Abstract
Main conclusion
Germination of primary dormant wild oat caused by KAR1 or GA3 is associated with ACC accumulation and increased ethylene production shortly before radicle protrusion as a result of the non-transcriptional and transcriptional activation of ACS and ACO enzymes, respectively. Response to both compounds involves the modulation of ethylene sensitivity through ethylene receptor genes.
Harvested Avena fatua caryopses are primary dormant and, therefore, germinated poorly at 20 °C. Karrikin 1 (KAR1), which action probably requires endogenous gibberellins (GAs), and gibberellin A3 (GA3) was found to induce dormant caryopses to germinate. The stimulatory effects were accompanied by the activation of the ethylene biosynthesis pathway and depended on undisturbed ethylene perception. KAR1 and GA3 promoted 1-aminocyclopropane-1-carboxylic acid (ACC) accumulation during coleorhizae emergence and ethylene production shortly prior to the radicle protrusion, which resulted from the enhanced activity of two ethylene biosynthesis enzymes, ACC synthase (ACS) and ACC oxidase (ACO). The inhibitor of ACS adversely affected beneficial impacts of both KAR1 and GA3 on A. fatua caryopses germination, while the inhibitor of ACO more efficiently impeded the GA3 effect. The inhibitors of ethylene action markedly lowered germination in response to KAR1 and GA3. Gene expression studies preceded by the identification of several genes related to ethylene biosynthesis (AfACS6, AfACO1, and AfACO5) and perception (AfERS1b, AfERS1c, AfERS2, AfETR2, AfETR3, and AfETR4) provided further evidence for the engagement of ethylene in KAR1 and GA3 induced germination of A. fatua caryopses. Both AfACO1 and AfACO5 were upregulated, whereas AfACS6 remained unaffected by the treatment. This suggests the existence of different regulatory mechanisms of enzymatic activity, transcriptional for ACO and non-transcriptional for ACS. During imbibition in water, AfERS1b was stronger expressed than other receptor genes. In the presence of KAR1 or GA3, the expression of AfETR3 was substantially induced. Differential expression of ethylene receptor genes implies the modulation of caryopses sensitivity adjusted to ethylene availability and suggests the functional diversification of individual receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary dormancy (PD) is established in the seeds of numerous, although not all species, during their development in the mother plant (Bewley et al. 2013). After shedding, these seeds are unable to germinate due to some internal block, even under favorable conditions. For germination to begin, seed dormancy must be lost, so that non-dormant seeds can germinate in a suitable environment specific for each species. Different treatments, such as after-ripening, chilling, alternate temperatures, exposure to light and also chemicals including respiratory inhibitors, sulfhydryl and nitrogenous compounds, oxidants, growth regulators, or smoke may allow dormant seeds to germinate (Bewley et al. 2013). The regulation of the seed dormancy state involves the hormonal balance between abscisic acid (ABA) and gibberellins (GAs) and/or sensitivity to these hormones. ABA promotes the establishment of seed dormancy during seed maturation and its maintenance, whereas GAs, by acting antagonistically to ABA, stimulate seed germination. In addition, the regulatory mechanisms of dormancy status and the germination potential of a seed are postulated to also comprise the other plant hormones, including ethylene, auxins, jasmonates, and brassinosteroids (Linkies and Leubner-Metzger 2012; Corbineau et al. 2014). Ethylene has been reported to break primary and secondary dormancy (Kępczyński and Kępczyńska 1997, 2003; Corbineau et al. 2014). Several studies have shown the correlation between ethylene production and/or action and the ability to germinate (Kępczyński and Kępczyńska 1997; Matilla and Matilla-Vázquez 2008; Corbineau et al. 2014). Ethylene seems to act antagonistically to ABA and in concert with GAs (Corbineau et al. 2014). However, the particulars of a molecular mechanism of the action of ethylene alone or its interplay with other phytohormones in breaking dormancy as well as in regulating germination still need further elucidation, especially in case of monocotyledonous species.
Ethylene in seeds is produced via the same pathway as described for the other plant organs (Corbineau et al. 2014). It begins from S-adenosyl-methionine (SAM) which is converted to 1-aminocyclopropane-1-carboxylic acid (ACC) by ACC synthase (ACS) and ends with oxidation of ACC to ethylene by ACC oxidase (ACO) (Yang and Hoffman 1984). ACS is commonly recognized as a key enzyme in the regulation of ethylene production. It is suggested that seed dormancy may result from an insufficient ACC level due to low ACS activity (Corbineau et al. 2014). Likewise, ACO by controlling the ethylene evolution may play a role in dormancy release and/or during seed germination (Matilla and Matilla-Vázquez 2008). The correlation between the abundance of ACO transcripts and the ACO enzymatic activity suggests its regulation at a transcriptional level during dormancy breaking of beechnut seeds (Calvo et al. 2004) as well as during non-dormant seed germination, e.g., of Pisum sativum (Petruzzelli et al. 2000), Arabidopsis thaliana, or Lepidium sativum (Linkies et al. 2009). Genes encoding both ACS and ACO form multigene families (Booker and DeLong 2015), the members of which can be differentially expressed and regulated in seeds during the transition from the dormant to the non-dormant state (Ruduś et al. 2013; Corbineau et al. 2014). After-ripening decreases transcript abundance of both SoACS7 and SoACO2 in Sisymbrium officinale dry seeds (Iglesias-Fernández and Matilla 2009). Breaking seed dormancy by cold stratification leads to downregulation of two out of several AtACS homologues, i.e., AtACS2 and AtACS11 and all AtACOs in A. thaliana (Narsai et al. 2011; Linkies and Leubner-Metzger 2012) but a slight increase of FsACO1 transcripts in Fagus sylvatica (Calvo et al. 2004). The expression of several TaACOs is higher during imbibition of after-ripened relative to dormant wheat grains (Chitnis et al. 2014). The levels of ACO transcripts have been shown to be upregulated by ethylene itself and GAs in stratified F. sylvatica and after-ripened S. officinale seeds (Calvo et al. 2004; Iglesias-Fernández and Matilla 2010). Interestingly, GA has gene-specific effects in A. thaliana GA-deficient mutant seeds, including upregulation of AtACO1 and downregulation of AtACO2 (Linkies and Leubner-Metzger 2012).
Once produced, ethylene may only exert its effect through binding to a receptor which is the first step in ethylene signaling (Shakeel et al. 2013). There are several types of ethylene-sensing receptors in plants exhibiting structural and functional diversity; e.g., there are five ethylene receptors in A. thaliana: ETR1, ERS1, EIN4, ETR2 and ERS2 (Shakeel et al. 2013; Gallie 2015b). All of them function as negative regulators of ethylene responses, i.e., ethylene is the signaling molecule that switches off the negative regulation by the receptors. As concluded from the loss-of-function mutant studies in A. thaliana, some components of ethylene signaling are involved in multi-signal regulation of seed dormancy and germination (Chiwocha et al. 2005; Wilson et al. 2014). Seeds of ethylene-insensitive (ein) or ethylene-resistant (etr) mutants exhibit deeper dormancy relative to wild type (Subbiah and Reddy 2010). Interestingly, the impairment of the ethylene signaling pathway in etr1-2 mutant results in a higher demand for GAs to promote seed germination (Chiwocha et al. 2005). It has been demonstrated that the level of receptor expression influences ethylene responses (Gallie 2015b), but only scarce data are available for transcriptional profiles of ethylene receptor genes during dormancy release or germination. The microarray data have revealed the upregulation of ERS1 in imbibing after-ripened wheat seeds that suggested the transcriptional activation of ethylene signaling as one of the mechanisms underlying seed dormancy release by after-ripening (Chitnis et al. 2014). In addition, the report of Ogawa et al. (2003) has shown that one of the ethylene receptor encoding genes, ERS1, is upregulated in A. thaliana gibberellin-defective mutant ga1-3 seeds germinating in the presence of GA4 which supports the idea that GA activates ethylene response.
Avena fatua L. and other weeds are important constraints for agricultural production in many cereal-growing regions in the world due to the long-term infestation of the soil. PD caryopses of this weed that are shed from the mother plant accumulate in the soil to form a soil seed bank and may remain viable for several years (Simpson 2007). Findings concerning the mechanisms of seed dormancy maintenance and its release not only ensure our better understanding of this developmental arrest in plant life cycle but also may have some practical implications on effectiveness of weed control strategies. Therefore, A. fatua has long been used as a model system to investigate the fundamental basis for dormancy in weed seeds (Simpson 2007; Kępczyński 2018), although the research is still impeded by the restricted genetic information of this hexaploid species.
Like in other grass species, the dormancy of A. fatua PD caryopses may be overcome by after-ripening (Foley 1994; Kępczyński and Van Staden 2012; Kępczyński et al. 2013). Also the application of various chemicals, e.g., a classical phytohormone gibberellin A3 (GA3) (Adkins et al. 1986; Kępczyński et al. 2006, 2010), a butenolide compound found in smoke karrikin 1 (KAR1) (Daws et al. 2007; Stevens et al. 2007; Kępczyński et al. 2010) or signaling molecules such as reactive oxygen species (ROS) (Cembrowska-Lech et al. 2015) are proved to effectively release dormancy in A. fatua caryopses. Ethylene alone, unlike KAR1 or GA3, is not sufficient to induce the complete germination of fully dormant wild oat florets or caryopses (Adkins and Ross 1981; Lalonde and Saini 1992; Kępczyński and Van Staden 2012; Kępczyński 2018). However, breaking of dormancy in A. fatua caryopses due to KAR1 requires ethylene action (Kępczyński and Van Staden 2012) and also endogenous GAs (Kępczyński et al. 2013). It is, however, unknown whether the action of GAs in the regulation of dormancy status and germination of A. fatua caryopses is assisted by ethylene, similarly as in case of KAR1. The underlying molecular mechanisms of KAR1—ethylene and possibly also GAs—ethylene interactions remain elusive so far.
Although several authors postulate the existence of a GA–ethylene synergism for dormancy release, after-ripening and germination promotion as a phylogenetically widespread phenomenon (Matilla and Matilla-Vázquez 2008; Corbineau et al. 2014), there are still not sufficient data on the control of ethylene biosynthesis and receptor genes by GAs in relation to seed dormancy releasing and the progress of seed germination. Those available are focused on dicotyledonous plants, mostly Brassicaceae species, A. thaliana and L. sativum (Ogawa et al. 2003; Linkies et al. 2009) or S. officinale (Iglesias-Fernández and Matilla 2010) with the exemption of F. sylvatica representing another plant taxa (Calvo et al. 2004). There is hardly any evidence on a molecular level of ethylene involvement and/or its interplay with GAs as a part of the regulatory mechanism in dormancy and germination of caryopses of grass species. A valuable exception is the report of Chitnis et al. (2014) showing the changes in transcript levels of genes related to hormonal control induced by after-ripening in wheat seeds. To our knowledge, the information on the regulation of expression of ethylene-related genes in seeds of any taxa by KAR1 is completely unavailable.
The general goal of the undertaken studies was to provide further evidence for the involvement of endogenous ethylene in dormancy release and germination of A. fatua caryopses in response to KAR1 and GA3. For this purpose, the dependence between endogenous ethylene availability/perception and PD caryopses’ germination induced by KAR1 or GA3 was compared by integrated enzymatic and gene expression profiling in relation to physiological effects. First, germination tests with ethylene direct biosynthesis precursor (ACC) and inhibitors of ethylene synthesis (AVG, AIB) or binding (NBD, 1-MCP) were conducted using caryopses treated either with KAR1 or GA3. Their ability to produce ethylene was measured and also the content of ACC was determined. As ethylene production depends on enzymatic reactions, the activities of ACS and ACO in relation to KAR1 or GA3 treatments were analyzed. As none of ethylene-related genes had yet been identified in A. fatua, their identification became a pre-requisite for gene expression studies. The putative genes encoding one ACS-like protein (AfACS6), two ACO isoforms (AfACO1 and AfACO5), and six ethylene receptors (AfERS1b, AfERS1c, AfERS2, and AfETR2-4) were successfully identified by homology. Finally, the expression profiles of these newly identified genes were assessed to reveal the molecular basis of KAR1 and/or GA3 dependence on ethylene in the induction of PD wild oat caryopses germination. In addition, to provide more information on the involvement of endogenous GAs in KAR1 action as a germination stimulant, also some experiments with the inhibitor of GAs synthesis (PAC) were performed.
Materials and methods
Plant material
Avena fatua L. (wild oat) spikelets were collected in July 2011, during the time of their natural dispersal, in the vicinity of Szczecin (Poland). The spikelets contained 2–3 florets covered with glumes. Each floret was a single caryopsis (fruit) covered by the lemma and palea (Simpson 2007). After collection, the florets were dried at room temperature for 7 days to a constant moisture content (ca. 11%) and then stored at − 20 °C until use to maintain their primary dormancy after harvest. Only caryopses, i.e., dehulled florets, were used in the experiments.
Germination conditions and treatment experiments
In all experiments, PD caryopses (25 in each of three biological replicates per treatment) were incubated at 20 °C (the temperature at which A. fatua dormancy is expressed) in the dark, in Petri dishes (ø 6 cm) on one layer of filter paper (Whatman no. 1) moistened with 1.5 ml of distilled water or a solution. Germinated caryopses were counted every day up to day 5 of incubation. Caryopses were regarded as germinated when the radicle protruded through the coleorhiza was above 1 mm in length. In one experiment, also the coat rupture and coleorhiza emergence (at least 1 mm in length) were recorded (Fig. 1). All manipulations were performed under a green safe light at 0.5 µmol m−2 s−1, which did not affect germination.
For determination of water uptake by the incubated caryopses which is a useful depiction of the progress of germination, the water content (WC) was determined every 4 h up to 36 h and then every 2 h up to 48 h of incubation. It was expressed on the fresh weight (FW) basis to represent the percentage of water in the total mass, and calculated as WC = [(FW − DW)/FW] × 100; dry weight (DW) of caryopses was determined gravimetrically on fully dried (105 °C for 24 h) specimens.
Treatment with KAR1 or GA3 in combination with ACC, AVG, or AIB
Caryopses were incubated in Petri dishes on filter paper moistened either with distilled water or the solution of KAR1 (3 × 10−9 M) or GA3 (10−5 M), applied alone or in combination with 1-aminocyclopropane-1-carboxylic acid (ACC 10−4 M), aminoethoxyvinylglycine (AVG, 10−3 M) or α-amino-isobutyric acid (AIB, 10−3 M). KAR1 used in experiments was synthesized as described previously (Cembrowska-Lech et al. 2015).
Treatment with KAR1 or GA3 in combination with NBD or 1-MCP
Three uncovered Petri dishes with caryopses on filter paper moistened either with distilled water or the solution of KAR1 (3 × 10−9 M) or GA3 (10−5 M), were placed in tightly closed glass containers (0.5 dm3) with air or air enriched with 2,5-norbornadiene (NBD) or 1-methylcyclopropene (1-MCP) as described previously (Kępczyński and Karssen 1985; Kępczyński and Van Staden 2012). Liquid NBD (2.5 or 10 μl) was applied by syringe via the stopper onto filter paper placed under the lid of the container. The liquid evaporated completely and the final gas concentration was 5 × 10−5 or 2 × 10−4 M, as calculated, respectively. Portions of powdered 1-MCP in concentrations producing 100 or 200 µl l−1 gas were placed in open glass vials suspended under the lid of containers. To liberate 1-MCP to the atmosphere, 2 ml of sterile water were injected through the stopper into the vials containing powdered 1-MCP. Each day during the 5-day incubation period, the containers were opened, and the caryopses were transferred to Petri dishes with filter paper moistened with fresh solution and then replaced in glass containers with NBD or 1-MCP applied again.
Treatment with KAR1, GA3, and PAC
Some caryopses were incubated at 20 °C in Petri dishes on filter paper moistened either with distilled water or the solution of KAR1 (3 × 10−9 M) or GA3 (10−5 M), applied alone or in combination with paclobutrazol (PAC 10−4 M) (Sigma-Aldrich). Other caryopses were preincubated at 4 °C in darkness for 12 or 24 h in the presence of PAC (10−4 M). Thereafter, they were rinsed once with 100 ml of distilled water and transferred to new Petri dishes containing distilled water, KAR1 (3 × 10−9 M) or GA3 (10−5 M), and further incubated at 20 °C. In addition, some caryopses were preincubated at 20 °C in darkness for 12, 24, or 28 h in the presence of KAR1 (3 × 10−9 M). Then, after rinsing once with distilled water (100 ml), transferred to new Petri dishes containing distilled water or PAC (10−4 M) and further incubated under the same conditions. In all these experiments, germination of caryopses was determined after 5 days of incubation at 20 °C.
Determination of ethylene production
Caryopses (25 in each of ten biological replicates per treatment) were incubated for 6, 22, 34, or 39 h either in water or in the solution of KAR1 (3 × 10−9 M) or GA3 (10−5 M), applied alone or in combination with ACC (10−4 M). Following these treatments, they were transferred to 7 ml glass vials containing filter paper moistened with 0.6 ml of fresh solutions and incubated in the same conditions (20 °C in the dark) for 2 h before ethylene measurement. After incubation, gas samples of 1 ml were taken with a syringe and injected into a Hewlett–Packard 5980 GC equipped with FID and stainless steel column (2 m; ø 1/8 inch) with a Poropack Q 80/100 mesh. The isothermal separation was conducted at 60 °C oven temperature. Ethylene production was expressed in pl h−1 25 caryopses−1. During 2 h incubation in water preceding ethylene measurement, no changes in coleorhizae emergence took place. No caryopses with the coleorhiza pierced by radicle were observed in the glass vials after ethylene determination.
In a follow-up experiment, caryopses imbibed for 39 h were divided based on the emergence of coleorhizae. Ethylene production was determined, by the method described above, separately in each sample comprising 25 caryopses either with or without emerged coleorhizae.
Quantification of ACC
Caryopses (25 in each of 3 biological replicates per treatment) were incubated either in water or in the solution of KAR1 (3 × 10−9 M) or GA3 (10−5 M) for various time. Caryopses, collected at different time-points of incubation, were ground to a fine powder in liquid nitrogen using a pre-chilled mortar and pestle in ice-cold 5% (v/v) sulfosalicylic acid (FW: extraction buffer, 1:6, w/v). The homogenate was left in the rotator for 30 min at 4 °C, following which it was centrifuged at 15,000g for 20 min at 4 °C. ACC content was determined by chemical conversion to ethylene using Lizada and Yang (1979) protocol. Samples were vortexed for 5 s to release ethylene into the vial headspace. Ethylene was determined using Hewlett–Packard 5980 GC. The ACC content was expressed in µmol g−1 FW.
In vitro activity of ACC synthase (EC 4.4.1.14)
ACS activity was assayed following Yip et al. (1991). Caryopses (25 in each of five biological replicates per treatment) were incubated either in water or in the solution of KAR1 (3 × 10−9 M) or GA3 (10−5 M). Caryopses, collected at different time-points of incubation, were ground to a fine powder in liquid nitrogen using a pre-chilled mortar and pestle in ice–cold 200 mM Tricine extraction buffer (pH 8.5) containing 10 mM DTT, 2% (w/v) PVP and 2 mM pyridoxal phosphate (PLP) (FW: extraction buffer, 1:8, w/v). The homogenates were centrifuged for 20 min at 15,000g at 4 °C. The assay mixtures containing: extract, 200 mM Tricine reaction buffer (pH 8.0), and 100 µM SAM chloride (AdoMet) were incubated at 37 °C for 1 h to convert SAM to ACC. The reaction was stopped by adding 100 mM HgCl2. The product of the reaction, ACC, was determined by ethylene production measurement according to Lizada and Yang (1979) protocol using Hewlett–Packard 5980 GC. The ACS activity was expressed as ethylene production in pl h−1 mg−1 protein.
In vitro activity of ACC oxidase (EC 1.14.17.4)
ACO activity was measured according to Mathooko et al. (1993). Caryopses (25 in each of five biological replicates per treatment) were incubated either in water or in the solution of KAR1 (3 × 10−9 M) or GA3 (10−5 M). Caryopses, collected at different time-points of incubation, were ground to a fine powder in liquid nitrogen using a pre-chilled mortar and pestle in ice-cold 100 mM Tris–HCl (pH 7.2) containing 10% (v/v) glycerol, 5 mM DTT and 30 mM Na-ascorbate (FW: extraction buffer, 1:8, w/v). The homogenates were centrifuged for 20 min at 15,000g at 4 °C. The assay mixtures containing: extract, 100 mM Tris–HCl (pH 7.2), 10% (v/v) glycerol, 5 mM Na–ascorbate, 20 mM NaHCO3, 0.02 mM FeSO4, 1 mM ACC and 1 mM DTT were incubated with shaking at 37 °C for 1 h. Next, samples were vortexed for 5 s to release ethylene into the vial headspace. Ethylene was determined using Hewlett–Packard 5980 GC. The ACO activity was expressed as ethylene production in pl h−1 mg−1 protein.
Protein assay
The protein content in the enzymatic extracts was assayed by Bradford’s method (1976), using bovine serum albumin (BSA) as a standard.
Sequence identification and analysis
Homologous sequences of target genes were retrieved from our A. fatua RNA-seq assembled transcriptome data (Ruduś and Kępczyński 2018). First, a cDNA library was constructed using a pooled sample of RNA isolated from caryopses and seedlings. Next, it was sequenced on the Illumina HiSeq 2000 genome analyzer at customer service of BGI-Tech Solutions (Hong Kong, China). Finally, transcriptome de-novo assembly was carried out with Trinity, a short reads’ assembling programme (Grabherr et al. 2011). The generated data set of assembled contigs was then searched for homologues of genes identified by Dahleen et al. (2012) in Hordeum vulgare. The sequence MegaBLAST search with the set parameter: E score < 1e−20 performed in Geneious R6 software (Biomatters Ltd., Auckland, New Zealand) using H. vulgare cDNA sequences obtained from GenBank database enabled the identification of A. fatua homologous transcripts (Table S1).
In case when retrieved contigs accounted for fragmented transcripts, PCR reactions were set up with GoTaq® Colorless Master Mix (Promega) and primers located on putative neighbours, to check whether they represent one gene sequence or separate homologues (Table S2). The amplification products were directly used for sequencing by Genomed S.A. (Warsaw, Poland). All sequencing data were analyzed using Chromas (Technelysium Pty Ltd., South Brisbane, Australia) and Geneious R6 software. The compiled sequences were verified via BLAST searching in the GeneBank database and multiple sequence alignments with representative orthologue sequences in closely related species. The nomenclature of identified A. fatua genes has been based on the highest homology of their predicted open reading frames (ORFs) to the corresponding orthologous genes from H. vulgare.
Phylogenetic trees were constructed using the deduced amino acid sequences of the newly identified genes of A. fatua by the neighbour-joining method with Jukes–Cantor genetic distance model in Geneious R6. The sequences of homologous proteins used for the construction of phylogenetic trees were obtained from UniProt database and their accession numbers have been used for unambiguous gene identity assignment.
To identify the conserved domains and residues in putative AfACS6 and two ACOs, the amino acid sequence alignments were performed using the PRALINE multiple sequence alignment web-based tool (Simossis and Heringa 2005). The following settings were applied for analysis: gap open penalty 12.0; gap extension 1.0; BLOSUM62 matrix; alignment strategy, homology-extended alignment. The outputs of PRALINE program, i.e., amino acid residue conservation in AfACS6 (cf. Fig. S4) and AfACOs (cf. Fig. S5) are presented with default colour schemes. For the alignments, amino acid sequences deposited in UniProt database were used.
RNA extraction, cDNA synthesis, and RT-qPCR
To estimate ethylene biosynthesis and ethylene receptor genes expression levels, total RNA was extracted from A. fatua caryopses at different time-points of incubation (0, 8, 24, 36, and 41 h). Caryopses (25 in each of three biological replicates per treatment) were incubated either in water or in the solution of KAR1 (3 × 10−9 M) or GA3 (10−5 M). The RNA extraction was conducted as described by Ruduś and Kępczyński (2018). Aliquots (1 μg) of total RNA were treated with DNase I (Ambion) and used to synthesize the first-strand cDNA with a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). cDNA samples were ten times diluted with nuclease-free water and used for RT-qPCR performed using SYBRSelect Master Mix (Applied Biosystems) on a StepOne Real-Time PCR System (Applied Biosystems). qPCR reactions were done in triplicate for each of three biological replicates per treatment.
The primers used in RT-qPCR analyses are listed in Table S3. The efficiency of each primer pair and the quantification cycle (Cq) determination were done by LinRegPCR method (Ruijter et al. 2009). Raw fluorescence data generated with the StepOne software (Applied Biosystems) were used for these calculations. LinRegPCR calculates individual efficiency values for each RT-qPCR reaction and averages them to obtain mean efficiency value for each gene. After running the LinReg algorithm, Cq values were transferred as a Microsoft Excel file for further gene expression analysis.
For each gene, the relative transcript abundance was calculated using the GED method (Schefe et al. 2006) and expressed as fold change relative to dry caryopses (for time-course profile during incubation in water) or to caryopses incubated in water (for KAR1 or GA3 treatments). In the latter case, PD caryopses imbibed in water served as calibrators at each time-point from 0 to 41 h. Their relative expression levels were set arbitrarily to 1, thus allowing observing the fold changes in gene expression in caryopses treated with KAR1 and GA3. This allowed showing directly the effect of dormancy releasing compounds on the expression levels of the genes of interest. A geometric mean of three genes (AfGAPDH1, AfUBC1, and AfUBC2) was used as a normalizing factor (Ruduś and Kępczyński 2018).
Statistical analysis
All the experiments were carried out in three, five, or ten biological replicates and the results are expressed as mean ± SD. The means were analyzed for significance using one–way or two–way analysis of variance, ANOVA (Statistica for Windows v. 12.0, Stat–Soft Inc., Tulsa, OK, USA). Duncan’s multiple range test was used to test for significance of differences (P ≤ 0.05) in germination experiments, ethylene production analyses, and enzymatic activity assays. Similar results were obtained in two or three independent experiments. Statistical analyses of gene expression fold changes were performed using a post hoc Tukey`s (HSD) test with confidence interval 0.05 and confidence level 95%. Differences between the mean values were considered to be significant at P < 0.01 or P < 0.05.
Results
Effect of KAR1 or GA3 on water uptake and germination of primary dormant caryopses
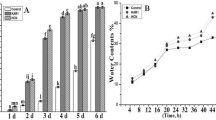
The germination tests are conducted in strictly controlled conditions, but, due to unknown environmental factors during caryopses development, the time-course of germination events may differ, especially in case of field-collected batches from natural populations. Therefore, as a pre-requisite for further analyses, the germination phases were determined for PD A. fatua caryopses harvested in 2011 (the batch used in all experiments), based on water uptake and morphological events: coat rupture, coleorhiza, and root emergence (Fig. 1).
Water uptake by PD A. fatua caryopses incubated in water or in the presence of KAR1 at 3 × 10−9 M or GA3 at 10−5 M increased rapidly during the initial 16 h of incubation up to the level of 30–35% (Fig. 1a). This period of imbibition can be considered as phase I of germination. Starting from 16 h, when phase II of germination began, the uptake of water by the caryopses slowed down. In the caryopses incubated in water, the water content remained nearly unchanged till the end of incubation time, 48 h. When either KAR1 or GA3 was applied, the water content in caryopses also hardly changed within the period from 16 to 42 h, but then increased to reach around 50% during further 6 h of incubation. Starting between 16 and 20 h, the percentage of coat rupture advanced with incubation time in caryopses treated with KAR1 or GA3, and then, at 32 h, the beginning of coleorhiza emergence was observed (Fig. 1b). The end of phase II equivalent with the onset of phase III (post-germination) was marked by radicle protrusion through the coleorhiza which took place only in caryopses incubated in the presence of KAR1 or GA3 later than 42 h. At this time-point, the coleorhiza emergence reached already over 50%. About 40% of treated caryopses germinated within 48 h of incubation, while the untreated caryopses showed no visible sign of germination during that period. If the incubation prolonged up to 5 days, around 15% germination was achieved in water, whereas the application of KAR1 or GA3 brought germination of nearly all caryopses.
Effect of PAC on germination induced by KAR1 or GA3
It was previously suggested by Kępczyński et al. (2013) that the stimulatory effects of KAR1 on the germination of dormant A. fatua caryopses depended on endogenous GAs. A widely used inhibitor of GAs biosynthesis (PAC) was used to further substantiate this idea (Table 1). PAC used alone at a concentration of 10−4 M totally inhibited the germination of A. fatua caryopses, reduced their germination by nearly 70% in the presence of KAR1 but had no effect if applied in combination with GA3 (Table 1a). Even a relatively short, 12 or 24 h pretreatment with PAC, strongly reduced the percent germination of caryopses further incubated in presence of KAR1. In comparison, that inhibitory effect of PAC was totally overcome by GA3. Interestingly, the preincubation of A. fatua caryopses from 12 up to 28 h in the presence of KAR1 was sufficient to totally release their dormancy (Table 1b). Subsequent incubation in PAC solution, however, decreased the germination of KAR1-treated caryopses down to less than ca. 60 or 70% in case of 12 or 24 h of the preincubation time, respectively.
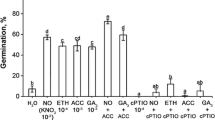
Effect of KAR1 or GA3 on germination in the presence of ACC, AVG, or AIB
To find out whether ethylene biosynthesis is required for the response of PD caryopses to KAR1 or GA3, the direct precursor (ACC) as well as two inhibitors (AVG and AIB) of ethylene biosynthesis were applied alone or in combination with KAR1 or GA3 (Table 2). The substrate in the final reaction of ethylene production, ACC applied at 10−4 M, had no significant effect on the germination of A. fatua caryopses. AVG, the inhibitor of ACS activity, used alone at a concentration of 10−3 M did not affect the germination. However, it decreased the stimulatory effects of both KAR1 and GA3, i.e., over 10% fewer caryopses germinated 3 days after the onset of imbibition. That inhibitory effect became even more pronounced at day 5 of imbibition, the percentage of caryopses germination was ca. 70 instead of about 95 in the absence of AVG. At the same time, AIB, the inhibitor of ACO activity, applied at 10−3 M, decreased the final percentage of germination (scored at day 5 of imbibition) of caryopses incubated in water. It slowed down the germination of caryopses due to KAR1, i.e., fewer of them germinated at day 3 in comparison with control, but the final percentage did not differ. AIB reduced the response of caryopses to GA3; from 96% down to ca. 80% of the final germination.
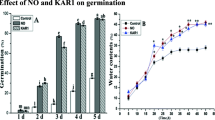
Effect of KAR1 or GA3 on germination in the presence of NBD or 1-MCP
To determine whether ethylene action is involved in the response of dormant caryopses to KAR1 and GA3, the competitive inhibitors of ethylene binding to its receptors, either reversible NBD (5 × 10−5 and 2 × 10−4 M) or irreversible 1-MCP (100 and 200 µl l−1), were applied. As in the previous experiment, PD caryopses germinated poorly at 20 °C in water (ca. 15%) (Fig. 2). The treatment with KAR1 (3 × 10−9) or GA3 (10−5 M) led to above 90% germination in comparison to control. Both ethylene signaling inhibitors, NBD and 1-MCP, markedly repressed germination of caryopses induced by KAR1 and only 20–36% germination was recorded. NBD applied at both concentrations in the presence of GA3 decreased germination but to a lesser extent than in combination with KAR1, resulting in 46–56% germination. 1-MCP also markedly antagonized the stimulatory effect of GA3 with a concentration-dependent effect. Its application at 100 or 200 µl l−1 caused that ca. 45 or only 20% of GA3-treated caryopses germinated, respectively.
Effect of KAR1 or GA3 in the absence or presence of NBD (a) or 1-MCP (b) on the germination of A. fatua caryopses after 5 days at 20 °C. Vertical bars indicate ± SD. Two-way ANOVA with the Duncan’s post hoc test was used to determine significance of differences. Mean values with different letters (a–g) are significantly different (P < 0.05, n = 3)
Ethylene production by caryopses treated with KAR1 or GA3
The rates of ethylene production by caryopses, either untreated or KAR1/GA3-treated, were determined at different time-points of imbibition (Fig. 3a). Caryopses were incubated in water or in the presence of KAR1 or GA3 for 6, 22, 34, or 39 h, and then for 2 h in water before measurement. No ethylene was detected at 8 h of incubation regardless of the treatment. Starting from 24 h, untreated caryopses produced ethylene at a relatively low stable level (about 3–4 pl h−1 25 caryopses−1) during further imbibition. No significant changes in ethylene production were observed with the application of exogenous ACC. Both KAR1 and GA3 increased ethylene production in caryopses shortly, from 3 to 5 h, before root protrusion (41 h) in comparison to control by about twofold. When applied in combination with ACC, further increase in ethylene production was observed indicating the activation of ACO in vivo. To distinguish whether ethylene production is associated with coleorhizae elongation, additional experiment was conducted (Fig. 3b). The caryopses incubated for 39 h were grouped into separated samples comprising either those with or without emerged coleorhizae and after 2 h incubation ethylene was measured. Regardless of the emergence of coleorhizae, ethylene production was stimulated in dormant caryopses due to KAR1 or GA3 and was further enhanced by the application of ACC. However, those with emerged coleorhizae produced more ethylene than those without.
Effect of KAR1 (3 × 10−9 M), GA3 (10−5 M) or ACC (10−4 M) on ethylene production by A. fatua caryopses incubated at 20 °C for various times (a) or 41 h (b). a Percentage of coleorhizae emergence noted at the time of caryopses transfer (34, 39 h) from Petri dishes to vials for ethylene accumulation: 38.8 ± 4.5 and 54.4 ± 3.4 (KAR1-treated), 52.4 ± 4.4, and 53.2 ± 5.0 (GA3-treated) did not change after the measurements (36 and 41 h). b After incubation for 39 h, caryopses without and with emerged coleorhizae were separated and transferred to GC vials with fresh solutions and incubated for 2 h before ethylene measurement. Vertical bars indicate ± SD. One-way ANOVA with the Duncan’s post hoc test was used to determine the significance of differences. Mean values with different letters (a–d) are significantly different (P < 0.05, n = 10)
ACC content in caryopses treated with KAR1 or GA3
Ethylene biosynthesis depends on the availability of its direct precursor, i.e., ACC, so the time-course of changes in ACC content in whole caryopses was determined during their imbibition (Fig. 4b). After 8 h of imbibition in water, the amount of ACC was ca. 0.3 μmol per g FW and remained constant throughout the incubation period. The presence of KAR1 and GA3 had no effect on the amount of ACC during the initial 24 h. However, later on, there was a gradual accumulation of ACC due to KAR1 and GA3. The ACC contents measured at 36 and 41 of imbibition in the presence of either KAR1 or GA3 in caryopses were above 0.4 and 0.8 μmol g−1 FW, respectively.
Effect of KAR1 or GA3 on ACS activity (a), ACC content (b), and ACO activity (c) in A. fatua caryopses incubated at 20 °C for various times. Vertical bars indicate ± SD. One-way ANOVA with the Duncan’s post hoc test was used to determine significance of differences. Mean values with different letters (a–d) are significantly different (P < 0.05, n = 3 or 5 in b and a/c, respectively)
ACS and ACO activities in caryopses treated with KAR1 or GA3
Next, we were interested in evaluating whether KAR1 or GA3 participates in the regulation of ACS (Fig. 4a) and ACO (Fig. 4c) activities which were determined in vitro. The activities of both ACS and ACO isolated from caryopses imbibed in water remained relatively stable during the whole incubation period. Under these conditions, ACO showed about three times higher activity in vitro than ACS. During the first 24 h of incubation, KAR1 and GA3 did not affect, but beginning from 36 h increased ACS activity, nearly two times when compared with control (Fig. 4a). During further incubation up to 41 h, ACS activity due to KAR1 or GA3 decreased but was still about 1.3-fold higher than in control. The significant stimulatory effect of KAR1 or GA3 on ACO activity in vitro was observed only at the end of imbibition period (41 h) shortly preceding radicle protrusion through the coleorhiza (Fig. 4c).
Identification and analysis of genes involved in ethylene biosynthesis and perception
In the view of genetics and molecular studies, wild oat belongs to the group of non-model organisms lacking fully sequenced and well-annotated genomes. Therefore, it was essential to identify the homologues of genes encoding ethylene biosynthetic enzymes and receptors prior to the expression studies. The A. fatua transcriptome data set was queried for the homologous sequences of ACC synthase, ACC oxidase, and ethylene receptors using the publicly available gene sequences from barley which belongs to Poaceae family similarly as A. fatua. The MegaBLAST search in RNA-seq A. fatua data set of assembled contigs allowed to obtain several homologous fragments of the searched genes (Table S1). Further analyses revealed that at least some of the retrieved contigs were parts of the same gene and longer sequences could have been identified, except for one of the putative ethylene receptors, AfETR4 (Table 3, S1). In total, cDNAs of three ethylene biosynthesis-related genes (AfACS6, AfACO1, and AfACO5) and six ethylene receptor genes (AfERS1b, AfERS1c, AfERS2, AfETR2, AfETR3, and AfETR4) were identified and their sequences submitted to GeneBank (Table 3). Each gene was named after its orthologue of the highest sequence similarity from barley.
The phylogenetic relationships between the newly identified wild oat genes and the known homologues representing whole gene families from Hordeum vulgare, Oryza sativa, and A. thaliana, as well as selected members from other species, were analyzed based on their predicted protein sequences (Figs. S1–S3). The amino acid sequence of the solely identified putative ACS in A. fatua clustered with rice OsACS6 and two homologous barley ACSs (Fig. S1). Together with A. thaliana ACS-like proteins, i.e., AtACS10 and AtACS12, they formed a clade separated from three ACS clades. The two ACOs found in wild oat, grouped separately on the phylogenetic tree to form distinct clades of ACO1/3 and ACO5 (Fig. S2). The former being a sub-clade within a diverse cluster comprising also ACO2 enzymes from other monocotyledonous species. Phylogenetic analysis assigned putative A. fatua ethylene receptors to two groups comprising exclusively either ERS or ETR homologues (Fig. S3). AfERS1b and AfERS1c close paralogues grouped to one clade, whereas, AfERS2 to another one, both nested within the group of ERS-like receptors. In comparison, A. fatua ETR-like receptors were to be found in three separate sister clades within their distinct group.
Analysis of the deduced translational sequences of putative AfACS and AfACOs
The fact that, AfACS6, the only transcript representing homology to ACS genes found in other species, clustered with ACS-like not ACS proteins prompted further comparative analyses of specific active site regions and/or residues in putative AfACS6. The inferred protein sequence for the partial wild oat AfACS6 gene product was aligned with several ACS enzymes having experimentally proven activity (Fig. S4). Seven regions (boxes 1–7), highly conserved in plant ACS enzymes, were distinguished in almost identical relative locations in the primary structure of AfACS6. Five of these seven regions of AfACS6 proved to be highly conserved showing identity of 60–86%. Only in two of them, fewer (37.5%) residues were identical within the corresponding regions of plant ACSs. Nearly all of the residues critical for ACS activity: directly participating in the catalytic reaction, responsible for cofactor (PLP) or substrate (SAM) positioning as well as involved in substrate (SAM) recognition were found to be conserved also in AfACS6 fragment, apart from four denoted substitutions (P/S33L, F35Y, Y255F, and S297G). The tyrosine residue Y255 replaced by phenylalanine in DEIY motif located between the conserved regions 4 and 5, that subsequently changed to DEVF, was distinctly not conserved in AfACS6. Other three residues were also substituted in the bacterial PcACS.
From deduced translations of both putative ACO transcripts, amino acid sequences were also aligned and compared with the experimentally proved functional ACOs from other species (Fig. S5) The putative wild oat ACO proteins had overall high similarity to other ACOs, especially within their central part. All critical residues involved in binding iron in the active site, and also binding bicarbonate (co-factors) as well as ACC (the substrate) during catalysis were conserved in both AfACO1 and AfACO5. Three residues located in C-terminus and involved in the mechanism of CO2 activation, although not totally conserved in ACO homologues in the other species, were found in A. fatua putative ACOs. Most of eight, otherwise, conserved lysine residues probably taking part in ascorbate activation were also found to be conserved in either AfACO1 or AfACO5 or both.
Relative expression of A. fatua ethylene-related genes in caryopses treated with KAR1 or GA3
At first, the relative expression of genes encoding biosynthetic enzymes, one ACC synthase AfACS6 and two ACC oxidases AfACO1 and AfACO5, and also those of ethylene receptors (AfERS1b, AfERS1c, AfERS2, AfETR2, AfETR3, and AfETR4) was analyzed during the incubation of caryopses in water to reveal the basic gene expression profile related to the changes in their water status (Fig. 5). When compared to dry (incubation time-point 0 h) caryopses, the level of AfACS6 transcripts remained, roughly speaking, unchanged during the whole period of incubation (Fig. 5a). In comparison, the expression of both AfACO genes showed a nearly twofold induction at 41 h of incubation. Four out of six identified ethylene receptor genes: AfERS1c, AfERS2, AfETR2, and AfETR3 were upregulated to a different extent during imbibition (Fig. 5b, c). AfERS1c showed the highest stable fourfold induction of its transcriptional activity when measured from 8 to 41 h of imbibition (Fig. 5b). About threefold change in the transcript levels was observed for AfERS2, AfETR2, and AfETR3, while neither AfERS1b nor AfETR4 was activated in PD caryopses in the course of their incubation in water (Fig. 5b, c).
Relative expression patterns of ethylene biosynthesis genes (a) and ethylene receptor genes (b, c) in A. fatua caryopses incubated in water at 20 °C. Transcript levels for each gene were estimated by qRT-PCR. The normalization factor was calculated as a geometric mean of Cq values of AfUBC1, AfUBC2, and AfGAPDH1. The fold changes indicate the expression patterns of analyzed genes relative to their transcript levels in dry PD caryopses (at 0 h time-point of incubation) with assumed value of 1. Statistical analyses: two-way ANOVA with post hoc Tukey’s (HSD) test with confidence interval 0.05, significance between groups indicated as * for P ≤ 0.05, ** for P ≤ 0.01 and *** for P ≤ 0.001. Bars indicate ± SD
To find out the molecular basis of ethylene involvement in the induction of germination in PD caryopses due to the application of either KAR1 or GA3, the expression levels of ethylene-related genes were assessed (Figs. 6, 7). Contrasting results were obtained for genes encoding biosynthetic enzymes (Fig. 6). Despite the application of a germination-inducing agent, KAR1, AfACS6 was expressed at a similar level as in water (Fig. 6a). Quite differently, exogenous GA3 which also stimulates A. fatua caryopses germination slightly downregulated the AfACS6 expression. In comparison to ACS gene, both AfACO1 and AfACO5 were substantially upregulated by the application of either KAR1 or GA3 (Fig. 6b, c), with the peak in transcript level of AfACO5 at 36 h of incubation due to GA3 (Fig. 6c). AfACO5 gene showed higher activation in imbibing caryopses due to KAR1 or GA3 than AfACO1. In addition, AfACO5 was over two times stronger induced in the presence of GA3 than KAR1.
Effect of KAR1 (3 × 10−9 M) and GA3 (10−5 M) on the expression of ethylene biosynthesis genes AfACS6 (a), AfACO1 (b) and AfACO5 (c) in A. fatua caryopses incubated at 20 °C. Transcript level determination and statistical analyses as in Fig. 4 with exception that the fold changes indicate the expression levels of analyzed genes relative to control treatment (incubation of PD caryopses on water) with assumed value of 1 at each time-point
Effect of KAR1 (3 × 10−9 M) and GA3 (10−5 M) on the expression of ethylene receptor genes AfERS1b (a), AfERS1c (b), AfERS2 (c), AfETR2 (d), AfETR3 (e), and AfETR4 (f) in A. fatua caryopses incubated at 20 °C (a–f). Transcript-level determination and statistical analyses as in Fig. 5
All of A. fatua putative ethylene receptor genes displayed different patterns of transcriptional activity in caryopses incubated either in the presence of KAR1 or GA3, although, generally, the level of their regulation, if any, was not much pronounced (Fig. 7). Two identified A. fatua ERS1 paralogues were slightly downregulated by both KAR1 or GA3 but at different time-points, i.e., AfERS1b at the end, whereas AfERS1c at the beginning of imbibition (Fig. 7a, b). Similarly as AfERS1c and belonging to the same group of receptors, AfERS2 had faintly higher transcript level at 36 h of incubation in the presence of GA3 (Fig. 7b, c). None of A. fatua ETR genes was downregulated in caryopses treated with KAR1 or GA3 (Fig. 7d–f). AfETR2 remained unaffected by any of these regulators during the whole incubation period (Fig. 7d). Contrastingly, AfETR3 expression was induced by both KAR1 and GA3, starting from 36 or even 24 h of incubation, respectively (Fig. 7e). In comparison, AfETR4 was upregulated only at 24 h of incubation due to KAR1 (Fig. 7f).
Discussion
The obtained data have confirmed that the harvested A. fatua caryopses are in a state of primary dormancy which is marked by their poor germination at 20 °C (Fig. 1). As in the previous studies, both KAR1 and GA3 are able to induce their germination (Kępczyński et al. 2006, 2010, 2013; Kępczyński and Van Staden 2012; Cembrowska-Lech et al. 2015; Cembrowska-Lech and Kępczyński 2016, 2017; Kępczyński 2018; Figs. 1, 2 and Tables 1, 2). The experiments with PAC (Table 1), the well-known inhibitor of GAs biosynthesis, provided additional evidence that the induction of germination of dormant A. fatua caryopses by KAR1 involves endogenous GAs as suggested by Kępczyński et al. (2013). It seems highly probable that, upon KAR1 treatment, GAs indispensable for the completion of germination are being synthesized in the imbibed caryopses starting from phase I of this process until the beginning of phase II, before coleorhiza emergence (Table 1, Fig. 1). Since a certain level of ethylene appears to be necessary for the stimulatory effect of KAR1 on the germination of dormant A. fatua caryopses (Kępczyński and Van Staden 2012), this study has focused on the clarification of physiological and molecular basis of ethylene function in releasing dormancy in A. fatua caryopses by both KAR1 and GA3.
Ethylene-related genes of A. fatua
To our knowledge, none of the genes involved in ethylene biosynthesis and reception have been identified so far in wild oat. It is a characteristic trait of plant genomes to contain a high proportion of duplicated genes as a result of numerous whole, segmental, and local duplications which lead up to the formation of gene families with a diverse number of paralogous genes that are very often differentially expressed and not always fully redundant in their function (Armisén et al. 2008). Ethylene-related genes are not unique in this respect (Jakubowicz 2002; Ruduś et al. 2013; Gallie 2015a) and accordingly, except for a single putative ACS, at least two ACO homologues and several putative ethylene receptor genes have been detected in A. fatua (Tables 3, S1).
ACC synthase
The ACS genes code for enzymes dependent on pyridoxal phosphate (PLP) in the formation of the direct ethylene precursor ACC. The Arabidopsis ACS gene family (AtACS 1–12) contains nine functional genes, plus a pseudogene (AtACS3), and two aminotransferases (AtACS10 and 12) (Yamagami et al. 2003). Out of six paralogous ACS genes identified in rice, one (OsACS6) shows high similarity to AtACS10 and AtACS12 (Rzewuski and Sauter 2008). In comparison, the five barley HvACS genes include two that are highly similar to OsACS6, and none that match OsACS3 and OsACS4 (Dahleen et al. 2012). The analysis of A. fatua caryopses transcriptome has allowed us to identify only one homologue of ACS gene with high similarity to HvACS6b (Tables 3, S1). It is nearly certain that wild oat possesses a multigene ACS gene family like other plant species, although we have failed to isolate others. It appears that AfACS6 is the only one expressed in the wild oat seeds.
Based on the sequence of their C-termini and the presence of putative calcium-dependent and/or mitogen-activated protein kinase phosphorylation sites, the ACS enzymes are divided into three classes/types (Booker and DeLong 2015). The phylogenetic analysis has shown that the newly identified AfACS6 gene does not encode any of them (Fig. S1). Instead, its predicted amino acid sequence shows higher similarity to barley and rice ACSs, i.e., HvACS6a, HvACS6b, and OsACS6, which, based on their homology, are considered rather as aminotransferases like AtACS10 and AtACS12 than active ACC synthases. However, further analysis has revealed the presence of highly conserved signature elements (regions and single residues) characteristic for active ACSs from several other species even as phylogenetically distant as bacteria (Fig. S4). The only substitution in residues critical for ACS catalytic properties that has been found distinctly in AfACS6 when compared with the active ACSs, either plant or bacterial, is the replacement of tyrosine (Y255) by phenylalanine (F255). However, this residue, which is a part of the PLP-binding site, is not absolutely conserved in all ACS enzymes (Tarun et al. 1998). Kinetic studies of a similar mutant in apple, Y233F, show an active enzyme with 24-fold increase in Km for SAM substrate but no change in kcat (White et al. 1994). Likewise in tomato, the corresponding substitution Y240F reduces by 36% but not totally abolishes the ACS catalytic activity. Thus, from the sequence analysis alone, AfACS6 might be assumed as active ACC synthase, yet without a direct experimental prove, it can be categorized only as ACS-like protein. According to Booker and DeLong (2015), the ACS-like subfamily includes both true ACS isozymes and a group of related proteins with ancestral function of aminotransferase activity.
ACC oxidase
The ACO genes code for enzymes responsible for the final oxygen-dependent conversion of ACC to ethylene. ACO isozymes are members of a large Fe(II)-requiring dioxygenase/oxidase superfamily encompassing roughly 100 Arabidopsis proteins (Zhang et al. 2004; Booker and DeLong 2015). However, the ACO gene families are less numerous and usually comprise up to seven members (Ruduś et al. 2013), e.g., five in A. thaliana and seven including a pseudogene OsACO6 in rice (Iwai et al. 2006). In H. vulgare, eight ACO genes were identified and mapped to six of the barley chromosomes (Dahleen et al. 2012). In this study, two members of the wild oat ACO gene family have been identified (Tables 3, S1), namely AfACO1 and AfACO5.
The phylogenetic analysis has revealed (Fig. S2) that putative AfACO1 is closer related to plant ACOs with experimentally proven activity than AfACO5. However, despite the lack of experimental evidence for their activity as ACC oxidases on the protein level, both A. fatua putative ACOs—although belonging to separate clades—display sequence features essential for the enzymatic activity of ACC oxidases (Fig. S5), i.e., conserved residues constituting the active site of these enzymes (Lay et al. 1996; Zhang et al. 2004; Binnie and McManus 2009).
Ethylene receptors
There are several types of ethylene-sensing receptors in plants exhibiting structural and functional diversity (Shakeel et al. 2013; Gallie 2015a, b). In A. thaliana, ethylene is perceived by a family of five receptors (ETR1, ETR2, ERS1, ERS2, and EIN4), the founding member being the ethylene receptor ETR1 (Shakeel et al. 2013). Based on phylogenetic analysis and some shared structural features, the genes encoding ethylene receptors can be divided into subfamily 1 (S1) and 2 (S2) (Shakeel et al. 2013; Gallie 2015a, b). The receptors of both subfamilies may be further distinguished into different types by the presence (+R) or absence (−R) of a receiver domain. In contrast, monocot genomes lack S1+R receptors homologous to ETR1 and contain only S1−R- and S2+R-type receptor genes (Yau et al. 2004; Chen and Gallie 2010). Three putative genes encoding ethylene receptors in A. fatua belong to S1 subfamily (Tables 3, S1 and Fig. S3) and the InterPro analysis has confirmed that two of them lack a receiver domain (AfERS1b and AfERS2) thus can be classified as S1-R. As only partial sequence of AfERS1c (C-terminus missing), no such confirmation is possible for this putative receptor at the moment. Similarly, as in genomes of rice (Yau et al. 2004; Iwai et al. 2006; Rzewuski and Sauter 2008) and barley (Dahleen et al. 2012), also three distinct ETR2-like receptors are present in wild oat (AfETR2-4) (Tables 3, S1). They are considered as belonging to S2 subfamily, although they lie outside AtEIN4, AtETR2, and AtERS2 clade (Fig. S3) which is consistent with the observation that monocot subfamily 2 receptors predate the appearance of distinct EIN4 and ETR2 receptors (Gallie 2015b). Each of three wild oat S2 receptors is grouped into subclades suggesting gene duplication prior to speciation. In case of AfERS1c, also for AfETR3 and AfETR4 only fragments of their coding sequences could be retrieved thus only AfETR2 has been confirmed to possess a receiver domain, indicating that it is an S2+R-type receptor.
Ethylene biosynthesis pathway in response to KAR1 and GA3
Several reports demonstrate that various factors releasing seed dormancy also increase ethylene production (Kępczyński and Kępczyńska 1997; Matilla and Matilla-Vázquez 2008; Corbineau et al. 2014). However, the role of ethylene in the removal of dormancy and transition to germination is still controversial (Matilla and Matilla-Vázquez 2008). Earlier reports show that germination preventing barriers present in PD A. fatua caryopses cannot be fully overcome by naturally produced or exogenous ethylene, indicating that this phytohormone is unlikely to be a major cause of dormancy breaking in this species (Adkins and Ross 1981; Kępczyński and Van Staden 2012). Our studies, comprising the application of ethylene biosynthesis inhibitors and the thorough analysis of the biosynthetic pathway, have confirmed the previous findings that undisturbed ethylene production is indispensable for KAR1 (Kępczyński and Van Staden 2012) and demonstrated such a necessity in GA3-mediated (Table 2 and Figs. 3, 4) induction of PD A. fatua caryopses germination.
The usage of two inhibitors of ethylene biosynthesis AVG (Yang and Hoffman 1984) and AIB (Satoh and Esashi 1982), inhibiting ACS and ACO activity, respectively, has given the clue that both KAR1 and GA3 modes of action in the stimulation of A. fatua PD caryopses germination depend on the availability of endogenous ethylene (Table 2). In this study, the adverse effect of AVG is more pronounced than that of AIB. Earlier studies demonstrated that AVG did not affect the germination of dormant A. fatua L. caryopses in the presence of KAR1 (Kępczyński and Van Staden 2012) or GA3 (Lalonde and Saini 1992). The lack of effect of this ethylene biosynthesis inhibitor might probably be attributed to higher concentration of KAR1 and also GA3 applied previously. Moreover, the influence of harvest on response of caryopses cannot be excluded.
Both KAR1 and GA3 induce the ethylene biosynthesis pathway (Fig. 4) which results in enhanced ethylene production (Fig. 3) preceding radicle protrusion through coleorhiza but not strictly connected with coleorhizae emergence (Fig. 1). Increased ethylene emission due to KAR1 or GA3 can be detected for caryopses without emerged coleorhizae, although the amount of produced ethylene is lower if compared to those showing coleorhiza emergence (Fig. 3b). This evolution of ethylene biosynthesis correlates with the elevated enzymatic activity of ACO (Fig. 4c). Likewise, amount and activity of ACO have been found to increase during the germination of non-dormant pea seeds with the maximum reached when germination was complete (Petruzzelli et al. 2000; Wang et al. 2012). KAR1 or GA3-activated ACO produces higher ethylene amounts (41 h) using ACC, the accumulation of which starts earlier, at 36 h of imbibition (Fig. 4b) due to the activation of ACS (Fig. 4a). No such an increase can be observed when PD caryopses are imbibed in water (Figs. 3, 4). This supports the idea that the lack of germination of dormant seeds might result from insufficient ACC level due to low ACS activity, which was formulated previously based on the data showing the improvement of seed germination in numerous species by exogenous ACC (Kępczyński and Kępczyńska 1997; Corbineau et al. 2014). In comparison, in A. fatua, the application of ACC causes only slight stimulation (Kępczyński and van Staden 2012) or does not affect germination of PD caryopses (Table 2). Its effect is similar to that of ethylene itself but much weaker than KAR1 or GA3, indicating not self-sufficient role of ethylene in the induction of germination in PD wild oat caryopses.
Accordingly to the rate of ethylene production (Fig. 3) and the catalytic activity of both biosynthetic enzymes in caryopses imbibed in water (Fig. 4a, c), the AfACS6 transcript level remains relatively constant and only a slight increase in two AfACO homologues has been observed (Fig. 5a). However, when caryopses are treated with KAR1 or GA3, despite the apparent stimulation of ACS activity by both compounds, it could not have been attributed to the increase in AfACS6 transcript level (Fig. 6a). Assuming that it is the only ACS gene expressed in A. fatua caryopses and encodes the active ACS enzyme, the results may imply the existence of a non-transcriptional regulatory mechanism of ACS activity in A. fatua caryopses. Newly emerging evidence strongly suggests that the regulation of ACS protein stability, by the processes of post-translational modifications, e.g., phosphorylation or ubiquitination, is an alternative mechanism that controls ethylene production (Yoon 2015).
In contrast to ACS, the increase in ACO enzyme activity correlates with the corresponding transcript accumulation, as both identified AfACO1 and AfACO5 paralogous genes are upregulated, although at different rates (Fig. 6b, c). Similar results suggesting the transcriptional regulation of ACO by GA during germination of seeds were also obtained in A. thaliana GA-deficient mutant seeds (Ogawa et al. 2003; Linkies and Leubner-Metzger 2012). In accordance, Calvo et al. (2004) have reported that regulatory cross-talk involving ethylene and GA3 affects the transition from seed dormancy to germination in F. sylvatica L. seeds. They have detected a drastic increase in FsACO1 expression in seeds treated with GA3 or ethephon, but the stimulatory effect of ethephon is reversed by PAC, a GAs biosynthesis inhibitor, suggesting that GAs positively regulates the expression of FsACO1 and thus activates the ethylene biosynthesis pathway. On the other hand, the FsGA20ox1 expression falls to the basal level in the presence of AOA, and thus, GA biosynthesis is down regulated if ethylene synthesis is impaired. However, GA stimulatory effect on germination not always involves changes in the expression of ethylene biosynthesis genes, e.g., in S. officinale, neither SoACS7 nor SoACO2 is affected by the application of gibberellin GA4+7 (Iglesias-Fernández and Matilla 2010).
Ethylene perception and expression of its receptor genes in response to KAR1 and GA3
The promoting effects of KAR1 and GA3 on PD A. fatua caryopses germination seem to be dependent on ethylene signaling, since they are heavily blocked in the presence of the inhibitors of ethylene action (Fig. 2), such as 2,5-NBD or 1-MCP, both compounds known to inhibit dormancy releasing by other factors as well as germination of non-dormant seeds (Kępczyński and Karssen 1985; Lalonde and Saini 1992; Gniazdowska et al. 2010; Kępczyński and Van Staden 2012). These observations may indicate that the functional ethylene receptors constitute a part of the regulatory mechanisms of KAR1 and GA3 in germination induction of dormant caryopses, which, to some extent, may also be dependent on ACS and/or ACO activity (Table 2). In addition, these findings are consistent with the previous suggestion that ethylene action may be either involved in breaking dormancy by KAR1 or required for the germination process after the release of dormancy by this compound (Kępczyński and Van Staden 2012). The necessity of ethylene binding for the appearance of the response of caryopses to GA3 (Fig. 2) may reflect the existence of the interaction between these two phytohormones in regulating seed dormancy and germination. The connection between ethylene and GA signaling pathways can be provided by DELLA proteins, as suggested by Achard et al. (2003) in relation to ethylene inhibition of root growth and GA involvement in apical hook maintenance in A. thaliana. They have observed that ethylene influences the rate at which the DELLA proteins disappear in response to GA.
Ethylene responses depend on the rate of receptor expression as shown in tomato transgenic plants where the degree of ethylene insensitivity was controlled by the mutant etr1-1 receptor expression (Gallie 2010). In primary dormant A. fatua caryopses, four (AfERS1c, AfERS2, AfETR2, and AfERS3) out of six identified ethylene receptor genes are upregulated upon imbibition in water (Fig. 5b, c) which might be the cause of their low sensitivity to ethylene. Ethylene receptors are negative regulators of the ethylene signaling and they inhibit the signal transduction pathway when not bound to ethylene (Shakeel et al. 2013). Therefore, the more receptors are synthesized in caryopses, the higher ethylene concentration is needed for their response to ethylene. Since ethylene production in dormant caryopses imbibed in water does not increase during imbibition (Fig. 3), any ethylene-dependent events which might have occurred during dormancy release and/or germination are probably inhibited.
Upon application of either KAR1 or GA3, a differential expression of ethylene receptor genes has been observed in A. fatua caryopses (Fig. 7), a slight downregulation of AfERS1c and AfERS1b at either the middle of phase I or the end of phase II of germination, respectively (Fig. 7a, b) accompanied in contrast by high upregulation of AfETR3 several hours before the radicle protrusion (Fig. 7e). Similar paradoxical situation of the simultaneous up and downregulation of different ethylene receptor homologues by the same factor have been also observed by others, e.g., in experiments with rice seedlings (Yau et al. 2004). The presence of several ethylene receptor genes in plant genomes, on one hand, assures their functional redundancy, and, on the other hand, allows for sub-functionalization (Shakeel et al. 2013; Gallie 2015a). Specialized roles for receptor family members are supported by the finding that A. thaliana receptor loss-of-function mutants exhibit increased expression of the remaining receptor isoforms at the level of mRNA and ethylene-binding activity but not at the level of receptor signaling (O’Malley et al. 2005). Likewise in A. fatua, the ethylene receptors may have special functions, e.g., AfETR4 gene is transiently upregulated only due to KAR1 but not GA3 treatment (Fig. 7). Thus, the differences in expression patterns of wild oat ethylene receptor homologues may depict their distinctive involvement in ethylene signaling indispensable for germination of dormant caryopses and/or perhaps also may account for preventing an excessive response to ethylene the production of which is stimulated by both KAR1 or GA3 (Fig. 3). Nevertheless, our results remain in agreement with the other reports, suggesting the stimulatory effect of exogenous GAs on seed germination to be associated with affecting ethylene signaling pathway, i.e., the detection of the upregulation of AtERS1, encoding a member of ethylene receptor gene family in the seeds of A. thaliana GA synthesis mutant, due to GA4 application (Ogawa et al. 2003) and of an EIN-3 like, a gene encoding one of transcription factors involved in the activation of ethylene response genes, in F. sylvatica dormant seeds (Lorenzo et al. 2000).
Summing up and perspectives
The induction of germination of PD A. fatua caryopses in response to both KAR1 and GA3 involves the participation of endogenous ethylene as summarized in the proposed model (Fig. 8). Ethylene very likely serves as an important agent allowing the action of KAR1 and GA3 as germination stimulants. With the application of GA3, the pool of active GAs in caryopses is probably modulated which, in turn, stimulate ethylene production (Fig. 3) by non-transcriptional regulation of ACS activity (Fig. 4a) and transcriptional regulation of ACO activity (Fig. 4c) due to the upregulation of AfACO1 and AfACO5 genes (Fig. 6b, c). GA3 also modulates ethylene sensitivity by acting on ethylene receptor genes (Fig. 7). As a result of a hypothesized GA-ethylene cross-talk, the completion of germination can be observed (Figs. 1, 2 and Tables 1, 2). Similar to GA3, also KAR1 stimulates dormant A. fatua caryopses to germinate (Figs. 1, 2 and Tables 1, 2) which as shown previously and now confirmed requires GAs synthesis (Kępczyński et al. 2013; Table 1) which probably starts during phase I of germination (Table 1). Therefore, it may be assumed that KAR1 mimics GA3 and, due to its application, similar mechanisms are activated. However, it would need further studies to reveal the components and discriminate the hierarchy of these two hormones signal transduction pathways and also to identify the response target genes which should elucidate the precise roles of GAs and ethylene in A. fatua germination process.
Summary of KAR1 or GA3 effects on water content, coleorhizae, and root emergence, ACS or ACO activities, expression of ethylene biosynthesis genes, and expression of ethylene receptor genes in A. fatua caryopses. The stimulatory effects are marked in green, while the inhibitory effects in red. CE coleorhiza emergence, CR coat rupture, RE root emergence
Author contribution statement
JK conceived and supervised the research as well as provided the funding, analyzed the results, and revised the manuscript. IR performed RNA and cDNA sample preparation, genes identification, determination of gene expression, bioinformatics, and statistical analysis, and wrote the manuscript. DC-L carried out physiological experiments, enzymes activity measurements, GC analysis, RNA, and cDNA sample preparation, and contributed to statistical analyses and writing the manuscript. AJ performed RNA and cDNA sample preparation for certain genes identification and contributed to the identification of selected genes.
Abbreviations
- ACC:
-
1-Aminocyclopropane-1-carboxylic acid
- ACO:
-
ACC oxidase
- ACS:
-
ACC synthase
- AIB:
-
α-Amino-isobutyric acid
- AVG:
-
Aminoethoxyvinylglycine
- ERS:
-
Ethylene response sensor
- ETR:
-
Ethylene response
- GA3 :
-
Gibberellic acid, gibberellin A3
- KAR1 :
-
Karrikin 1
- 1-MCP:
-
Methylcyclopropene
- NBD:
-
2,5-Norbornadien
- PAC:
-
Paclobutrazol
- PD:
-
Primary dormancy, primary dormant
- SAM:
-
S-Adenosyl-methionine
References
Achard P, Vriezen WH, Van der Straeten D, Harberd NP (2003) Ethylene regulates Arabidopsis development via the modulation of DELLA protein growth repressor function. Plant Cell 15:2816–2825
Adkins SW, Ross JD (1981) Studies in wild oat seed dormancy. I. The role of ethylene in dormancy breakage and germination of wild oat seeds (Avena fatua L.). Plant Physiol 67:358–362
Adkins SW, Loewen M, Symons SJ (1986) Variations within pure lines of wild oat (Avena fatua) in relation to degree of primary dormancy. Weed Sci 34:859–864
Armisén D, Lecharny A, Aubourg S (2008) Unique genes in plants: specificities and conserved features throughout evolution. BMC Evol Biol 8:280
Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H (2013) Seeds: physiology of development, germination and dormancy. Springer, New York
Bidonde S, Ferrer MA, Zegzouti H, Ramassamy S, Latché A, Pech JC, Hamilton AJ, Grierson D, Bouzayen M (1998) Expression and characterization of three tomato 1-aminocyclopropane-1-carboxylate oxidase cDNAs in yeast. Eur J Biochem 253:20–26
Binnie JE, McManus MT (2009) Characterization of the 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase multigene family of Malus domestica Borkh. Phytochemistry 70:348–360
Booker MA, DeLong A (2015) Producing the ethylene signal: regulation and diversification of ethylene biosynthetic enzymes. Plant Physiol 169:42–50
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Calvo AP, Nicolás C, Lorenzo O, Nicolás G, Rodríguez D (2004) Evidence for positive regulation by gibberellins and ethylene of ACC oxidase expression and activity during transition from dormancy to germination in Fagus sylvatica L. seeds. J Plant Growth Regul 23:44–53
Cembrowska-Lech D, Kępczyński J (2016) Gibberellin-like effects of KAR1 on dormancy release of Avena fatua caryopses include participation of non-enzymatic antioxidants and cell cycle activation in embryos. Planta 243:531–548
Cembrowska-Lech D, Kępczyński J (2017) Plant-derived smoke induced activity of amylases, DNA replication and β-tubulin accumulation before radicle protrusion of dormant Avena fatua L. caryopses. Acta Physiol Plant 39:39. https://doi.org/10.1007/s11738-016-2329-x
Cembrowska-Lech D, Koprowski M, Kępczyński J (2015) Germination induction of dormant Avena fatua caryopses by KAR1 and GA3 involving the control of reactive oxygen species (H2O2 and O2·−) and enzymatic antioxidants (superoxide dismutase and catalase) both in the embryo and the aleurone layers. J Plant Physiol 176:169–179
Chen JF, Gallie DR (2010) Analysis of the functional conservation of ethylene receptors between maize and Arabidopsis. Plant Mol Biol 74:405–421
Chitnis VR, Gao F, Yao Z, Jordan MC, Park S, Ayele BT (2014) After-ripening induced transcriptional changes of hormonal genes in wheat seeds: the cases of brassinosteroids, ethylene, cytokinin and salicylic acid. PLoS One 9:e87543
Chiwocha SDS, Cutler AJ, Abrams SR, Ambrose SJ, Yang J, Ross ARS, Kermode AR (2005) The etr1-2 mutation in Arabidopsis thaliana affects the abscisic acid, auxin, cytokinin and gibberellin metabolic pathways during maintenance of seed dormancy, moist-chilling and germination. Plant J 42:35–48
Corbineau F, Xia Q, Bailly C, El-Maarouf-Bouteau H (2014) Ethylene, a key factor in the regulation of seed dormancy. Front Plant Sci 5:539
Dahleen LS, Tyagi N, Bregitzer P, Brown RH, Morgan WC (2012) Developing tools for investigating the multiple roles of ethylene: identification and mapping genes for ethylene biosynthesis and reception in barley. Mol Genet Genom 287:793–802
Daws MI, Davies J, Pritchard HW, Brown NAC, Van Staden J (2007) Butenolide from plant-derived smoke enhances germination and seedling growth of arable weed species. Plant Growth Regul 51:73–82
Foley ME (1994) Temperature and water status of seed affect after-ripening in wild oat (Avena fatua). Weed Sci 42:200–204
Gallie DR (2010) Regulated ethylene insensitivity through the inducible expression of the Arabidopsis etr1-1 mutant ethylene receptor in tomato. Plant Physiol 152:1928–1939
Gallie DR (2015a) Appearance and elaboration of the ethylene receptor family during land plant evolution. Plant Mol Biol 87:521–539
Gallie DR (2015b) Ethylene receptors in plants—why so much complexity? F1000Prime Rep 7:39
Gniazdowska A, Krasuska U, Bogatek R (2010) Dormancy removal in apple embryos by nitric oxide or cyanide involves modifications in ethylene biosynthetic pathway. Planta 232:1397–1407
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, Chen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A (2011) Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat Biotechnol 29:644–652
Huang P-L, Parks JE, Rottmann WH, Theologis A (1991) Two genes encoding 1-aminocyclopropane-1-carboxylate synthase in zucchini (Cucurbita pepo) are clustered and similar but differentially regulated. Proc Natl Acad Sci USA 88:7021–7025
Iglesias-Fernández R, Matilla AJ (2009) After-ripening alters the gene expression pattern of oxidases involved in ethylene and gibberellin pathways during early imbibition of Sisymbrium officinale L. seeds. J Exp Bot 60:1645–1661
Iglesias-Fernández R, Matilla AJ (2010) Genes involved in ethylene and gibberellins metabolism are required for endosperm-limited germination of Sisymbrium officinale L. seeds. Planta 231:653–664
Iwai T, Miyasaka A, Seo S, Ohashi Y (2006) Contribution of ethylene biosynthesis for resistance to blast fungus infection in young rice plants. Plant Physiol 142:1202–1215
Jakubowicz M (2002) Structure, catalytic activity and evolutionary relationships of 1-aminocyclo propane-1-carboxylate synthase, the key enzyme of ethylene synthesis in higher plants. Acta Biochim Pol 49:757–774
Kakuta Y, Igarashi T, Murakami T, Ito H, Matsui H, Honma M (2001) 1-Aminocyclopropane-1-carboxylate synthase of Penicillium citrinum: primary structure and expression in Escherichia coli and Saccharomyces cerevisiae. Biosci Biotechnol Biochem 65:1511–1518
Kępczyński J (2018) Induction of agricultural weed seed germination by smoke and smoke-derived karrikin (KAR1), with a particular reference to Avena fatua L. Acta Physiol Plant 40:87. https://doi.org/10.1007/s11738-018-2663-2
Kępczyński J, Karssen CM (1985) Requirement for the action of endogenous ethylene during germination of non-dormant seeds of Amaranthus caudatus. Physiol Plant 63:49–52
Kępczyński J, Kępczyńska E (1997) Ethylene in seed dormancy and germination. Physiol Plant 101:720–726
Kępczyński J, Kępczyńska E (2003) Ethylene and secondary seed dormancy. In: Vendrell M, Klee H, Pech JC, Romojaro F (eds) Biology and biotechnology of the plant hormone ethylene III. IOS Press, Amsterdam, pp 209–215
Kępczyński J, Van Staden J (2012) Interaction of karrikinolide and ethylene in controlling germination of dormant Avena fatua L. caryopses. Plant Growth Regul 67:185–190
Kępczyński J, Białecka B, Light ME, Van Staden J (2006) Regulation of Avena fatua seed germination by smoke solution, gibberellin A3 and ethylene. Plant Growth Regul 49:9–16
Kępczyński J, Cembrowska D, Van Staden J (2010) Releasing primary dormancy in Avena fatua L. caryopses by smoke-derived butenolide. Plant Growth Regul 62:85–91
Kępczyński J, Cembrowska-Lech D, Van Staden J (2013) Necessity of gibberellin for stimulatory effect of KAR1 on germination of dormant Avena fatua L. caryopses. Acta Physiol Plant 35:379–387
Lalonde S, Saini HS (1992) Comparative requirement for endogenous ethylene during seed germination. Ann Bot 69:423–428
Lay VJ, Prescott AG, Thomas PG, John P (1996) Heterologous expression and site-directed mutagenesis of the 1-aminocyclopropane-1-carboxylate oxidase from kiwi fruit. Eur J Biochem 242:228–234
Linkies A, Leubner-Metzger G (2012) Beyond gibberellins and abscisic acid: how ethylene and jasmonates control seed germination. Plant Cell Rep 31:253–270
Linkies A, Müller K, Morris K, Turečková V, Wenk M, Cadman CSC, Corbineau F, Strnad M, Lynn JR, Finch-Savage WE, Leubner-Metzger G (2009) Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana. Plant Cell 21:3803–3822
Lizada MCC, Yang SF (1979) A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem 100:140–145
Lorenzo O, Rodriguez D, Nicolas C, Nicolas G (2000) Characterization and expression of two protein kinase and an EIN3-like genes, which are regulated by ABA and GA3 in dormant Fagus sylvatica seeds. In: Black M, Bradford KJ, Vazquez-Ramos J (eds) Seed biology: advances and applications. CAB International, Wallingford, pp 329–340
Mathooko FM, Kubo Y, Inaba A, Nakamura R (1993) Partial characterization of 1-aminocyclopropane-1-carboxylate oxidase from excised mesocarp tissue of winter squash fruit. Sci Rep Fac Agric Okayama Univ 82:49–59
Matilla AJ, Matilla-Vázquez MA (2008) Involvement of ethylene in seed physiology. Plant Sci 175:87–97
Narsai R, Law SR, Carrie C, Xu L, Whelan J (2011) In depth temporal transcriptome profiling reveals a crucial developmental switch with roles for RNA processing and organelle metabolism that are essential for germination in Arabidopsis. Plant Physiol 157:1342–1362
O’Malley RC, Rodriguez FI, Esch JJ, Binder BM, O’Donnell P, Klee HJ, Bleecker AB (2005) Ethylene-binding activity, gene expression levels, and receptor system output for ethylene receptor family members from Arabidopsis and tomato. Plant J 41:651–659
Ogawa M, Tanada A, Yamauchi Y, Kuwahara A, Kamiya Y, Yamaguchi S (2003) Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 15:1591–1604
Petruzzelli L, Coraggio I, Leubner-Metzger G (2000) Ethylene promotes ethylene biosynthesis during pea seed germination by positive feedback regulation of 1-aminocyclo-propane-1-carboxylic acid oxidase. Planta 211:144–149
Ruduś I, Kępczyński J (2018) Reference gene selection for molecular studies of dormancy in wild oat (Avena fatua L.) caryopses by RT-qPCR method. PLOS One 13:e0192343
Ruduś I, Sasiak M, Kępczyński J (2013) Regulation of ethylene biosynthesis at the level of 1-aminocyclopropane-1-carboxylate oxidase (ACO) gene. Acta Physiol Plant 35:295–307
Ruijter JM, Ramakers C, Hoogaars WMH, Karlen Y, Bakker O, van den Hoff MJB, Moorman AFM (2009) Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 37:e45
Rzewuski G, Sauter M (2008) Ethylene biosynthesis and signaling in rice. Plant Sci 175:32–42
Satoh S, Esashi Y (1982) Effect of α-aminoisobutyric acid and d- and l-amino acids on ethylene production and content of 1-aminocyclopropane-1-carboxylic acid in cotyledonary segments of cocklebur seeds. Physiol Plant 54:147–152
Schefe JH, Lehmann KE, Buschmann IR, Unger T, Funke-Kaiser H (2006) Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression’s CT difference” formula. J Mol Med (Berl) 84:901–910
Shakeel SN, Wang X, Binder BM, Schaller GE (2013) Mechanisms of signal transduction by ethylene: overlapping and non-overlapping signalling roles in a receptor family. AoB Plants 5:plt010
Simossis VA, Heringa J (2005) PRALINE: a multiple sequence alignment toolbox that integrates homology-extended and secondary structure information. Nucleic Acids Res 33:W289–W294
Simpson GM (2007) Seed dormancy in grasses. Cambridge University Press, Cambridge
Stevens JC, Merritt DJ, Flematti GR, Ghisalberti EL, Dixon KW (2007) Seed germination of agricultural weeds is promoted by the butenolide 3-methyl-2H-furo[2,3-c]pyran-2-one under laboratory and field conditions. Plant Soil 298:113–124
Subbiah V, Reddy KJ (2010) Interactions between ethylene, abscisic acid and cytokinin during germination and seedling establishment in Arabidopsis. J Biosci 35:451–458
Tarun AS, Lee JS, Theologis A (1998) Random mutagenesis of 1-aminocyclopropane-1-carboxylate synthase: a key enzyme in ethylene biosynthesis. Proc Natl Acad Sci USA 95:9796–9801
Wang WQ, Møller IM, Song SQ (2012) Proteomic analysis of embryonic axis of Pisum sativum seeds during germination and identification of proteins associated with loss of desiccation tolerance. J Proteom 77:68–86
White MF, Vasquez J, Yang SF, Kirsch JF (1994) Expression of apple 1-aminocyclopropane-1-carboxylate synthase in Escherichia coli: kinetic characterization of wild-type and active-site mutant forms. Proc Natl Acad Sci USA 91:12428–12432
Wilson RL, Bakshi A, Binder BM (2014) Loss of the ETR1 ethylene receptor reduces the inhibitory effect of far-red light and darkness on seed germination of Arabidopsis thaliana. Front Plant Sci 5:433
Yamagami T, Tsuchisaka A, Yamada K, Haddon WF, Harden LA, Theologis A (2003) Biochemical diversity among the 1-amino-cyclopropane-1-carboxylate synthase isozymes encoded by the Arabidopsis gene family. J Biol Chem 278:49102–49112
Yang SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35:155–189
Yau CP, Wang L, Yu M, Zee SY, Yip WK (2004) Differential expression of three genes encoding an ethylene receptor in rice during development, and in response to indole-3-acetic acid and silver ions. J Exp Bot 55:547–556
Yip WK, Dong JG, Yang SF (1991) Purification and characterization of 1-aminocyclopropane-1-carboxylate synthase from apple fruits. Plant Physiol 95:251–257
Yoon GM (2015) New insights into the protein turnover regulation in ethylene biosynthesis. Mol Cells 38:597–603
Zhang Z, Ren J-S, Clifton IJ, Schofield CJ (2004) Crystal structure and mechanistic implications of 1-aminocyclopropane-1-carboxylic acid oxidase—the ethylene-forming enzyme. Chem Biol 11:1383–1394
Acknowledgements
The study was partly supported by the Ministry of Science and Higher Education Grant NN 310 726340. We would like to express our gratitude to Prof. Ewa Kępczyńska for reading and commenting on the manuscript draft.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ruduś, I., Cembrowska-Lech, D., Jaworska, A. et al. Involvement of ethylene biosynthesis and perception during germination of dormant Avena fatua L. caryopses induced by KAR1 or GA3. Planta 249, 719–738 (2019). https://doi.org/10.1007/s00425-018-3032-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-018-3032-5