) Q gene variability in wheat species with different spike morphology. Genet Resour Crop Evol 62:837–852. https://doi.org/10.1007/s10722-014-0195-1
Wendt T, Holme I, Dockter C, Preuss A, Thomas W, Druka A, Waugh R, Hansson M, Braumann I (2016) HvDep1 is a positive regulator of culm elongation and grain size in barley and impacts yield in an environment-dependent manner. PLoS ONE 11:e0168924. https://doi.org/10.1371/journal.pone.0168924
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Heredity 93:77–78. https://doi.org/10.1093/jhered/93.1.77
Xu Y, Jia Q, Zhou G, Zhang XQ, Angessa T, Broughton S, Yan G, Zhang W, Li C (2017) Characterization of the sdw1 semi-dwarf gene in barley. BMC Plant Biol 17:11. https://doi.org/10.1186/s12870-016-0964-4
Zakhrabekova S, Dockter C, Ahmann K, Braumann I, Gough SP, Wendt T, Lundqvist U, Mascher M, Stein N, Hansson M (2015) Genetic linkage facilitates cloning of Ert-m regulating plant architecture in barley and identified a strong candidate of Ant1 involved in anthocyanin biosynthesis. Plant Mol Biol 88:609–626. https://doi.org/10.1007/s11103-015-0350-x
Zakhrabekova S, Gough SP, Braumann I, Muller AH, Lundqvist J, Ahmann K, Dockter C, Matyszczak I, Kurowska M, Druka A, Waugh R, Graner A, Stein N, Steuernagel B, Lundqvist U, Hansson M (2012) Induced mutations in circadian clock regulator Mat-a facilitated short-season adaptation and range extension in cultivated barley. Proc Natl Acad Sci 109:4326–4331. https://doi.org/10.1073/pnas.1113009109
Zhang J (1998) Allelism tests for the dwarf genes in the three main dwarf sources of barley. Acta Agron Sin 24:42–46
Acknowledgements
This work was financially supported by the Green Development and Demonstration Programme (GUDP) at the Ministry of Environment and Food of Denmark, project no. 34009-12-0522, the Carlsberg Foundation, The Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS 2018-01026), The Swedish Research Council (VR 2018-05117), The Erik Philip-Sörensen's Foundation and The Nilsson-Ehle Foundation.
Funding
Open access funding provided by Lund University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
U.L.: passed away shortly before submission of this work.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lu, Q., Dockter, C., Sirijovski, N. et al. Analysis of barley mutants ert-c.1 and ert-d.7 reveals two loci with additive effect on plant architecture. Planta 254, 9 (2021). https://doi.org/10.1007/s00425-021-03653-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-021-03653-w