Abstract
The protease renin, the key enzyme of the renin–angiotensin–aldosterone system, is mainly produced and secreted by juxtaglomerular cells in the kidney, which are located in the walls of the afferent arterioles at their entrance into the glomeruli. When the body’s demand for renin rises, the renin production capacity of the kidneys commonly increases by induction of renin expression in vascular smooth muscle cells and in extraglomerular mesangial cells. These cells undergo a reversible metaplastic cellular transformation in order to produce renin. Juxtaglomerular cells of the renin lineage have also been described to migrate into the glomerulus and differentiate into podocytes, epithelial cells or mesangial cells to restore damaged cells in states of glomerular disease. More recently, it could be shown that renin cells can also undergo an endocrine and metaplastic switch to erythropoietin-producing cells. This review aims to describe the high degree of plasticity of renin-producing cells of the kidneys and to analyze the underlying mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The kidneys are the main production site for renin, the key enzyme of the systemic renin–angiotensin–aldosterone system (RAAS). The systemic RAAS plays an essential role in regulating blood pressure and maintaining electrolyte and extracellular volume homeostasis. The protease renin is the limiting determinant of the RAAS by cleaving the decapeptide angiotensin (Ang) I from angiotensinogen which is mainly produced in the liver. Endothelial cell-derived angiotensin-converting enzyme (ACE) shortens Ang I to Ang II that exerts its effects mainly via Ang II type I (AT1) receptors. Ang II has a strong vasoconstrictor effect and thus directly influences blood pressure. Additionally, it regulates the production of aldosterone in the adrenal cortex and the release of antidiuretic hormone from the pituitary gland, thereby ensuring salt and water homeostasis [104, 106].
Renin production and secretion are mainly controlled by three different mechanisms. These are the renal perfusion pressure, the tubular sodium chloride concentration sensed by macula densa cells, and the activity of β-adrenergic receptors on renin-producing cells. More generally renin production and secretion are controlled in the sense of negative feedback loops involving blood pressure, sodium balance, and angiotensin II [9, 96].
Most of the circulating renin is produced by specialized myoendocrine cells located in the walls of afferent arterioles directly at the entrance into the glomerulus. Due to this characteristic position in the adult, renal renin-producing cells are called juxtaglomerular cells. When the demand for renin increases due to threats to sodium or blood pressure homeostasis, additional cells in the afferent vessel wall and in the juxtaglomerular area are recruited to adjust the production of renin accordingly. Once homeostasis is restored, renin production ceases in recruited cells which retransform into their former phenotype [27, 100, 106]. This regulatory mechanism hints at a high plasticity of renal renin-producing cells.
Plasticity is not only relevant for the physiological regulation of renin production. Renin-lineage cells can also fulfill a kind of stem cell function as progenitor cells for the regeneration of mesangial cells and podocytes after kidney injury [43, 86, 105]. Moreover, it was found that juxtaglomerular renin cells can transform into erythropoietin (EPO)-producing cells due to genetic activation of the hypoxia signaling pathway [6, 48].
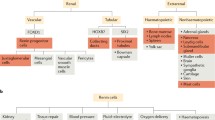
This review will specifically focus on the aspect of renin-cell plasticity (Fig. 1). The identity of classic juxtaglomerular renin+ cells and the newly described interstitial renin-expressing cells will be outlined in more detail. Particular attention will be paid to processes involved in the reversible metaplastic transformation of cells into renin-producing cells. Another focus will be the involvement of renin-lineage cells in regeneration and the endocrine transformation of renin+ cells into EPO-producing cells.
Schematic overview of different aspects of renin-lineage cell plasticity. Renin-lineage cells show a high plasticity and can fulfill different functions in order to maintain renal function, blood pressure, and water and salt balance in the body. Topics highlighted in this review are indicated in bold font
Identity of renin-expressing cells
“Classic” renin cells at juxtaglomerular position
The classic renin-producing cells are located directly at the entrance of the afferent arteriole into the glomerulus, hence their labeling as juxtaglomerular cells. Together with the adjacent parts of the afferent and efferent arteriole, the extraglomerular mesangium (EGM) and the specialized macula densa cells of the distal tubule, they form the juxtaglomerular apparatus (JGA)—a functional unit, which coordinates the secretion rate of renin, the glomerular filtration rate and the tubuloglomerular feedback. Thus, it ultimately regulates systemic blood pressure as well as salt and extracellular volume balance, by integrating various signals.
In the adult mammal, only about 4–8 renin-producing cells per glomerulus can be detected under basal physiologic conditions [106]. The juxtaglomerular renin-producing cells originate in the FoxD1+ stromal progenitor cell population which also gives rise to interstitial fibroblasts and pericytes, mesangial cells, and vascular smooth muscle cells (VSMCs) [21, 37, 101]. Renin-producing cells can first be detected sporadically in the undifferentiated mesenchyme. With the onset of kidney vascularization, renin-producing cells are involved in the development of the renal vascular tree. Before a new arterial branch grows out, renin cells accumulate at the branching point. During outgrowth and elongation of the vessel, the renin cells constitute the mural cells of the newly developing vascular segments. With ongoing vessel maturation, the renin-producing cells differentiate into VSMCs. Thus, the renin+ cells are always associated with the youngest vessel segments. With the completion of the vascular tree, they are restricted to the juxtaglomerular position. Genetic defects in the RAAS cascade or deletion of the renin gene during nephrogenesis lead to severe vascular malformations [10, 24, 44, 92, 102]. In adults, the renin-lineage cells still retain their developmental plasticity and retransform into the renin cell phenotype when homeostasis is threatened [27, 100].
Renin-producing cells are located in the media layer of the afferent arterioles and have a cuboidal shape. The cells are packed with storage vesicles both small individual vesicles and also voluminous interconnected caverns [107]. Renin is synthesized as the preproprotein prorenin, which is glycosylated in the endoplasmic reticulum and then proteolytically processed to active renin in the storage vesicles [71], from which it is released by compound exocytosis on demand [107]. In addition, prorenin is constitutively secreted. Upon binding to the (pro)renin receptor, prorenin can mediate either RAS-dependent or RAS-independent effects [77]. The cells forming the juxtaglomerular apparatus are interconnected by numerous gap junctions. Within the JGA, gap junctions are formed by four different connexin (Cx) isoforms, Cx37, Cx40, Cx43, and Cx45, with renin-producing cells expressing only Cx37 and Cx40 [49, 50, 118]. Expression of Cx40 seems to be crucial for determining the juxtaglomerular position of renin-producing cells, as well as for the blood pressure-dependent regulation of renin synthesis and secretion [47, 51, 61, 112, 113]. Inducible deletion of Cx40 in adult mice results in misplaced renin cells that are no longer located in the media layer of afferent arterioles but in the extraglomerular mesangium [22]. In addition, the inhibitory effect of elevated blood pressure on renin synthesis and secretion is absent, resulting in increased plasma renin concentrations despite hypertension [22, 113]. Thus, Cx40 apparently mediates the pressure control of renin secretion [61]. A study with mice harboring a missense mutation in the Cx40 gene, first observed in a human patient displaying hypertension, suggests that correct Cx40 gap junction coupling of renin-producing cells is also essential for renin secretion in the human kidney [58]. A study, in which Cx40 was replaced by Cx45, however shows that not the expression of Cx40 itself is crucial for the regulation of renin release from juxtaglomerular cells but the gap junctional coupling with neighboring cells [98]. Moreover, not only cell–cell communication via gap junctions seems to be important for the regulation of renin secretion but also signaling via connexin hemichannels [36]. A recent study suggests that pannexin 1 could also be involved in the regulation of basal renin secretion through mediating intercellular communication [14]. Another cell adhesion molecule essential for mediating cell–cell or cell–matrix interactions is β1-integrin, which is abundantly expressed in the kidneys including renin-lineage cells. Deletion of β1-integrin in the renin-lineage results in apoptosis and therefore leads to a decrease in circulating renin, hypotension, and vascular alterations in the kidney [67].
To this date, a number of molecular markers have been determined that are characteristically expressed in juxtaglomerular renin-producing cells. They strongly express the AT1 receptor that mediates the effects of Ang II [95]. Moreover, they express aldo–keto reductase 1b7 (Akr1b7), an enzyme that reduces carbonyl groups to their respective alcohols [54, 57]. Prolyl-4-hydroxylase (PHD) 3, an enzyme involved in the hypoxia signaling pathway, is also expressed in renin-producing cells [6]. Cell type-specific deletion of the respective marker, however, has no direct effect on renin secretion [6, 60, 76].
Through numerous studies in mice two major signaling pathways have emerged that play a key role in determining and maintaining the renin cell phenotype. One is the Notch signaling pathway and the other is the cAMP signaling pathway. Notch signaling is a cell–cell communication pathway involved in regulating cell fate and thereby enabling cell plasticity. Ligand binding to the transmembrane Notch receptor leads to cleavage of the extracellular receptor domain followed by a second cleavage step that releases the intracellular domain of Notch (NICD). NICD translocates into the nucleus and binds to the recombination signal binding protein for Ig-kJ region (RBP-J), an important downstream effector of Notch signaling. The DNA-binding protein RBP-J recruits a coactivator complex and thereby activates the transcription of Notch target genes [4, 23]. In juxtaglomerular cells, RBP-J activates genes that are characteristic for their myo-endocrine phenotype and simultaneously inhibits the expression of genes typical for other cell lineages like hematopoietic markers. After deletion of RBP-J, renin-lineage cells stop producing renin, lose the typical renin vesicles, and no longer express Akr1b7 or contractile proteins [7, 8]. Another factor critically involved in Notch signaling and renin cell identity is the enzyme Dicer which is responsible for microRNA-processing [18, 39, 103]. miRNAs regulate many cellular processes by targeting specific mRNAs to either suppress or activate their expression [17, 81]. For the identity of renin cells, two distinct miRNAs, miR-330 and miR-125b-5p, seem to be important [68].
The second major pathway which is not only crucial for the synthesis and secretion of renin but also for the differentiation and maintenance of renin-producing cells is cAMP signaling. cAMP activates protein kinase A, which subsequently leads to the phosphorylation of cAMP-responsive element binding protein (CREB) in the nucleus. Phosphorylated CREB as a complex with the co-factors CBP (CREB-binding protein) and p300 then binds to the cAMP-responsive element in the promoter region of target genes like renin and Akr1b7 [54, 99]. A rise in intracellular cAMP to stimulate renin expression can be mediated either by the Gs/AC-coupled prostaglandin E2 receptors EP2 and EP4 or Gs/AC-coupled β-adrenergic receptors (Gs/AC, G protein stimulating cAMP signaling by adenylyl cyclase (AC) activation) [9, 97]. Deletion of either β-adrenergic receptors, both EP2 and EP4 receptors or the intracellular Gsα (G protein subunit α) results in significantly decreased renin expression and secretion in adult mice [11, 15, 16, 45]. Also, combined deletion of the two histone acetyltransferases CBP and p300 in renin-lineage cells results in a loss of renin-expressing cells [26].
These findings are further supported by more recent studies revealing the molecular fingerprint of renin-producing cells through analysis of the chromatin structure and epigenetic status of juxtaglomerular cells, recruited renin cells, and the renin cell line As4.1. Renin-lineage cells possess a unique super-enhancer at the renin gene locus which directly influences renin expression. In addition, they share 91 super-enhancers that determine the renin cell phenotype. More in-depth data analyses further confirmed the importance of Notch and cAMP signaling for determining renin cell identity [64]. In this context, it was shown that the 3D structure of chromatin and, in particular, chromatin structural protein Ctcf (CCCTC-binding factor) are also crucial for the expression of renin and the number of renin-producing cells [63].
Interstitial renin-expressing cells
Recently, two groups independently reported active renin gene transcription in tubulointerstitial cells of the adult mouse kidney [6, 70]. Renal interstitial fibroblast-like cells are involved in maintaining tissue architecture and integrity by producing extracellular matrix and mediating the cross-talk between different renal cell types. In many kidney diseases, interstitial fibroblasts and pericytes play a crucial role in the development of interstitial fibrosis as matrix-producing myofibroblasts [37]. Moreover, in healthy kidneys, a subset of interstitial fibroblasts produce the hormone erythropoietin (EPO), which regulates erythropoiesis [1, 67]. Like juxtaglomerular renin-producing cells, renal interstitial fibroblasts/pericytes originate from the FoxD1+ stromal progenitor cell population [101].
Under basal conditions, about 250 interstitial cells expressing renin mRNA per transverse kidney section of wild-type mice (C57/Bl6J) can be detected using the high-resolution RNAscope in situ hybridization technique. Most of these interstitial renin-expressing cells are found throughout the outer medulla and some are dispersed throughout the tubulointerstitium of the cortex. These interstitial renin-expressing cells coexpress the fibroblast marker platelet-derived growth factor receptor β (PDGFR-β) (Fig. 2) [6, 70] which is also expressed by EPO-producing fibroblasts [20]. Within the pool of PDGFR-β+ interstitial cells, a number of different subpopulations exists that are characterized by the expression of a distinct additional marker like CD73, Gli1, smooth-muscle myosin heavy chain (SM-MHC), or tenascin-C [5]. Whether interstitial renin expression is restricted to one of these subpopulations has not yet been determined. Like typical renal fibroblasts, interstitial renin+ cells possess a flat, elongated cell body with processes which often wrap around tubules or capillaries [52]. In contrast to juxtaglomerular cells, interstitial renin-expressing cells are negative for renin immunohistochemistry in the kidneys of wild-type mice under basal conditions. However, lineage tracing using a GFP reporter mouse confirmed the activity of the renin gene promoter [6]. Therefore, it could be speculated that interstitial renin-expressing cells do not store renin in granules but instead constitutively release prorenin [71]. Whether the interstitial renin+ cells also possess the typical markers of juxtaglomerular renin-producing cells, like Akr1b7, AT1A, Cx40 or distinct miRNAs requires further investigation.
Interstitial renin-expressing cells on kidney sections of wild-type mice. A Spatial distribution pattern of interstitial renin-expressing cells was visualized with RNAscope, a high resolution in situ hybridization technology. Renin mRNA-expressing cells were highlighted with yellow dots on a kidney section of a wild-type mouse. Interstitial renin mRNA-expressing cells are mainly distributed in the outer medulla and to a lesser extent in the renal cortex. Nuclei were counterstained with DAPI (gray). Scale bar 500 μm. B Medullary detail of a co-RNAscope for renin (green) and PDGFR-β (red) mRNA on a wild-type kidney section. Colocalization of both mRNAs identifies interstitial renin+ cells as fibroblast-like cells. Nuclei were counterstained with DAPI (gray). Scale bar 20 μm
So far, the functions of renin expression in interstitial cells under physiological or pathophysiological conditions are not understood. An initial study suggests that hypotension caused by anemia or treatment with an angiotensin II receptor blocker increases the number of interstitial renin-expressing cells, whereas hypertension decreases their number compared to control animals. Additional interstitial renin+ cells are recruited throughout the cortex and outer medulla. In this context, it was speculated that interstitial renin expression may contribute to systemic renin production to maintain blood pressure homeostasis. Notably, some interstitial renin+ cells coexpress EPO in the anemic hypotension mouse model (please see part IV for detailed discussion). Furthermore, an increased number of interstitial renin-expressing myofibroblasts is observed in an experimental fibrosis model [70]. However, in a mouse model of aldosterone synthase deficiency leading to hypotension and significantly increased plasma renin concentrations, the number of interstitial renin+ cells is unchanged compared to wild-type animals [6, 62]. The increased levels of circulating renin in these mice are produced mainly by recruited cells along the afferent arterioles, in the extraglomerular mesangium, and in perivascular cells [62]. Therefore, it is conceivable that juxtaglomerular renin production and interstitial renin expression are two separate systems that are regulated independently. While juxtaglomerular renin regulates the systemic RAAS, interstitial renin expression may belong to a local RAS, which apart from the kidney has also been described in the heart, brain, or the adrenal glands [82, 117]. The intrarenal RAS is a local paracrine system, which contains all elements required for Ang II production and is involved in the pathogenesis of hypertension and renal disease. In diabetic nephropathy or Ang II-induced hypertension, for example, components of the RAS like angiotensinogen, ACE, (pro)renin, and AT1 receptors are upregulated along the nephron and in the medullary region leading to high levels of intratubular Ang II [28, 73, 117]. However, the detailed functions of the intrarenal RAS and its regulation are not yet fully understood.
Cells reversibly recruited for renin production
Vascular smooth muscle cells in afferent arterioles
If a homeostatic threat to blood pressure or sodium balance develops that cannot be compensated by increasing the secretion rate of the existing juxtaglomerular renin-producing cells, additional cells are recruited for renin production rather than renin synthesis per cell is upregulated. This is the case, for example in states of hypotension, salt depletion, or restrictions of extracellular volume which ultimately lead to a reduction of renal blood flow [3, 25, 29]. The recruitment of additional renin-producing cells does not occur by proliferation of the existing juxtaglomerular renin cells, but largely by retransformation of cells that had already expressed renin during renal development and then differentiated into other cell types after completion of kidney development [31]. Depending on the strength of the stimulus, initially vascular smooth muscle cells along the afferent arterioles are recruited for renin synthesis. If the stimulus persists for a longer period of time, subsequently more and more cells are recruited also along larger vessels and in the intra- and extraglomerular mesangium [100]. Recently, evidence was provided suggesting that a small proportion (≤ 10%) of renin-producing cells in the adult mouse also arise by neogenesis, that is, de novo differentiation from other cells [35]. Once homeostasis is restored, the recruited cells retransform back to their previous phenotype [27].
In general, VSMCs are crucial for the control of blood pressure and blood distribution as well as for maintaining the blood vessel integrity. Due to their contractile function, they express a distinct set of contractile proteins including α-SMA (smooth muscle α-actin), SM22α, SM-MHC (smooth-muscle myosin heavy chain), smoothelin, and calponin [56]. Contractile VSMCs show an elongated spindle-like shape and possess a large number of contractile filaments [89]. Once VSMCs are recruited for renin synthesis, they become more epitheloid. They acquire renin storage granules and the endoplasmic reticulum as well as the Golgi apparatus are more pronounced, whereas the number of myofibrils decreases [27]. Along with renin, the recruited VSMCs begin to express typical renin cell markers, such as Akr1b7 and Cx40. In contrast, VSMC marker expression is downregulated. Translation of typical proteins such as Cx45, α-SMA, SM-MHC, and smoothelin can no longer be observed in these cells (Fig. 3) [42, 47, 84].
Prerequisite for the transformation of a cell into a renin-producing cell seems to be the ingrained epigenetic memory of the renin cell phenotype. This molecular memory resides in a set of 91 super-enhancers common to renin-lineage cells and an additional super-enhancer located at the renin gene locus. This super-enhancer seems to directly control renin gene expression and to integrate various signals regarding blood pressure and salt and volume homeostasis [64]. Sox6 is a transcription factor that appears to be important in this context. Sox6 binds to the renin promoter within the super-enhancer region. After deletion of Sox6 in renin-lineage cells, recruitment of VSMCs is impaired under salt restriction and dehydration or in renal arterial stenosis [90, 91]. Also, posttranscriptional modifications play an important role in retransformation. Deletion of the RNase III endonuclease Dicer, an enzyme necessary for the maturation of miRNAs, leads to a decreased number of renin-producing cells [103]. Two distinct miRNAs are involved in balancing the myoendocrine phenotype of renin-producing cells. Usually, all renin-lineage cells express miR-125-5p which maintains their contractile functions. When homeostasis is threatened, the expression of miR-125-5p decreases in VSMCs in favor of the endocrine phenotype. In juxtaglomerular renin cells, however, miR-125-5p expression is maintained. In addition, they start to express miR-330 which enhances their endocrine features [68].
Moreover, Notch/RBP-J signaling and the cAMP/CBP/p300 pathway that are involved in determining the renin cell phenotype are also important for the retransformation of VSMCs into renin-producing cells. RBP-J acts as a transcription factor thereby regulating not only genes that confer the endocrine phenotype (renin and Akr1b7), but also the contractile phenotype (α-SMA, SM-MHC, and smoothelin). Thus, RBP-J activity is essential for the recruitment of VSMCs during homeostatic challenges. Mice with deletion of RBP-J in renin-lineage cells were unable to recruit VSMCs when treated with the ACE inhibitor captopril in combination with a low sodium diet [8].
Activation of the cAMP/CBP/p300 pathway contributes to the transformation of VSMCs into renin-producing cells [83]. In contrast to juxtaglomerular renin cells, cAMP in VSMCs does not seem to be generated by activation of EP2/EP4 receptors or β-adrenergic receptors [42]. Deletion of β-adrenergic receptors in renin-lineage cells does not impair the recruitment of VSMCs in mice treated with a low sodium diet and an ACE inhibitor [75]. Intracellular cAMP can also be increased by inhibiting phosphodiesterase 3. This inhibition can be mediated by increased cGMP levels, generated through the activation of the NO-sensitive soluble guanylate cyclase (sGC) [65]. This NO-sGC-mediated pathway seems to be of relevance for the transformation of VSMCs. Deletion of either endothelial NO-synthase (eNOS) or sGC leads to an impaired recruitment of VSMCs in mice on a low sodium diet in combination with an ACE inhibitor. But, the recruitment of EGM cells is unaffected in these mouse models (Fig. 3) [74].
Homeostatic threats lead to recruitment of VSMCs and EGM cells for additional renin production. The transformation of EGM cells depends on the activation of prostaglandin receptors EP2 and EP4 and subsequently cAMP/CBP/p300 signaling as well as Notch signaling. Transformed EGM cells lose PDGFR-β and instead express Akr1b7. VSMCs are recruited by the activation of cAMP/CBP/p300 signaling through NO-sGC and Notch signaling. The recruited VSMCs lose their contractile features and become more cuboid in their appearance. Moreover, they start to express Cx40 instead of Cx45.
Extraglomerular mesangial cells
Extraglomerular mesangial cells are a part of the juxtaglomerular apparatus. Like renin-producing cells, they express Cx40, AT1, and the prostaglandin E2 (PGE2) receptors EP2 and EP4 [16, 95]. Moreover, they are positive for PDGFR-β, the common marker of renal interstitial cells and pericytes. Transformation of extraglomerular mesangial cells to renin-producing cells is often observed in situations of chronic salt wasting, for example, due to genetic defects in the aldosterone synthase or in chloride channels, as in Bartter syndrome [2, 30, 62, 108]. After transformation into renin-producers, EGM cells start to express Akr1b7 [54]. PDGFR-β expression however is downregulated (Fig. 3) [41].
Renin production by EGM cells seems to be preferentially regulated through the activation of EP2 and EP4 receptors. These Gs-coupled receptors are activated through binding of PGE2 which is produced by cyclooxygenase 2 (Cox-2) in the macula densa cells. PGE2 production is upregulated by salt deficiency. Activation of EP2 and EP4 receptors leads to an increase in the intracellular cAMP levels, thereby elevating renin expression [9]. Increased plasma renin levels in humans on a low salt diet could be suppressed by the administration of selective Cox-2 inhibitors [40]. The importance of the Cox-2/PGE2/EP axis for renin expression is further supported by the successful treatment of hyperreninemia with Cox-2 inhibitors in patients with Bartter syndrome [80, 88]. Deletion of both EP2 and EP4 receptors or treatment with a Cox-2 inhibitor in mice lacking aldosterone synthase significantly downregulates extraglomerular renin expression but renin production by VSMCs is not affected [42]. The persistent renin expression by VSMCs in these mice is supported by a recent study reporting a lack of EP2 and EP4 receptors in these cells [16]. In contrast, signaling via NO-sGC, which is important for VSMC recruitment, has no effect on EGM recruitment (Fig. 3) [74].
In unstressed wild-type mice, the expression of Cx40 seems to suppress the expression of renin in EGM cells. Deletion of Cx40 leads to renin expression in extraglomerular mesangial cells, while the typical juxtaglomerular renin expression is absent [22]. However, recruitment of VSMCs for renin production seems unaffected in Cx40-deficient mice [61].
Renin cell plasticity in pathophysiology
Besides the physiological recruitment, renin-lineage cells have been suggested to play a role in the regeneration of different glomerular cell types after injury. In a model of focal segmental glomerular sclerosis, tracking of renin-lineage cells with reporter mice demonstrated that inhibition of the RAS by administration of the ACE inhibitor enalapril or the AT1 receptor inhibitor losartan stimulated the proliferation of renin-lineage cells in the juxtaglomerular region. These cells then migrated into the glomerulus and differentiated into intraglomerular mesangial cells, podocytes, or epithelial cells of Bowman’s capsule. After transdifferentiation, the cells no longer express renin, but instead express typical markers of the respective cell types. The mesangial cells differentiated from the renin-lineage cells are positive for integrin α8, the podocytes express synaptopodin, nephrin, podocin, and Wilms tumor protein, and the parietal epithelial cells are positive for PAX8 and claudin [43, 53, 86]. These findings are supported by another study, where after induction of renal damage and a loss of podocytes due to 5/6 nephrectomy, renin-lineage cells also migrated into the glomeruli and transformed into podocytes [85]. These findings may explain why therapies with RAS inhibitors (RASi) have been shown to be beneficial in patients with glomerular disease [32, 59, 115]. The signaling pathways that induce the migration and the phenotypic shift of renin-lineage cells into podocytes and parietal epithelial cells are not yet known [53].
In another model of mesangial cell injury, extraglomerular renin-lineage cells could also compensate for the loss of intraglomerular mesangial cells through migration and retransformation. It has been speculated that pathways which mediate the differentiation of renin-lineage cells during nephrogenesis could be involved in this repair process [105].
A quite recent study showed that prolonged treatment with RASi activates renin-producing cells and leads to arterial hypertrophy, a concentric thickening of the intrarenal arteries and arterioles in mice and humans. The renin+ cells adapt a more VSMC-like phenotype with upregulated expression of α-SMA, SM-MHC, and calponin-1, while maintaining renin expression. Based on these findings, further studies are needed to determine more precisely the morphologic and functional consequences of RASi treatment to be able to balance its positive and negative effects in the future [72, 114].
In rare cases, transformation of juxtaglomerular renin cells into tumor cells occurs. Juxtaglomerular cell tumors are benign and are also referred to as reninomas. They secrete renin autonomously and persistently, leading to increased plasma renin activities and elevated serum aldosterone levels. Affected patients therefore suffer from hypertension, hyperreninemia, and secondary aldosteronism [55, 109]. Findings suggest that impaired expression of RBP-J could lead to overexpression of renin in juxtaglomerular cell tumors [38]. After transformation reninoma cells newly express CD34, a marker for hematopoietic stem cells and collagen type VI [55, 66]. To this date, the signals and regulatory mechanisms involved in the regenerative or aberrant transformation await elucidation.
Endocrine plasticity: transformation into erythropoietin producers
Recently, it has been shown that a subset of interstitial fibroblast-like cells expresses renin in mouse kidneys. In situations of stimulated renal erythropoietin (EPO) production, a partial coexpression of renin and EPO in interstitial cells has been observed [6, 70].
Apart from renin, the kidneys are also the main expression site for the hormone EPO which triggers erythropoiesis. EPO is produced by tubulointerstitial fibroblasts located in the deep cortex along the cortico-medullary border. These cells are characterized by the expression of PDGFR-β and CD73. EPO expression is stimulated by a fall of the local oxygen tension as a consequence of arterial hypoxia or anemia. EPO production is transcriptionally controlled by the dimeric transcription factor HIF-2. Under normoxic conditions, the HIF-2α subunit is continuously hydroxylated by prolyl-4-hydroxylase 2 (PHD-2). Hydroxylated HIF-2α gets ubiquitinylated by the ubiquitin E3 ligase von Hippel-Lindau (Vhl) protein and eliminated by proteasomal degradation. If tissue oxygenation declines, HIF-2α is no longer hydroxylated and degraded but instead translocates into the nucleus. There, HIF-2α dimerizes with the HIF-1β subunit and together with other co-factors, and activates the transcription of its target genes, including EPO [33, 79].
It has been observed in mice that during anemia or after pharmacological inhibition of PHDs a subpopulation of interstitial cells coexpresses EPO and renin suggesting an endocrine plasticity of these cells [70]. In addition, renin cell-specific deletion of PHD2 also induces EPO expression in interstitial cells [79], indicating that interstitial renin+ cells are able to express EPO. Altogether, these findings suggest that at least a subpopulation of interstitial renin+ cells rapidly turns on EPO expression in response to an acute stimulus that leads to HIF-2α stabilization [6]. In contrast to the transformation of VSMCs for renin production, no phenotypic shift can be observed after the induction of EPO expression in interstitial renin+ cells. The renin and EPO coexpressing interstitial cells still express the typical fibroblast marker PDGFR-β (Fig. 4) [6].
In contrast to interstitial renin+ cells, EPO expression is not induced in juxtaglomerular renin-producing cells after treatment with a single-dose of a PHD inhibitor or after renin cell-specific deletion of PHD2 [6]. At first glance, these findings appear contradictory to the original observation that renin cell-specific deletion of Vhl induces EPO expression in juxtaglomerular cells [19, 48]. It turned out, that it requires a codeletion of PHD2 with PHD3 to induce EPO expression in juxtaglomerular renin cells [6]. PHD3 is a PHD isoform known to cooperate with PHD2 in some cell types to modulate the HIF response [69, 111]. Clear HIF-2α stabilization is observed in juxtaglomerular renin cells with Vhl or with PHD2/PHD3 deletions, while deletion of PHD2 alone, only causes a minor HIF-2α stabilization. Although single-dose application of PHD inhibitors leads to HIF-2α stabilization in juxtaglomerular cells, it fails to induce EPO gene expression in these cells [6, 46]. These findings suggest that EPO expression in juxtaglomerular renin cells requires prolonged HIF-2α stabilization. This could indicate that a metaplastic cell transformation is a prerequisite for the inducibility of EPO expression in juxtaglomerular cells.
After Vhl-deletion or PHD2/3 codeletion juxtaglomerular renin+ cells lose their renin storage granules and no longer appear epithelioid. Instead, they have a rather flattened and elongated appearance similar to fibroblast-like cells. Moreover, not only the expression of renin but also the expressions of the typical markers Akr1b7 and Cx40 are strongly downregulated. Conversely, typical markers of interstitial EPO-producing cells such as PDGFR-β and CD73 are clearly upregulated in transformed juxtaglomerular cells (Fig. 4) [6, 19, 46]. Whether this metaplastic transformation of juxtaglomerular renin+ cells into EPO+ cells is reversible, like for recruited VSMCs, still needs to be determined.
Juxtaglomerular renin cells (top, left), expressing Cx40 and Akr1b7, are transformed into EPO-producing cells by a prolonged genetic stabilization of HIF-2α. In juxtaglomerular renin cells, HIF-2α leads to a phenotypic as well as an endocrine shift. After transformation, these cells no longer have their distinct cuboid shape, but appear flattened, and instead of Cx40 and Akr1b7, these cells then express PDGFR-β and CD73 (top, right). Whether a re-transformation of EPO cells in juxtaglomerular position, back into renin-producing cells is possible, is not yet known.
Interstitial renin-expressing cells (bottom, left) can undergo an endocrine shift in response to an acute stabilization of HIF-2α due to anemia or the administration of PHD inhibitors. Interstitial renin-expressing cells are able to coexpress renin and EPO simultaneously (bottom right). If the acute hypoxic stimulus is removed, interstitial EPO/renin+ cells revert to only renin expression.
The mechanisms underlying the transformation of juxtaglomerular cells in EPO-producing cells are still unknown. So far, it is only known that the metaplastic transformation of renin+ cells into EPO-producing cells is dependent on HIF-2α and can be prevented by the deletion of HIF-2α in these cells. However, the pathways interacting with the hypoxia signaling pathway to mediate this transformation are still elusive [19, 46]. It is conceivable that activation of the hypoxia signaling pathway via HIF-2 leads to a change in the microRNA expression pattern [48, 63]. miRNAs can directly affect chromatin structure by targeting factors like DNA methyltransferases or histone deacetylases [4]. Previous studies have already demonstrated that the chromatin structure and the expression of certain miRNAs are essential for renin cell identity, as well as for the recruitment of VSMCs for renin production [67, 68, 101]. If the expression of miRNAs changes, this could lead to a remodeling of chromatin structure and thus ultimately influence cell identity.
It can also be speculated that HIF-2α activation could lead to a de-differentiation of juxtaglomerular renin+ cells to a less specialized fibroblast-like cell. It was shown in tumor cells that chronic HIF-2 stabilization can lead to a phenotype shift of differentiated cells towards a more de-differentiated phenotype. For example, neuroblastoma cells lose typical neuroendocrine and neuronal markers after HIF-2 activation [78]. HIF-2 has also been shown to activate the expression of the transcription marker Oct-4 which is associated with stem cell pluripotency and de-differentiation [13]. However, juxtaglomerular renin-producing cells not only lose characteristic markers during the transformation into EPO-producing cells but also gain markers typical of the new phenotype. It should also be mentioned in this context that not every cell type can be transformed into an EPO-expressing cell by HIF-2α stabilization [87, 93, 94]. Even in close relatives of renin+ cells, like VSMCs which develop from renin-producing cells during nephrogenesis, EPO expression cannot be induced by HIF-2α stabilization [5, 100, 106]. Only after a prior transformation of VSMCs and EGM cells into renin+ cells due to genetic RAAS activation, these cells can be further transformed into EPO-producing cells [6].
Further aspects on EPO-expressing cells are reviewed in the article of Wenger et al. also published in this Special Issue on “Kidney Control of Homeostasis.”
Renin cell plasticity: opportunities beyond renin production
The plasticity of renin+ cells is evident in the reversible recruitment of VSMCs and EGM cells during homeostatic challenges, in their ability to replace specialized intraglomerular cells and their ability to change their endocrine function by producing EPO instead of renin. This plasticity could offer a number of possibilities for therapeutic approaches in the future.
With regard to glomerular diseases, a treatment with RAS inhibitors has been shown to be beneficial in patients [32, 59, 110, 116]. Promising data from studies in mice indicate that juxtaglomerular renin-lineage cells could be used to replace intraglomerular mesangial cells or podocytes [53, 85, 105]. However, this repair mechanism seems to be insufficient in chronic progressive glomerular disease. A deeper understanding of the factors and signaling pathways that control this regeneration process will be necessary to specifically activate cells of the renin-lineage for glomerular repair.
With regard to the potential EPO-producing ability of renin-producing cells, one could speculate about new therapeutic opportunities to treat anemia in patients with chronic kidney disease. If the complex transformation mechanism could be unraveled, a targeted endocrine phenotype switch in juxtaglomerular renin cells could be induced for treatment. Previous findings suggest that the loss of renin production due to transformation of juxtaglomerular cells into EPO-producing cells may be compensated by recruitment of VSMCs, as observed in physiological stress situations [19, 48]. In this context, it will also be important to elucidate if the transformation of juxtaglomerular renin+ cells into EPO+ cells is reversible as observed for recruited VSMCs or EGM cells.
A possible transformation into EPO-producing cells should be considered, when evaluating the long-term effects of PHD inhibitor treatment on renin-producing cells. PHD inhibitors are a novel class of drugs that have recently been approved for the treatment of anemia in chronic kidney disease (Roxadustat is the first PHD inhibitor approved in the EU since September 2021) [12]. They interfere with the hypoxia signaling pathway and lead to the stabilization of hypoxia-inducible factors [34]. Therefore, it would be possible that EPO induction could occur in juxtaglomerular renin cells.
The existence of interstitial renin-expressing cells adds a new layer to the field of renin research. So far only few aspects are known about these cells. It appears that additional interstitial cells are recruited to express renin under certain conditions, for example, during anemia-induced hypotension or in experimental renal injury. Interestingly, these cells can also fulfill multiple endocrine functions simultaneously [6, 70].
Challenges for future renin research
To this date, several typical markers and signaling pathways involved in conveying the identity of juxtaglomerular renin-producing cells and the recruitment of additional cells have been elucidated. However, less is known about the detailed regulatory mechanisms that govern the migration of renin cells into damaged glomeruli and their differentiation into another cell type. Similarly, little is known about the endocrine shift into EPO-producing cells. Therefore, it will be of future interest to unravel the specific pathways that govern the transformational processes and phenotypic changes of renin-expressing cells. This would allow the development of selective drugs to specifically manipulate these cells and influence their transformation.
Furthermore, it will be of interest to determine the function and relevance of interstitial renin expression. To this aim, the cells should be characterized in more detail to identify differences and similarities to juxtaglomerular cells in terms of their phenotype and regulation. One challenge will be to analyze the interstitial renin+ cells without simultaneously affecting the juxtaglomerular cells to determine whether the interstitial cells cooperate with the systemic RAAS or function independently.
References
Bachmann S, Le Hir M, Eckardt KU (1993) Co-localization of erythropoietin mRNA and ecto-5’-nucleotidase immunoreactivity in peritubular cells of rat renal cortex indicates that fibroblasts produce erythropoietin. J Histochem Cytochem Off J Histochem Soc 41:335–341. https://doi.org/10.1177/41.3.8429197
Bartter FC, Pronove P, Gill JR, MacCardle RC (1998) Hyperplasia of the juxtaglomerular complex with hyperaldosteronism and hypokalemic alkalosis. A new syndrome. 1962. J Am Soc Nephrol 9:516–528. https://doi.org/10.1681/ASN.V93516
Berka JL, Alcorn D, Coghlan JP, Fernley RT, Morgan TO, Ryan GB, Skinner SL, Weaver DA (1990) Granular juxtaglomerular cells and prorenin synthesis in mice treated with enalapril. J Hypertens 8:229–238
Borggrefe T, Oswald F (2009) The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol Life Sci CMLS 66:1631–1646. https://doi.org/10.1007/s00018-009-8668-7
Broeker KAE, Fuchs MAA, Schrankl J, Kurt B, Nolan KA, Wenger RH, Kramann R, Wagner C, Kurtz A (2020) Different subpopulations of kidney interstitial cells produce erythropoietin and factors supporting tissue oxygenation in response to hypoxia in vivo. Kidney Int 98:918–931. https://doi.org/10.1016/j.kint.2020.04.040
Broeker KAE, Fuchs MAA, Schrankl J, Lehrmann C, Schley G, Todorov VT, Hugo C, Wagner C, Kurtz A (2021) Prolyl-4-hydroxylases 2 and 3 control erythropoietin production in renin expressing cells of mouse kidneys. J Physiol. https://doi.org/10.1113/JP282615
Castellanos Rivera RM, Monteagudo MC, Pentz ES, Glenn ST, Gross KW, Carretero O, Sequeira-Lopez MLS, Gomez RA (2011) Transcriptional regulator RBP-J regulates the number and plasticity of renin cells. Physiol Genomics 43:1021–1028. https://doi.org/10.1152/physiolgenomics.00061.2011
Castellanos-Rivera RM, Pentz ES, Lin E, Gross KW, Medrano S, Yu J, Sequeira-Lopez MLS, Gomez RA (2015) Recombination signal binding protein for Ig-κJ region regulates juxtaglomerular cell phenotype by activating the myo-endocrine program and suppressing ectopic gene expression. J Am Soc Nephrol JASN 26:67–80. https://doi.org/10.1681/ASN.2013101045
Castrop H, Höcherl K, Kurtz A, Schweda F, Todorov V, Wagner C (2010) Physiology of kidney renin. Physiol Rev 90:607–673. https://doi.org/10.1152/physrev.00011.2009
Celio MR, Groscurth P, Inagami T (1985) Ontogeny of renin immunoreactive cells in the human kidney. Anat Embryol (Berl) 173:149–155. https://doi.org/10.1007/BF00316297
Chen L, Kim SM, Oppermann M, Faulhaber-Walter R, Huang Y, Mizel D, Chen M, Lopez MLS, Weinstein LS, Gomez RA, Briggs JP, Schnermann J (2007) Regulation of renin in mice with Cre recombinase-mediated deletion of G protein Gsα in juxtaglomerular cells. Am J Physiol-Ren Physiol 292:F27–F37. https://doi.org/10.1152/ajprenal.00193.2006
Chen N, Hao C, Peng X, Lin H, Yin A, Hao L, Tao Y, Liang X, Liu Z, Xing C, Chen J, Luo L, Zuo L, Liao Y, Liu B-C, Leong R, Wang C, Liu C, Neff T, Szczech L, Yu K-HP (2019) Roxadustat for Anemia in Patients with Kidney Disease Not Receiving Dialysis. N Engl J Med 381:1001–1010. https://doi.org/10.1056/NEJMoa1813599
Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu C-J, Labosky PA, Simon MC, Keith B (2006) HIF-2α regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev 20:557–570. https://doi.org/10.1101/gad.1399906
DeLalio LJ, Masati E, Mendu S, Ruddiman CA, Yang Y, Johnstone SR, Milstein JA, Keller TCS, Weaver RB, Guagliardo NA, Best AK, Ravichandran KS, Bayliss DA, Sequeira-Lopez MLS, Sonkusare SN, Shu XH, Desai B, Barrett PQ, Le TH, Gomez RA, Isakson BE (2020) Pannexin 1 channels in renin-expressing cells influence renin secretion and blood pressure homeostasis. Kidney Int 98:630–644. https://doi.org/10.1016/j.kint.2020.04.041
Facemire CS, Nguyen M, Jania L, Beierwaltes WH, Kim H-S, Koller BH, Coffman TM (2011) A major role for the EP4 receptor in regulation of renin. Am J Physiol Renal Physiol 301:F1035-1041. https://doi.org/10.1152/ajprenal.00054.2011
Fuchs MAA, Schrankl J, Leupold C, Wagner C, Kurtz A, Broeker K (2022) Intact prostaglandin signaling through EP2 and EP4 receptors in stromal progenitor cells is required for normal development of the renal cortex in mice. Am J Physiol-Ren Physiol. https://doi.org/10.1152/ajprenal.00414.2021
Gebert LFR, MacRae IJ (2019) Regulation of microRNA function in animals. Nat Rev Mol Cell Biol 20:21–37. https://doi.org/10.1038/s41580-018-0045-7
Georgi SA, Reh TA (2011) Dicer is required for the maintenance of notch signaling and gliogenic competence during mouse retinal development. Dev Neurobiol 71:1153. https://doi.org/10.1002/dneu.20899
Gerl K, Miquerol L, Todorov VT, Hugo CPM, Adams RH, Kurtz A, Kurt B (2015) Inducible glomerular erythropoietin production in the adult kidney. Kidney Int 88:1345–1355. https://doi.org/10.1038/ki.2015.274
Gerl K, Nolan KA, Karger C, Fuchs M, Wenger RH, Stolt CC, Willam C, Kurtz A, Kurt B (2016) Erythropoietin production by PDGFR-β+ cells. Pflüg Arch - Eur J Physiol 468:1479–1487. https://doi.org/10.1007/s00424-016-1829-2
Gerl K, Steppan D, Fuchs M, Wagner C, Willam C, Kurtz A, Kurt B (2017) Activation of hypoxia signaling in stromal progenitors impairs kidney development. Am J Pathol 187:1496–1511. https://doi.org/10.1016/j.ajpath.2017.03.014
Gerl M, Vöckl J, Kurt B, van Veen TAB, Kurtz A, Wagner C (2015) Inducible deletion of connexin 40 in adult mice causes hypertension and disrupts pressure control of renin secretion. Kidney Int 87:557–563. https://doi.org/10.1038/ki.2014.303
Giaimo BD, Gagliani EK, Kovall RA, Borggrefe T (2021) Transcription factor RBPJ as a molecular switch in regulating the notch response. Adv Exp Med Biol 1287:9–30. https://doi.org/10.1007/978-3-030-55031-8_2
Gomez RA (1979) (2017) Fate of renin cells during development and disease. Hypertens Dallas Tex 69:387–395. https://doi.org/10.1161/HYPERTENSIONAHA.116.08316
Gomez RA, Chevalier RL, Everett AD, Elwood JP, Peach MJ, Lynch KR, Carey RM (1990) Recruitment of renin gene-expressing cells in adult rat kidneys. Am J Physiol 259:F660-665. https://doi.org/10.1152/ajprenal.1990.259.4.F660
Gomez RA, Pentz ES, Jin X, Cordaillat M, Sequeira Lopez MLS (2009) CBP and p300 are essential for renin cell identity and morphological integrity of the kidney. Am J Physiol-Heart Circ Physiol 296:H1255–H1262. https://doi.org/10.1152/ajpheart.01266.2008
Gomez RA, Sequeira-Lopez MLS (2018) Renin cells in homeostasis, regeneration and immune defence mechanisms. Nat Rev Nephrol 14:231–245. https://doi.org/10.1038/nrneph.2017.186
Gonzalez AA, Prieto MC (2015) Roles of collecting duct renin and (pro)renin receptor in hypertension: mini review. Ther Adv Cardiovasc Dis 9:191–200. https://doi.org/10.1177/1753944715574817
Graham PC, Stewart HV, Downie I, Lindop GB (1990) The distribution of renin-containing cells in kidneys with renal artery stenosis–an immunocytochemical study. Histopathology 16:347–355. https://doi.org/10.1111/j.1365-2559.1990.tb01138.x
Grill A, Schießl IM, Gess B, Fremter K, Hammer A, Castrop H (2016) Salt-losing nephropathy in mice with a null mutation of the Clcnk2 gene. Acta Physiol Oxf Engl 218:198–211. https://doi.org/10.1111/apha.12755
Guessoum O, Zainab M, Sequeira-Lopez MLS, Gomez RA (2020) Proliferation does not contribute to murine models of renin cell recruitment. Acta Physiol 230:e13532. https://doi.org/10.1111/apha.13532
Guo S, Kowalewska J, Wietecha TA, Iyoda M, Wang L, Yi K, Spencer M, Banas M, Alexandrescu S, Hudkins KL, Alpers CE (2008) Renin-angiotensin system blockade is renoprotective in immune complex–mediated glomerulonephritis. J Am Soc Nephrol 19:1168–1176. https://doi.org/10.1681/ASN.2007050607
Haase VH (2013) Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev 27:41–53. https://doi.org/10.1016/j.blre.2012.12.003
Haase VH (2021) Hypoxia-inducible factor–prolyl hydroxylase inhibitors in the treatment of anemia of chronic kidney disease. Kidney Int Suppl 11:8–25. https://doi.org/10.1016/j.kisu.2020.12.002
Hickmann L, Steglich A, Gerlach M, Al-Mekhlafi M, Sradnick J, Lachmann P, Sequeira-Lopez MLS, Gomez RA, Hohenstein B, Hugo C, Todorov VT (2017) Persistent and inducible neogenesis repopulates progenitor renin lineage cells in the kidney. Kidney Int 92:1419–1432. https://doi.org/10.1016/j.kint.2017.04.014
Hong J, Yao J (2020) Connexin hemichannels contribute to the activation of cAMP signaling pathway and renin production. Int J Mol Sci 21:4462. https://doi.org/10.3390/ijms21124462
Humphreys BD, Lin S-L, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS (2010) Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176:85–97. https://doi.org/10.2353/ajpath.2010.090517
Inam R, Gandhi J, Joshi G, Smith NL, Khan SA (2019) Juxtaglomerular cell tumor: reviewing a cryptic cause of surgically correctable hypertension. Curr Urol 13:7–12. https://doi.org/10.1159/000499301
Junker F, Chabloz A, Koch U, Radtke F (2015) Dicer1 imparts essential survival cues in Notch-driven T-ALL via miR-21–mediated tumor suppressor Pdcd4 repression. Blood 126:993–1004. https://doi.org/10.1182/blood-2014-12-618892
Kammerl MC, Nüsing RM, Schweda F, Endemann D, Stubanus M, Kees F, Lackner KJ, Fischereder M, Krämer BK (2001) Low sodium and furosemide-induced stimulation of the renin system in man is mediated by cyclooxygenase 2. Clin Pharmacol Ther 70:468–474. https://doi.org/10.1016/S0009-9236(01)49789-X
Karger C, Kurtz F, Steppan D, Schwarzensteiner I, Machura K, Angel P, Banas B, Risteli J, Kurtz A (2013) Procollagen I-expressing renin cell precursors. Am J Physiol Renal Physiol 305:F355-361. https://doi.org/10.1152/ajprenal.00079.2013
Karger C, Machura K, Schneider A, Hugo C, Todorov VT, Kurtz A (2018) COX-2-derived PGE2 triggers hyperplastic renin expression and hyperreninemia in aldosterone synthase-deficient mice. Pflugers Arch 470:1127–1137. https://doi.org/10.1007/s00424-018-2118-z
Kaverina NV, Kadoya H, Eng DG, Rusiniak ME, Sequeira-Lopez MLS, Gomez RA, Pippin JW, Gross KW, Peti-Peterdi J, Shankland SJ (2017) Tracking the stochastic fate of cells of the renin lineage after podocyte depletion using multicolor reporters and intravital imaging. PLoS ONE 12:e0173891. https://doi.org/10.1371/journal.pone.0173891
Kessel F, Steglich A, Hickmann L, Lira Martinez R, Gerlach M, Sequeira-Lopez MLS, Gomez RA, Hugo CPM, Todorov VT (2021) Patterns of differentiation of renin lineage cells during nephrogenesis. Am J Physiol Renal Physiol 321:F378–F388. https://doi.org/10.1152/ajprenal.00151.2021
Kim SM, Chen L, Faulhaber-Walter R, Oppermann M, Huang Y, Mizel D, Briggs JP, Schnermann J (2007) Regulation of renin secretion and expression in mice deficient in β1- and β2-adrenergic receptors. Hypertension 50:103–109. https://doi.org/10.1161/HYPERTENSIONAHA.107.087577
Kurt B, Gerl K, Karger C, Schwarzensteiner I, Kurtz A (2015) Chronic hypoxia-inducible transcription factor-2 activation stably transforms juxtaglomerular renin cells into fibroblast-like cells in vivo. J Am Soc Nephrol JASN 26:587–596. https://doi.org/10.1681/ASN.2013111152
Kurt B, Kurtz L, Sequeira-Lopez ML, Gomez RA, Willecke K, Wagner C, Kurtz A (2011) Reciprocal expression of connexin 40 and 45 during phenotypical changes in renin-secreting cells. Am J Physiol - Ren Physiol 300:F743–F748. https://doi.org/10.1152/ajprenal.00647.2010
Kurt B, Paliege A, Willam C, Schwarzensteiner I, Schucht K, Neymeyer H, Sequeira-Lopez MLS, Bachmann S, Gomez RA, Eckardt K-U, Kurtz A (2013) Deletion of von Hippel-Lindau protein converts renin-producing cells into erythropoietin-producing cells. J Am Soc Nephrol 24:433–444. https://doi.org/10.1681/ASN.2012080791
Kurtz L, Janssen-Bienhold U, Kurtz A, Wagner C (2009) Connexin expression in renin-producing cells. J Am Soc Nephrol 20:506–512. https://doi.org/10.1681/ASN.2008030252
Kurtz L, Madsen K, Kurt B, Jensen BL, Walter S, Banas B, Wagner C, Kurtz A (2010) High-level connexin expression in the human juxtaglomerular apparatus. Nephron Physiol 116:p1–p8. https://doi.org/10.1159/000315658
Kurtz L, Schweda F, de Wit C, Kriz W, Witzgall R, Warth R, Sauter A, Kurtz A, Wagner C (2007) Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J Am Soc Nephrol JASN 18:1103–1111. https://doi.org/10.1681/ASN.2006090953
Lemley KV, Kriz W (1991) Anatomy of the renal interstitium. Kidney Int 39:370–381. https://doi.org/10.1038/ki.1991.49
Lichtnekert J, Kaverina NV, Eng DG, Gross KW, Kutz JN, Pippin JW, Shankland SJ (2016) Renin-angiotensin-aldosterone system inhibition increases podocyte derivation from cells of renin lineage. J Am Soc Nephrol 27:3611–3627. https://doi.org/10.1681/ASN.2015080877
Lin EE, Pentz ES, Sequeira-Lopez MLS, Gomez RA (2015) Aldo-keto reductase 1b7, a novel marker for renin cells, is regulated by cyclic AMP signaling. Am J Physiol-Regul Integr Comp Physiol 309:R576–R584. https://doi.org/10.1152/ajpregu.00222.2015
Lin S-Y, Liu W-Y, Chen W-C, Chen R-H (2010) Secondary hypertension due to a renin-secreting juxtaglomerular cell tumor. J Formos Med Assoc 109:237–240. https://doi.org/10.1016/S0929-6646(10)60047-2
Liu M, Gomez D (2019) Smooth muscle cell phenotypic diversity. Arterioscler Thromb Vasc Biol 39:1715–1723. https://doi.org/10.1161/ATVBAHA.119.312131
Liu M-J, Takahashi Y, Wada T, He J, Gao J, Tian Y, Li S, Xie W (2009) The aldo-keto reductase Akr1b7 gene is a common transcriptional target of xenobiotic receptors pregnane X receptor and constitutive androstane receptor. Mol Pharmacol 76:604–611. https://doi.org/10.1124/mol.109.057455
Lübkemeier I, Machura K, Kurtz L, Neubauer B, Dobrowolski R, Schweda F, Wagner C, Willecke K, Kurtz A (2011) The connexin 40 A96S mutation causes renin-dependent hypertension. J Am Soc Nephrol JASN 22:1031–1040. https://doi.org/10.1681/ASN.2010101047
Macconi D, Sangalli F, Bonomelli M, Conti S, Condorelli L, Gagliardini E, Remuzzi G, Remuzzi A (2009) Podocyte repopulation contributes to regression of glomerular injury induced by ace inhibition. Am J Pathol 174:797–807. https://doi.org/10.2353/ajpath.2009.080227
Machura K, Iankilevitch E, Neubauer B, Theuring F, Kurtz A (2013) The aldo-keto reductase AKR1B7 coexpresses with renin without influencing renin production and secretion. Am J Physiol-Ren Physiol 304:F578–F584. https://doi.org/10.1152/ajprenal.00617.2012
Machura K, Neubauer B, Müller H, Tauber P, Kurtz A, Kurtz L (2015) Connexin 40 is dispensable for vascular renin cell recruitment but is indispensable for vascular baroreceptor control of renin secretion. Pflüg Arch - Eur J Physiol 467:1825–1834. https://doi.org/10.1007/s00424-014-1615-y
Makhanova N, Lee G, Takahashi N, Sequeira Lopez ML, Gomez RA, Kim H-S, Smithies O (2006) Kidney function in mice lacking aldosterone. Am J Physiol-Ren Physiol 290:F61–F69. https://doi.org/10.1152/ajprenal.00257.2005
Martinez MF, Martini AG, Sequeira-Lopez MLS (1979) Gomez RA (2020) Ctcf is required for renin expression and maintenance of the structural integrity of the kidney. Clin Sci Lond Engl 134:1763–1774. https://doi.org/10.1042/CS20200184
Martinez MF, Medrano S, Brown EA, Tufan T, Shang S, Bertoncello N, Guessoum O, Adli M, Belyea BC, Sequeira-Lopez MLS, Gomez RA (2018) Super-enhancers maintain renin-expressing cell identity and memory to preserve multi-system homeostasis. J Clin Invest 128:4787–4803. https://doi.org/10.1172/JCI121361
Martini AG, Danser AHJ (2017) Juxtaglomerular cell phenotypic plasticity. High Blood Press Cardiovasc Prev Off J Ital Soc Hypertens 24:231–242. https://doi.org/10.1007/s40292-017-0212-5
Martini AG, Xa LK, Lacombe M-J, Blanchet-Cohen A, Mercure C, Haibe-Kains B, Friesema ECH, van den Meiracker AH, Gross KW, Azizi M, Corvol P, Nguyen G, Reudelhuber TL, Danser AHJ (2017) Transcriptome analysis of human reninomas as an approach to understanding juxtaglomerular cell biology. Hypertension 69:1145–1155. https://doi.org/10.1161/HYPERTENSIONAHA.117.09179
Maxwell PH, Osmond MK, Pugh CW, Heryet A, Nicholls LG, Tan CC, Doe BG, Ferguson DJ, Johnson MH, Ratcliffe PJ (1993) Identification of the renal erythropoietin-producing cells using transgenic mice. Kidney Int 44:1149–1162. https://doi.org/10.1038/ki.1993.362
Medrano S, Monteagudo MC, Sequeira-Lopez MLS, Pentz ES, Gomez RA (2012) Two microRNAs, miR-330 and miR-125b-5p, mark the juxtaglomerular cell and balance its smooth muscle phenotype. Am J Physiol - Ren Physiol 302:F29–F37. https://doi.org/10.1152/ajprenal.00460.2011
Minamishima YA, Moslehi J, Padera RF, Bronson RT, Liao R, Kaelin WG (2009) A feedback loop involving the Phd3 prolyl hydroxylase tunes the mammalian hypoxic response in vivo. Mol Cell Biol 29:5729–5741. https://doi.org/10.1128/MCB.00331-09
Miyauchi K, Nakai T, Saito S, Yamamoto T, Sato K, Kato K, Nezu M, Miyazaki M, Ito S, Tamamoto M, Suzuki N (2021) Renal interstitial fibroblasts coproduce erythropoietin and renin under anaemic conditions. EBioMedicine 64:103209. https://doi.org/10.1016/j.ebiom.2021.103209
Morris BJ (1992) Molecular biology of renin. I: Gene and protein structure, synthesis and processing. J Hypertens 10:209–214. https://doi.org/10.1097/00004872-199203000-00002
Nagai Y, Yamabe F, Sasaki Y, Ishii T, Nakanishi K, Nakajima K, Shibuya K, Mikami T, Akasaka Y, Urita Y, Yamanaka N (2020) A study of morphological changes in renal afferent arterioles induced by angiotensin II type 1 receptor blockers in hypertensive patients. Kidney Blood Press Res 45:194–208. https://doi.org/10.1159/000505025
Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA (2011) Intratubular renin-angiotensin system in hypertension. Hypertension 57:355–362. https://doi.org/10.1161/HYPERTENSIONAHA.110.163519
Neubauer B, Machura K, Kettl R, Lopez MLSS, Friebe A, Kurtz A (2013) Endothelium-derived nitric oxide supports renin cell recruitment through the nitric oxide–sensitive guanylate cyclase pathway. Hypertension 61:400–407. https://doi.org/10.1161/HYPERTENSIONAHA.111.00221
Neubauer B, Machura K, Schnermann J, Wagner C (2011) Renin expression in large renal vessels during fetal development depends on functional β1/β2-adrenergic receptors. Am J Physiol-Ren Physiol 301:F71–F77. https://doi.org/10.1152/ajprenal.00443.2010
Neubauer B, Schrankl J, Steppan D, Neubauer K, Sequeira-Lopez ML, Pan L, Gomez RA, Coffman TM, Gross KW, Kurtz A, Wagner C (2018) Angiotensin II short-loop feedback. Hypertension 71:1075–1082. https://doi.org/10.1161/HYPERTENSIONAHA.117.10357
Nguyen G, Danser AHJ (2008) Prorenin and (pro)renin receptor: a review of available data from in vitro studies and experimental models in rodents. Exp Physiol 93:557–563. https://doi.org/10.1113/expphysiol.2007.040030
Nilsson H, Jögi A, Beckman S, Harris AL, Poellinger L, Påhlman S (2005) HIF-2α expression in human fetal paraganglia and neuroblastoma: relation to sympathetic differentiation, glucose deficiency, and hypoxia. Exp Cell Res 303:447–456. https://doi.org/10.1016/j.yexcr.2004.10.003
Nolan KA, Wenger RH (2018) Source and microenvironmental regulation of erythropoietin in the kidney. Curr Opin Nephrol Hypertens 27:277–282. https://doi.org/10.1097/MNH.0000000000000420
Nüsing RM, Seyberth HW (2004) The role of cyclooxygenases and prostanoid receptorsin furosemide-like salt losing tubulopathy: the hyperprostaglandin E syndrome. Acta Physiol Scand 181:523–528. https://doi.org/10.1111/j.1365-201X.2004.01326.x
O’Brien J, Hayder H, Zayed Y, Peng C (2018) Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol 9:402. https://doi.org/10.3389/fendo.2018.00402
Paul M, Poyan Mehr A, Kreutz R (2006) Physiology of local renin-angiotensin systems. Physiol Rev 86:747–803. https://doi.org/10.1152/physrev.00036.2005
Pentz ES, Cordaillat M, Carretero OA, Tucker AE, Sequeira Lopez MLS, Gomez RA (2012) Histone acetyl transferases CBP and p300 are necessary for maintenance of renin cell identity and transformation of smooth muscle cells to the renin phenotype. Am J Physiol - Heart Circ Physiol 302:H2545–H2552. https://doi.org/10.1152/ajpheart.00782.2011
Pentz ES, Sequeira Lopez MLS, Cordaillat M, Gomez RA (2008) Identity of the renin cell is mediated by cAMP and chromatin remodeling: an in vitro model for studying cell recruitment and plasticity. Am J Physiol-Heart Circ Physiol 294:H699–H707. https://doi.org/10.1152/ajpheart.01152.2007
Pippin JW, Kaverina NV, Eng DG, Krofft RD, Glenn ST, Duffield JS, Gross KW, Shankland SJ (2015) Cells of renin lineage are adult pluripotent progenitors in experimental glomerular disease. Am J Physiol-Ren Physiol 309:F341–F358. https://doi.org/10.1152/ajprenal.00438.2014
Pippin JW, Sparks MA, Glenn ST, Buitrago S, Coffman TM, Duffield JS, Gross KW, Shankland SJ (2013) Cells of renin lineage are progenitors of podocytes and parietal epithelial cells in experimental glomerular disease. Am J Pathol 183:542–557. https://doi.org/10.1016/j.ajpath.2013.04.024
Rankin EB, Tomaszewski JE, Haase VH (2006) Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res 66:2576–2583. https://doi.org/10.1158/0008-5472.CAN-05-3241
Reinalter SC, Jeck N, Brochhausen C, Watzer B, Nüsing RM, Seyberth HW, Kömhoff M (2002) Role of cyclooxygenase-2 in hyperprostaglandin E syndrome/antenatal Bartter syndrome. Kidney Int 62:253–260. https://doi.org/10.1046/j.1523-1755.2002.00435.x
Rensen SSM, Doevendans PAFM, van Eys GJJM (2007) Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Neth Heart J 15:100–108
Saleem M, Hodgkinson CP, Xiao L, Gimenez-Bastida JA, Rasmussen ML, Foss J, Payne AJ, Mirotsou M, Gama V, Dzau VJ, Gomez JA (2020) Sox6 as a new modulator of renin expression in the kidney. Am J Physiol Renal Physiol 318:F285–F297. https://doi.org/10.1152/ajprenal.00095.2019
Saleem M, Saavedra-Sánchez L, Barturen-Larrea P, Gomez JA (2021) The transcription factor Sox6 controls renin expression during renal artery stenosis. Kidney360 2:842–856. https://doi.org/10.34067/KID.0002792020
Sauter A, Machura K, Neubauer B, Kurtz A, Wagner C (2008) Development of renin expression in the mouse kidney. Kidney Int 73:43–51. https://doi.org/10.1038/sj.ki.5002571
Schietke RE, Hackenbeck T, Tran M, Günther R, Klanke B, Warnecke CL, Knaup KX, Shukla D, Rosenberger C, Koesters R, Bachmann S, Betz P, Schley G, Schödel J, Willam C, Winkler T, Amann K, Eckardt K-U, Maxwell P, Wiesener MS (2012) Renal tubular HIF-2α expression requires VHL inactivation and causes fibrosis and cysts. PLoS ONE 7:e31034. https://doi.org/10.1371/journal.pone.0031034
Schley G, Klanke B, Schödel J, Forstreuter F, Shukla D, Kurtz A, Amann K, Wiesener MS, Rosen S, Eckardt K-U, Maxwell PH, Willam C (2011) Hypoxia-inducible transcription factors stabilization in the thick ascending limb protects against ischemic acute kidney injury. J Am Soc Nephrol 22:2004–2015. https://doi.org/10.1681/ASN.2010121249
Schrankl J, Fuchs M, Broeker K, Daniel C, Kurtz A, Wagner C (2021) Localization of angiotensin II type 1 receptor gene expression in rodent and human kidneys. Am J Physiol Renal Physiol 320:F644–F653. https://doi.org/10.1152/ajprenal.00550.2020
Schweda F, Friis U, Wagner C, Skott O, Kurtz A (2007) Renin release. Physiol Bethesda Md 22:310–319. https://doi.org/10.1152/physiol.00024.2007
Schweda F, Klar J, Narumiya S, Nüsing RM, Kurtz A (2004) Stimulation of renin release by prostaglandin E2 is mediated by EP2 and EP4 receptors in mouse kidneys. Am J Physiol Renal Physiol 287:F427-433. https://doi.org/10.1152/ajprenal.00072.2004
Schweda F, Kurtz L, de Wit C, Janssen-Bienhold U, Kurtz A, Wagner C (2009) Substitution of connexin40 with connexin45 prevents hyperreninemia and attenuates hypertension. Kidney Int 75:482–489. https://doi.org/10.1038/ki.2008.637
Sequeira Lopez MLS, Gomez RA (2010) Novel mechanisms for the control of renin synthesis and release. Curr Hypertens Rep 12:26–32. https://doi.org/10.1007/s11906-009-0080-z
Sequeira López MLS, Pentz ES, Nomasa T, Smithies O, Gomez RA (2004) Renin cells are precursors for multiple cell types that switch to the renin phenotype when homeostasis is threatened. Dev Cell 6:719–728. https://doi.org/10.1016/s1534-5807(04)00134-0
Sequeira Lopez MLS, Pentz ES, Robert B, Abrahamson DR, Gomez RA (2001) Embryonic origin and lineage of juxtaglomerular cells. Am J Physiol-Ren Physiol 281:F345–F356. https://doi.org/10.1152/ajprenal.2001.281.2.F345
Sequeira-Lopez MLS, Gomez RA (2021) Renin cells, the kidney, and hypertension. Circ Res 128:887–907. https://doi.org/10.1161/CIRCRESAHA.121.318064
Sequeira-Lopez MLS, Weatherford ET, Borges GR, Monteagudo MC, Pentz ES, Harfe BD, Carretero O, Sigmund CD, Gomez RA (2010) The microRNA-processing enzyme dicer maintains juxtaglomerular cells. J Am Soc Nephrol JASN 21:460–467. https://doi.org/10.1681/ASN.2009090964
Sparks MA, Crowley SD, Gurley SB, Mirotsou M, Coffman TM (2014) Classical renin-angiotensin system in kidney physiology. Compr Physiol 4:1201–1228. https://doi.org/10.1002/cphy.c130040
Starke C, Betz H, Hickmann L, Lachmann P, Neubauer B, Kopp JB, Sequeira-Lopez MLS, Gomez RA, Hohenstein B, Todorov VT, Hugo CPM (2015) Renin lineage cells repopulate the glomerular mesangium after injury. J Am Soc Nephrol JASN 26:48–54. https://doi.org/10.1681/ASN.2014030265
Steglich A, Hickmann L, Linkermann A, Bornstein S, Hugo C, Todorov VT (2020) Beyond the paradigm: novel functions of renin-producing cells. In: Pedersen SHF (ed) Reviews of Physiology, Biochemistry and Pharmacology. Springer International Publishing, Cham, pp 53–81
Steppan D, Zügner A, Rachel R, Kurtz A (2013) Structural analysis suggests that renin is released by compound exocytosis. Kidney Int 83:233–241. https://doi.org/10.1038/ki.2012.392
Takahashi N, Chernavvsky DR, Gomez RA, Igarashi P, Gitelman HJ, Smithies O (2000) Uncompensated polyuria in a mouse model of Bartter’s syndrome. Proc Natl Acad Sci U S A 97:5434–5439
Trnka P, Orellana L, Walsh M, Pool L, Borzi P (2014) Reninoma: an uncommon cause of renin-mediated hypertension. Front Pediatr 2:89. https://doi.org/10.3389/fped.2014.00089
Tylicki L, Larczynski W, Rutkowski B (2005) Renal protective effects of the renin-angiotensin-aldosterone system blockade: from evidence-based approach to perspectives. Kidney Blood Press Res 28:230–242. https://doi.org/10.1159/000087842
Urrutia AA, Afzal A, Nelson J, Davidoff O, Gross KW, Haase VH (2016) Prolyl-4-hydroxylase 2 and 3 coregulate murine erythropoietin in brain pericytes. Blood 128:2550–2560. https://doi.org/10.1182/blood-2016-05-713545
Wagner C, Kurtz L, Schweda F, Simon AM, Kurtz A (2009) Connexin 37 is dispensable for the control of the renin system and for positioning of renin-producing cells in the kidney. Pflugers Arch 459:151–158. https://doi.org/10.1007/s00424-009-0707-6
Wagner C, de Wit C, Kurtz L, Grünberger C, Kurtz A, Schweda F (2007) Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res 100:556–563. https://doi.org/10.1161/01.RES.0000258856.19922.45
Watanabe H, Martini AG, Brown EA, Liang X, Medrano S, Goto S, Narita I, Arend LJ, Sequeira-Lopez MLS, Gomez RA (2021) Inhibition of the renin-angiotensin system causes concentric hypertrophy of renal arterioles in mice and humans. JCI Insight 6. https://doi.org/10.1172/jci.insight.154337
Wennmann DO, Hsu H-H, Pavenstädt H (2012) The Renin-Angiotensin-Aldosterone System in Podocytes. Semin Nephrol 32:377–384. https://doi.org/10.1016/j.semnephrol.2012.06.009
Wolf G, Butzmann U, Wenzel UO (2003) The renin-angiotensin system and progression of renal disease: from hemodynamics to cell biology. Nephron Physiol 93:p3–p13. https://doi.org/10.1159/000066656
Yang T, Xu C (2017) Physiology and pathophysiology of the intrarenal renin-angiotensin system: an update. J Am Soc Nephrol JASN 28:1040–1049. https://doi.org/10.1681/ASN.2016070734
Yao J, Oite T, Kitamura M (2009) Gap junctional intercellular communication in the juxtaglomerular apparatus. Am J Physiol-Ren Physiol 296:F939–F946. https://doi.org/10.1152/ajprenal.90612.2008
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by grants from the German Research Foundation (Ku 859/15–2 and SFB 1350, project number 387509280).
Author information
Authors and Affiliations
Contributions
K. A. E. B. and A. K. conceptualized the review, K. A. E. B. performed the literature search and wrote the first draft of the manuscript, all authors commented on previous versions of the manuscript, all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figure Design Schematic figures were created using biorender.com.
This article is part of the special issue on Kidney Control of Homeostasis in Pflügers Archiv—European Journal of Physiology
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Broeker, K.A.E., Schrankl, J., Fuchs, M.A.A. et al. Flexible and multifaceted: the plasticity of renin-expressing cells. Pflugers Arch - Eur J Physiol 474, 799–812 (2022). https://doi.org/10.1007/s00424-022-02694-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-022-02694-8