Abstract
Main conclusion
Of the three functional FPPS identified in maize, fpps3 is induced by herbivory to produce FDP important for the formation of the volatile sesquiterpenes of plant defense.
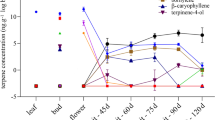
Sesquiterpenes are not only crucial for the growth and development of a plant but also for its interaction with the environment. The biosynthesis of sesquiterpenes proceeds over farnesyl diphosphate (FDP), which is either used as a substrate for protein prenylation, converted to squalene, or to volatile sesquiterpenes. To elucidate the regulation of sesquiterpene biosynthesis in maize, we identified and characterized the farnesyl diphosphate synthase (FPPS) gene family which consists of three genes. Synteny analysis indicates that fpps2 and fpps3 originate from a genome duplication in an ancient tetraploid ancestor. The three FPPSs encode active enzymes that produce predominantly FDP from the isopentenyl diphosphate and dimethylallyl diphosphate substrates. Only fpps1 and fpps3 are induced by elicitor treatment, but induced fpps1 levels are much lower and only increased to the amounts of fpps3 levels in intact leaves. Elicitor-induced fpps3 levels in leaves increase to more than 15-fold of background levels. In undamaged roots, transcript levels of fpps1 are higher than those of fpps3, but only fpps3 transcripts are induced in response to herbivory by Diabrotica virgifera virgifera. A kinetic of transcript abundance in response to herbivory in leaves provided further evidence that the regulation of fpps3 corresponds to that of tps23, a terpene synthase, that converts FDP to the volatile (E)-ß-caryophyllene. Our study indicates that the differential expression of fpps1 and fpps3 provides maize with FDP for both primary metabolism and terpene-based defenses. The expression of fpps3 seems to coincide with the herbivore-induced emission of volatile sesquiterpenes that were demonstrated to be important defense signals.






Similar content being viewed by others
Abbreviations
- DMADP:
-
Dimethylallyl diphosphate
- FARM:
-
First aspartate-rich motif
- FDP:
-
Farnesyl diphosphate
- FPPS:
-
Farnesyl diphosphate synthase
- GDP:
-
Geranyl diphosphate
- GGDP:
-
Geranylgeranyl diphosphate
- GGPPS:
-
Geranylgeranyl diphosphate synthase
- IDP:
-
Isopentenyl diphoshphate
- LG:
-
Linolenoyl-l-glutamine
- SARM:
-
Second aspartate-rich motif
- TPS:
-
Terpene synthase
References
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry, 4th edn. Allured Publ. Corp, Carol Stream
Ahn S, Anderson J, Sorrells M, Tanksley S (1993) Homoeologous relationships of rice, wheat and maize chromosomes. Mol Gen Genet 241 (5–6):483–490
Anderson MS, Yarger J, Burck C, Poulter C (1989) Farnesyl diphosphate synthetase. Molecular cloning, sequence, and expression of an essential gene from Saccharomyces cerevisiae. J Biol Chem 264:19176–19184
Ashby MN, Edwards P (1990) Elucidation of the deficiency in two yeast coenzyme Q mutants. Characterization of the structural gene encoding hexaprenyl pyrophosphate synthetase. J Biol Chem 265:13157–13164
Beck G, Coman D, Herren E, Ruiz-Sola MÁ, Rodríguez-Concepción M, Gruissem W, Vranová E (2013) Characterization of the GGPP synthase gene family in Arabidopsis thaliana. Plant Mol Biol 82:393–416
Cervantes-Cervantes M, Gallagher CE, Zhu C, Wurtzel ET (2006) Maize cDNAs expressed in endosperm encode functional farnesyl diphosphate synthase with geranylgeranyl diphosphate synthase activity. Plant Physiol 141:220–231
Closa M, Vranová E, Bortolotti C, Bigler L, Arró M, Ferrer A, Gruissem W (2010) The Arabidopsis thaliana FPP synthase isozymes have overlapping and specific functions in isoprenoid biosynthesis, and complete loss of FPP synthase activity causes early developmental arrest. Plant J 63:512–525
Correll CC, Edwards PA (1994) Mevalonic acid-dependent degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase in vivo and in vitro. J Biol Chem 269:633–638
Cunillera N, Arró M, Delourme D, Karst F, Boronat A, Ferrer A (1996) Arabidopsis thaliana contains two differentially expressed farnesyl-diphosphate synthase genes. J Biol Chem 271:7774–7780
Cunillera N, Boronat A, Ferrer A (1997) The Arabidopsis thaliana FPS1 gene generates a novel mRNA that encodes a mitochondrial farnesyl-diphosphate synthase isoform. J Biol Chem 272:15381–15388
Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P (1996) A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science 273(5279):1239–1241
Degenhardt J, Gershenzon J, Baldwin IT, Kessler A (2003) Attracting friends to feast on foes: engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr Opin Biotechnol 14:169–176. doi:10.1016/s0958-1669(03)00025-9
Degenhardt J, Hiltpold I, Kollner TG, Frey M, Gierl A, Gershenzon J, Hibbard BE, Ellersieck MR, Turlings TCJ (2009a) Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc Natl Acad Sci USA 106:13213–13218. doi:10.1073/pnas.0906365106
Degenhardt J, Koellner TG, Gershenzon J (2009b) Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 70:1621–1637. doi:10.1016/j.phytochem.2009.07.030
Delourme D, Fo Lacroute, Karst F (1994) Cloning of an Arabidopsis thaliana cDNA coding for farnesyl diphosphate synthase by functional complementation in yeast. Plant Mol Biol 26:1867–1873
Eisenreich W, Rohdich F, Bacher A (2001) Deoxyxylulose phosphate pathway to terpenoids. Trends Plant Sci 6:78–84
Fontana A, Reichelt M, Hempel S, Gershenzon J, Unsicker SB (2009) The effects of arbuscular mycorrhizal fungi on direct and indirect defense metabolites of Plantago lanceolata L. J Chem Ecol 35:833–843. doi:10.1007/s10886-009-9654-0
Fujisaki S, Hara H, Nishimura Y, Horiuchi K, Nishino T (1990) Cloning and nucleotide sequence of the ispA gene responsible for farnesyl diphosphate synthase activity in Escherichia coli1. J Biochem 108:995–1000
Gaffe J, Bru J-P, Causse M, Vidal A, Stamitti-Bert L, Carde J-P, Gallusci P (2000) LEFPS1, a tomato farnesyl pyrophosphate gene highly expressed during early fruit development. Plant Physiol 123:1351–1362
Gale MD, Devos KM (1998) Comparative genetics in the grasses. Proc Natl Acad Sci USA 95:1971–1974
Galichet A, Gruissem W (2003) Protein farnesylation in plants—conserved mechanisms but different targets. Curr Opin Plant Biol 6:530–535
Gaut BS (2001) Patterns of chromosomal duplication in maize and their implications for comparative maps of the grasses. Genome Res 11:55–66
Geisler K, Hughes RK, Sainsbury F, Lomonossoff GP, Rejzek M, Fairhurst S, Olsen C-E, Motawia MS, Melton RE, Hemmings AM, Bak S, Osbourn A (2013) Biochemical analysis of a multifunctional cytochrome P450 (CYP51) enzyme required for synthesis of antimicrobial triterpenes in plants. Proc Natl Acad Sci USA 110:E3360–E3367
Goritschnig S, Weihmann T, Zhang Y, Fobert P, McCourt P, Li X (2008) A novel role for protein farnesylation in plant innate immunity. Plant Physiol 148:348–357
Gupta P, Akhtar N, Tewari SK, Sangwan RS, Trivedi PK (2011) Differential expression of farnesyl diphosphate synthase gene from Withania somnifera in different chemotypes and in response to elicitors. Plant Growth Regul 65:93–100
Harborne JB (1991) Recent advances in the ecological chemistry of plant terpenoids. In: Harborne JB, Tomas-Barberan FA (eds) Ecological chemistry and biochemistry of plant terpenoids. Clarendon Press, Oxford, pp 399–426
Hassanali A, Herren H, Khan ZR, Pickett JA, Woodcock CM (2008) Integrated pest management: the push-pull approach for controlling insect pests and weeds of cereals, and its potential for other agricultural systems including animal husbandry. Phil Trans R Soc B Biol Sci 363:611–621
Helentjaris T, Weber D, Wright S (1988) Identification of the genomic locations of duplicate nucleotide sequences in maize by analysis of restriction fragment length polymorphisms. Genetics 118:353–363
Hemmerlin AA, Rivera SB, Erickson HK, Poulter CD (2003) Enzymes encoded by the farnesyl diphosphate synthase gene family in the big sagebrush Artemisia tridentata ssp. spiciformis. J Biol Chem 278:32132–32140
Hemmi H, Noike M, Nakayama T, Nishino T (2003) An alternative mechanism of product chain-length determination in type III geranylgeranyl diphosphate synthase. Eur J Biochem 270:2186–2194
Howe GA, Jander G (2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59:41–66
Huang M, Sanchez-Moreiras AM, Abel C, Sohrabi R, Lee S, Gershenzon J, Tholl D (2012) The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-ß-caryophyllene, is a defense against a bacterial pathogen. New Phytol 193:997–1008
Huffaker A, Kaplan F, Vaughan MM, Dafoe NJ, Ni X, Rocca JR, Alborn HT, Teal PE, Schmelz EA (2011) Novel acidic sesquiterpenoids constitute a dominant class of pathogen-induced phytoalexins in maize. Plant Physiol 156:2082–2097
Jiang F, Yang L, Cai X, Cyriac J, Shechter I, Wang Z (2001) Farnesyl diphosphate synthase is abundantly expressed and regulated by androgen in rat prostatic epithelial cells. J Steroid Biochem 78(2):123–130
Kim OT, Kim SH, Ohyama K, Muranaka T, Choi YE, Lee HY, Kim MY, Hwang B (2010) Upregulation of phytosterol and triterpene biosynthesis in Centella asiatica hairy roots overexpressed ginseng farnesyl diphosphate synthase. Plant Cell Rep 29:403–411
Köllner TG, Held M, Lenk C, Hiltpold I, Turlings TCJ, Gershenzon J, Degenhardt J (2008) A maize (E)-beta-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell 20:482–494. doi:10.1105/tpc.107.051672
Koyama T (1999) Molecular analysis of prenyl chain elongating enzymes. Biosci Biotechnol Biochem 63:1671–1676
Koyama T, Obata S, Osabe M, Takeshita A, Yokoyama K, Uchida M, Ogura T (1993) Thermostable farnesyl diphosphate synthase of Bacillus stearothermophilus: molecular cloning, sequence determination, overproduction, and purification. J Biochem 113:355–363
Lange BM, Ghassemian M (2003) Genome organization in Arabidopsis thaliana: a survey for genes involved in isoprenoid and chlorophyll metabolism. Plant Mol Biol 51:925–948
Li CP, Larkins BA (1996) Identification of a maize endosperm-specific cDNA encoding farnesyl pyrophosphate synthetase. Gene 171:193–196
Nagel R, Gershenzon J, Schmidt A (2012) Nonradioactive assay for detecting isoprenyl diphosphate synthase activity in crude plant extracts using liquid chromatography coupled with tandem mass spectrometry. Anal Biochem 422:33–38
Novelli G, D´Apice MR (2012) Protein farnesylation and disease. J Inherited Metab Dis 35:917–926
Odland W, Baumgarten A, Phillips R (2006) Ancestral rice blocks define multiple related regions in the maize genome. Crop Sci 46(Supplement_1):S-41–S-48
Ohnuma S-I, Nakazawa T, Hemmi H, Hallberg A-M, Koyama T, Ogura K, Nishino T (1996) Conversion from farnesyl diphosphate synthase to geranylgeranyl diphosphate synthase by random chemical mutagenesis. J Biol Chem 271:10087–10095
Okada K, Saito T, Nakagawa T, Kawamukai M, Kamiya Y (2000) Five geranylgeranyl diphosphate synthases expressed in different organs are localized into three subcellular compartments in Arabidopsis. Plant Physiol 122:1045–1056
Popjak G, Goodman DS, Cornforth J, Cornforth RH, Ryhage R (1961) Studies on the biosynthesis of cholesterol XV. Mechanism of squalene biosynthesis from farnesyl pyrophosphate and from mevalonate. J Biol Chem 236:1934–1947
Rasmann S, Köllner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TC (2005) Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434:732–737
Reed BC, Rilling HC (1975) Crystallization and partial characterization of prenyltransferase from avian liver. Biochemistry 14:50–54
Salse JRM, Piégu B, Cooke R, Delseny M (2004) New in silico insight into the synteny between rice (Oryza sativa L.) and maize (Zea mays L.) highlights reshuffling and identifies new duplications in the rice genome. Plant J 38:396–409
Sanmiya K, Iwasaki T, Matsuoka M, Miyao M, Yamamoto N (1997) Cloning of a cDNA that encodes farnesyl diphosphate synthase and the blue-light-induced expression of the corresponding gene in the leaves of rice plants. Biochim Biophys Acta 1350:240–246
Sanmiya K, Ueno O, Matsuoka M, Yamamoto N (1999) Localization of farnesyl diphosphate synthase in chloroplasts. Plant Cell Physiol 40:348–354
Schmidt A, Gershenzon J (2008) Cloning and characterization of two different types of geranyl diphosphate synthases from Norway spruce (Picea abies). Phytochemistry 69:49–57. doi:10.1016/j.phytochem.2007.06.022
Schnee C, Koellner TG, Held M, Turlings TCJ, Gershenzon J, Degenhardt J (2006) The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc Natl Acad Sci USA 103:1129–1134. doi:10.1073/pnas.0508027103
Schüler G, Görls H, Boland W (2001) 6-Substituted indanoyl isoleucine conjugates mimic the biological activity of coronatine. Eur J Org Chem 2001:1663–1668
Seidl-Adams I, Richter A, Boomer KB, Yoshinaga N, Degenhardt J, Tumlinson JH (2014) Emission of herbivore elicitor-induced sesquiterpenes is regulated by stomatal aperture in maize (Zea mays) seedlings. Plant, Cell Environ 38:23–34
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tan XP, Liang WQ, Liu CJ, Luo P, Heinstein P, Chen XY (2000) Expression pattern of (+)-delta-cadinene synthase genes and biosynthesis of sesquiterpene aldehydes in plants of Gossypium arboreum L. Planta 210:644–651
Tarshis L, Yan M, Poulter CD, Sacchettini JC (1994) Crystal structure of recombinant farnesyl diphosphate synthase at 2.6-Angstrom resolution. Biochemistry 33:10871–10877
Turlings TC, Tumlinson JH, Lewis W (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250(4985):1251–1253
Walter MH, Hans J, Strack D (2002) Two distantly related genes encoding 1-deoxy-d-xylulose 5-phosphate synthases: differential regulation in shoots and apocarotenoid-accumulating mycorrhizal roots. Plant J 31:243–254
Wei F, Coe E, Nelson W, Bharti AK, Engler F, Butler E, Kim H, Goicoechea JL, Chen M, Lee S (2007) Physical and genetic structure of the maize genome reflects its complex evolutionary history. PLoS Genet 3:e123
Wilkin DJ, Kutsunai S, Edwards P (1990) Isolation and sequence of the human farnesyl pyrophosphate synthetase cDNA. Coordinate regulation of the mRNAs for farnesyl pyrophosphate synthetase, 3-hydroxy-3-methylglutaryl coenzyme A reductase, and 3-hydroxy-3-methylglutaryl coenzyme A synthase by phorbol ester. J Biol Chem 265:4607–4614
Wilson WA, Harrington SE, Woodman WL, Lee M, Sorrells ME, McCouch SR (1999) Inferences on the genome structure of progenitor maize through comparative analysis of rice, maize and the domesticated panicoids. Genetics 153:453–473
Yalovsky S, Kulukian A, Rodríguez-Concepción M, Young CA, Gruissem W (2000) Functional requirement of plant farnesyltransferase during development in Arabidopsis. Plant Cell 12:1267–1278
Acknowledgments
The authors would like to thank Matthias Erb for D. virgifera-treated root material. We are indebted to Raimund Nagel and Michael Reichelt for help with LC-MS analysis. A. Richter and J. Degenhardt were supported by project B7 of the Collaborative Research Center 648 of the German Research Foundation (DFG). I. Seidl-Adams was supported by two travel Grants of the Collaborative Research Center 648 of the German Research Foundation (DFG).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Richter, A., Seidl-Adams, I., Köllner, T.G. et al. A small, differentially regulated family of farnesyl diphosphate synthases in maize (Zea mays) provides farnesyl diphosphate for the biosynthesis of herbivore-induced sesquiterpenes. Planta 241, 1351–1361 (2015). https://doi.org/10.1007/s00425-015-2254-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-015-2254-z