Abstract
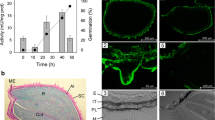
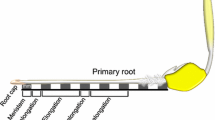
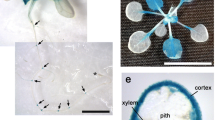
Mannans are hemicellulosic polysaccharides in the plant primary cell wall (CW). Mature seeds, specially their endosperm cells, have CWs rich in mannan-based polymers that confer a strong mechanical resistance for the radicle protrusion upon germination. The rupture of the seed coat and endosperm are two sequential events during the germination of Arabidopsis thaliana. Endo-β-mannanases (MAN; EC. 3.2.1.78) are hydrolytic enzymes that catalyze cleavage of β1 → 4 bonds in the mannan-polymer. In the genome of Arabidopsis, the endo-β-mannanase (MAN) family is represented by eight members. The expression of these eight MAN genes has been systematically explored in different organs of this plant and only four of them (AtMAN7, AtMAN6, AtMAN2 and AtMAN5) are expressed in the germinating seeds. Moreover, in situ hybridization analysis shows that their transcript accumulation is restricted to the micropylar endosperm and to the radicle and this expression disappears soon after radicle emergence. T-DNA insertion mutants in these genes (K.O. MAN7, K.O. MAN6, K.O. MAN5), except that corresponding to AtMAN2 (K.O. MAN2), germinate later than the wild type (Wt). K.O. MAN6 is the most affected in the germination time course with a t 50 almost double than that of the Wt. These data suggest that AtMAN7, AtMAN5 and specially AtMAN6 are important for the germination of A. thaliana seeds by facilitating the hydrolysis of the mannan-rich endosperm cell walls.







Similar content being viewed by others
Abbreviations
- CW:
-
Cell-wall
- ECWE:
-
Enhancing cell wall extensibility
- MAN:
-
Endo-β-mannanase
- ME:
-
Micropylar endosperm
References
Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:653–657
Arana MV, de Miguel LC, Sánchez RA (2006) A phytochrome dependent embryonic factor modulates gibberellin responses in the embryo and micropylar endosperm of Datura ferox seeds. Planta 223:847–857
Bailey TL, Williams N, Misleh C, Li WW (2006) MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acid Res 34:369–373
Bailly C (2004) Active oxygen species and antioxidants in seed biology. Seed Sci Res 14:93–107
Belotserkovsky H, Berger Y, Shahar R, Wolf S (2007) Specific role of LeMAN2 in the control of seed germination exposed by overexpression of the LeMAN3 gene in tomato plants. Planta 227:199–209
Bethke PC, Libourel IGL, Aoyama N, Chung YY, Still DW, Jones RL (2007) The Arabidopsis aleurone layer responds to nitric oxide, gibberellin and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol 143:1173–1188
Bewley JD (1997) Breaking down the walls: a role for endo-β-mannanase in release from seed dormancy? Trends Plant Sci 2:464–469
Bewley JD, Burton RA, Morohashi Y, Fincher GB (1997) Molecular cloning of a cDNA encoding a (1 → 4)-β-mannan endo-hydrolase from the seeds of germinated tomato (Lycopersicon esculentum). Planta 203:454–459
da Silva EAA, Toorop PE, van Aelst AC, Hilhorst HWM (2004) Abscisic acid controls embryo growth potential and endosperm cap weakening during coffee (Coffea arabica cv. Rubi) seed germination. Planta 220:251–261
Debeaujon I, Lepiniec L, Pourcel L, Routaboul J-M (2007) Seed development, dormancy and germination. In: Bradford K, Nonogaki H (eds) Seed coat development and dormancy. Blackwell, Oxford, pp 25–49
Dubreucq B, Berger N, Vincent E, Boisson M, Pelletier G, Caboche M, Lepiniec L (2003) The Arabidopsis AtEPR1 extensin-like gene is specifically expressed in endosperm during seed germination. Plant J 23:643–652
Emmanuelson O, Brunnak S, von Heijne G, Nielsen H (2007) Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc 2:953–971
Ferrandiz C, Liljegren S, Yanofsky M (2000) FRUITFULL negatively regulates the SHATTERPROOF genes during Arabidopsis fruit development. Science 289:436–438
Finch-Savage W, Leubner-Metzger G (2006) Seed dormancy and the control of germination. New Phytol 171:501–523
Finkelstein R, Reeves W, Ariizumi T, Steber C (2008) Molecular aspects of seed dormancy. Annu Rev Plant Biol 59:387–415
Gasteiger E, Gattiker A, Googland C, Ivanyi I, Appel RD, Bairoch A (2003) ExPASy: the proteomics server for in-depth protein knowledge and analysis. Nucleic Acid Res 31:3784–3788
Gong XM, Bewley JD (2007) Sorting out the LeMANs: endo-β-mannanase genes and their encoded proteins in tomato. Seed Sci Res 17:143–154
Gong XM, Bassel GW, Wang A, Greenwood JS, Bewley JD (2005) The emergence of embryos from hard seeds is related to the structure of the cell walls of the micropylar endosperm, and not to endo-β-mannanase activity. Ann Bot 96:1–9
Haughn G, Chaudhury A (2005) Genetic analysis of seed coat development in Arabidopsis. Trends Plant Sci 10:472–477
Holdsworth MJ, Soppe WJJ, Bentsink L (2008) Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol 179:33–54
Homrichhausen TM, Hewitt JR, Nonogaki H (2003) Endo-β-mannanase activity is associated with the completion of embryogenesis in imbibed carrot (Daucus carota L.) seeds. Seed Sci Res 13:219–227
Iglesias-Fernández R, Matilla AJ (2009) After-ripening alters the gene expression pattern of oxidases involved in the ethylene and gibberellins pathways during early imbibitions of Sisymbrium officinale L. seeds. J Exp Bot 6:1645–1661
Iglesias-Fernández R, Matilla AJ (2010) Genes involved in ethylene and gibberellins metabolism are required for endosperm-limited germination of Sisymbrium officinale L. seeds. Planta 231:653–664
Ikuma H, Thimann KV (1963) The role of the seed-coats in germination of photosensitive lettuce seeds. Plant Cell Physiol 4:169–185
Joshi CP, Zhou H, Huang XQ, Chiang VL (1997) Context sequences of translation initiation codon in plants. Plant Mol Biol 35:993–1001
Koornneef M, Bentsink L, Hilhorst H (2002) Seed dormancy and germination. Curr Opin Plant Biol 5:33–36
Kucera B, Cohn MA, Leubner-Metzger G (2005) Plant hormone interactions during seed dormancy release and germination. Seed Sci Res 15:281–307
Leubner-Metzger G, Meins F Jr (2000) Sense transformation reveals a novel role for class I β-1,3-glucanases in tobacco seed germination. Plant J 23:215–221
Li Y, Jones J, McQueen-Mason S (2003) Expansins and cell growth. Curr Opin Plant Biol 6:603–610
Linkies A, Müller K, Morris K, Turecková V, Wenk M, Cadman CSC, Corbineau F, Strnad M, Lynn JR, Finch-Savage WE, Leubner-Metzger G (2009) Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana. Plant Cell 21:3803–3822
Linkies A, Graeber K, Knight C, Leubner-Metzger G (2010) The evolution of seeds. New Phytol 186:817–831
Liu P-P, Koizuka N, Homrichhausen TM, Hewitt JR, Martin RC, Nonogaki H (2005) Large-scale screening of Arabidopsis enhancer-trap lines for seed germination-associated genes. Plant J 41:936–944
Matilla AJ, Matilla-Vázquez MA (2008) Involvement of ethylene in seed physiology. Plant Sci 175:87–97
Moreira LRS, Filho EXF (2008) An overview of mannan structure and mannan-degrading enzyme systems. Appl Microbiol Biotechnol 79:165–178
Müller K, Tintelnot S, Leubner-Metzger G (2006) Endosperm-limited Brassicaceae seed germination: abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana. Plant Cell Physiol 47:864–977
Müller K, Linkies A, Vreeburg RAM, Fry SC, Krieger-Liszkay A, Leubner-Metzger G (2009) In vivo cell wall loosening by hydroxyl radicals during cress (Lepidium sativum L.) seed germination and elongation growth. Plant Physiol 150:1855–1865
Müller K, Job C, Belghazi M, Job D, Leubner-Metzger G (2010) Proteomics reveal tissue-specific features of the cress (Lepidium sativum L.) endosperm cap proteome and its hormone-induced changes during seed germination. Proteomics 10:406–416
Nonogaki H, Morohashi Y (1999) Temporal and spatial pattern of the development of endo-β-mannanase activity in germinating and germinated seeds. J Exp Bot 50:1307–1313
Nonogaki H, Gee OH, Bradford KJ (2000) A germination-specific endo-beta-mannanase gene is expressed in the micropylar endosperm cap of tomato seeds. Plant Physiol 123:1235–1245
Nonogaki H, Chen F, Bradford KJ (2007) Mechanisms and genes involved in germination “senso stricto”. In: Bradford K, Nonogaki H (eds) Seed development, dormancy and germination. Blackwell, Oxford, pp 264–304
Nonogaki H, Bassel GW, Bewley JD (2010) Germination—still a mystery. Plant Sci. doi:10.1016/j.plantsci.2010.02.010
Penfield S, Li Y, Gilday A, Graham S, Graham I (2006) Arabidopsis ABA INSENSITIVE4 regulates lipid mobilization in the embryo and reveals repression of seed germination in the endosperm. Plant Cell 18:1887–1899
Pfaffl MW (2001) A new mathematical model for relative quantification in real time RT-PCR. Nucleic Acid Res 29:2002–2007
Ren Y, Bewley JD, Wang X (2008) Protein and gene expression patterns of endo-β-mannanase following germination of rice. Seed Sci Res 18:139–149
Rodríguez-Gacio MC, Matilla-Vázquez MA, Matilla AJ (2009) Seed dormancy and ABA signaling: the breakthrough goes on. Plant Signal Behav 4:1035–1048
Rosso MG, Li Y, Strizhov N, Reiss B, Dekker K, Weisshaar B (2003) An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Mol Biol 53:247–259
Sánchez RA, de Miguel L (1992) Phytochrome promotion of mannan-degrading enzyme activities in the micropylar endosperm of Datura ferox seeds requires the presence of embryo and gibberellin synthesis. Seed Sci Res 7:27–33
Schröder R, Wegrzyn TF, Sharma NN, Atkinson RG (2006) LeMAN4 endo-β-mannanase from ripe tomato fruit has dual enzyme activity and can act as a mannan transglycosylase and hydrolase. Planta 224:1091–1102
Schröder R, Atkinson RG, Redgwell RJ (2009) Re-interpreting the role of endo-β-mannanases as mannan endotransglycosylase/hydrolases in the plant cell wall. Ann Bot 104:197–204
Sliwinska E, Bassel GW, Bewley JD (2009) Germination of Arabidopsis thaliana seeds is not completed as a result of elongation of the radicle but of the adjacent transition zone and lower hypocoty. J Exp Bot 60:3587–3594
Tamura K, Dudley J, Nei M, Kumar C (2007) MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thompson J, Higgins D, Gibson T (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Toorop PE, Bewley JD, Hilhorst HWM (1996) Endo-β-mannanase isoforms are present in the endosperm and embryo of tomato seeds, but are not essentially linked to the completion of germination. Planta 200:153–158
Toorop PE, van Aelst AC, Hilhorst HWM (2000) The second step of the biphasic endosperm cap weakening that mediates tomato (Lycopersicon esculentum) seed germination is under control of ABA. J Exp Bot 51:1371–1379
Vicente-Carbajosa J, Carbonero P (2005) Seed maturation: developing and intrusive phase to accomplish a quiescent state. Int J Dev Biol 49:645–651
Wang AX, Li JR, Bewley JD (2004) Molecular cloning and characterization of an endo-β-mannanase gene expressed in the lettuce endosperm following radicle emergence. Seed Sci Res 14:267–276
Wu CT, Leubner-Metzger G, Meins F Jr, Bradford KJ (2001) Class I β-1,3-glucanases and chitinases are expressed in the micropylar endosperm of tomato seeds prior to radicle emergence. Plant Physiol 126:1299–1313
Yuan JS, Yang X, Lai J, Lin H, Cheng Z-M, Nonogaki H, Chen F (2007) The endo-β mannanase gene families in Arabidopsis, rice, and poplar. Funct Integr Genom 7:1–16
Acknowledgments
This work was financially supported by grants from Ministerio de Ciencia e Innovación (MICINN, Spain) (CGL2004-01996; CGL2009-11425 and Consolider CSD2007-00057). R I–F and C B–S are supported by post-doctoral contracts from CSD2007-00057 and JdC-UPM (BFU2006-07258), respectively. We thank B. Lueiro for expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Iglesias-Fernández, R., Rodríguez-Gacio, M.C., Barrero-Sicilia, C. et al. Three endo-β-mannanase genes expressed in the micropylar endosperm and in the radicle influence germination of Arabidopsis thaliana seeds. Planta 233, 25–36 (2011). https://doi.org/10.1007/s00425-010-1257-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-010-1257-z