Abstract
Muscle inactivity reduces muscle protein synthesis (MPS), whereas a subsequent period of rehabilitation resistance training (retraining) increases MPS. However, less is known regarding muscle protein breakdown (MPB) during such conditions. Furthermore, nonsteroidal anti-inflammatory drugs (NSAIDs) may have a dampening effect on MPB during periods of inactivity in older individuals. Thus, we measured the average MPB, by use of the deuterated water methodology, during an immobilization period and a subsequent retraining period in older individuals with and without NSAID treatment. Eighteen men (60–80 years: range) were randomly assigned to ibuprofen (1200 mg/d, Ibu) or placebo (Plc). One lower limb was immobilized in a cast for 2 weeks and retrained for 2 weeks, and 2 × 20 g of whey protein was ingested daily during both periods. Besides MPB, the protein expression of different muscle degradation signaling molecules was investigated. MPB was lower during immobilization compared to retraining (p < 0.01). NSAID treatment did not affect the MPB rate during immobilization or retraining (p > 0.05). The protein expression of muscle degradation signaling molecules changed during the study intervention but were unaffected by NSAID treatment. The finding that MPB was lower during immobilization than during retraining indicates that an increased MPB may play an important role in the muscle protein remodeling processes taking place within the initial retraining period. Moreover, NSAID treatment did not significantly influence the MPB rate during 2 weeks of lower limb immobilization or during 2 weeks of subsequent retraining in older individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Disuse of skeletal muscle either in the form of reduced use [7], bed rest [13, 14], or immobilization [11, 41] causes atrophy. While it is well-established that the muscle protein synthesis rate declines during immobilization, the role of muscle protein breakdown (MPB) in relation to inactivity-induced muscle atrophy is less clear. So far, only few attempts have been made to measure MPB after periods of muscle inactivity. By use of the arteriovenous balance model in combination with stable isotope infusion, it has been demonstrated that the MPB is unchanged after 14 days of bed rest in young men [14]. Furthermore, through pulse isotope infusions, it has been shown that the muscle fractional breakdown rate (FBR) is unchanged after 21 days of bed rest in young men [42]. However, another study reported that interstitial 3-methylhistidine, a biomarker of myofibrillar protein breakdown, was increased with 3 days of immobilization in young men [44]. Taken together, these findings indicate that the MPB may variate throughout the period of muscle inactivity with a transient elevation during the early inactivity period followed by a return to baseline levels during sustained periods of inactivity. However, the findings obtained with the tracer dilution methods provide a nonprotein-specific MPB measurement, and moreover, all reported values represent acute “snap shots” of the MPB rate. Therefore, these findings may not be fully representative of the MPB during daily life periods of immobilization. Although the tracer dilution methods can be advantageous in relation to measurement of net tissue balance with a high time resolution, the deuterated water methodology provides a protein specific and direct measurement of the average MPB over a period of daily living (days) [18].
Conduction of muscular contractions, e.g., resistance exercises, stimulates muscle protein turnover rates in the following hours/days of recovery [3, 30]. Moreover, muscle protein synthesis has been shown to be elevated during the first 8 days of resistance exercise training [50]. However, less is known regarding the specific fluctuations in MPB during prolonged periods with repeated resistance exercise sessions, although it seems possible that the MPB would increase due to the processes of skeletal muscle remodeling that occurs during the early period of unaccustomed resistance exercise [9]. Especially, the early period of rehabilitation resistance training, after a period of muscle inactivity, could be expected to have a significant impact on the overall muscle protein turnover and hence also muscle MPB. Nevertheless, as compared to the state of inactivity, early rehabilitation resistance training represents the complete opposite state, and hence, the two conditions make up two “extremes” within the normal life span of most people.
It has been demonstrated that NSAIDs may influence the muscle mass adaptation to periods of resistance training in healthy, older humans [45], as a consequence of alterations in muscle protein turnover kinetics [46]. Especially, the observation that NSAIDs inhibit the training induced increase in muscle gene expression of interleukin-6 (IL-6) and muscle RING finger protein 1 (MuRF-1) indicates that MPB may be affected by NSAID intake [46]. Moreover, supplementation with dietary omega-3 fatty acids (which can have anti-inflammatory effects [2, 16]) prior to and during 10 days of immobilization has been shown to alleviate muscle catabolism in healthy, adult rats [51]. This muscle-preserving effect was partly achieved by eliminating the increases in the muscle expression of the ubiquitin ligases, MuRF1 and Atrophy gene-1 (Atrogin-1) [51], which regulate muscle degradation via the ubiquitin-proteasome system [27, 35]. Somehow in line with that, it has been demonstrated that protein feeding induces an increase in Forkhead box O (FoxO)-3a phosphorylation, alongside a decrease in muscle degradation, in elderly inflamed rats treated with NSAID, but not in placebo-treated rats [33]. Taken together, these findings could indicate that anti-inflammatory treatments may have a diminishing effect on MPB in both catabolic and anabolic situations such as muscle inactivity, protein ingestion, and resistance training.
Even though the effect of NSAID on both muscle gene and protein signaling response to resistance exercise has been investigated [25, 46, 48], the information is still sparse. The nuclear factor-kappa B (NF-κB) transcriptional activator is regarded as a central regulator of muscle inflammatory signaling pathways that can induce transcription of the atrogens, which may ultimately lead to increased muscle degradation [20, 21, 36, 48]. Upon activation of the NF-κB complex, regulatory molecules, which keep the NF-κB transcription factors sequestered in the cytosol, are phosphorylated and removed, which leads to the transport of NF-κB transcription factors into the nucleus [20, 21, 36, 48]. Therefore, the relationship between the concentrations of NF-κB transcription factors, measured in the cytosol and the nucleus subcellular fractions, respectively, could give an indication of the NF-κB activation. Interestingly, the observation that NSAIDs may inhibit the training induced increase in MuRF-1 gene expression [46] has led to speculations of that this may occur through a pathway involving both prostaglandin (PG)E2 and NF-κB [38, 46]. However, other work could not clearly demonstrate that NSAID affected the activation NF-κB [48], and more work is needed to further clarify this.
The present study aimed to investigate the rate and intramyocellular regulation (protein signaling) of the MPB process during 2 weeks of lower limb immobilization and 2 weeks of subsequent retraining. Since immobilization may induce some degree of local muscle inflammation [40], NSAID treatment was applied to dampen local muscle inflammation during a period of immobilization and retraining. The deuterated (2H2O) water–based method was used to assess the gross average myofibrillar fractional breakdown rate, as previously described [18]. Furthermore, protein concentrations of signaling molecules related to MPB were measured both in the cytosolic and nuclear subcellular fractions at baseline, immediately after immobilization, 20 h after the first retraining session and after 2 weeks of retraining. We assumed that prolonged inactivity obtained by immobilization would decrease muscle protein turnover rates (also MPB), and therefore, it was hypothesized that MPB would be lower compared to during the subsequent retraining period. Furthermore, it was hypothesized that treatment with NSAID would have a dampening effect on MPB during immobilization but that it would not influence the retraining conditions.
Methods
Study design
Experimental protocol
Eighteen healthy, older males (age, 60–80 years; BMI, 20–30 kg/m2) were recruited through newspaper advertisements. The included subjects had no cancer and metabolic, cardiac, or neurological diseases; were nonsmokers and recreationally physically active; and had not taken part in any form of strenuous endurance or resistance training before trial participation. Furthermore, subjects gave their written informed consent before being enrolled in the experiment that were approved by the Copenhagen Ethics Committee (H-1-2010-007) and conformed to the Helsinki Declaration. Finally, subjects were instructed not to take any kind of analgesic medication at least 2 weeks before the beginning of the study.
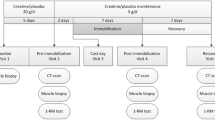
The experimental design is shown in Fig. 1. The present investigation was a part of another experiment that investigated the effect of ibuprofen treatment on muscle mass and strength adaptation during 2 weeks of lower limb immobilization and 6 weeks of subsequent retraining [11]. Moreover, the subject characteristic data of the included individuals have been used in previous studies [4, 11, 12], whereas all other presented data have not been reported previously.
Study intervention protocol. 18 older men completed 2 weeks of immobilization (2-week IM) and 2 weeks of retraining (2-week retrain) during which period they all ingested 2 × 20 g of whey protein per day. The participants were randomized into groups double blindly receiving either ibuprofen (n = 8) or placebo (n = 10) administration (2 × 600 mg/day). Measurements were performed at baseline, after 2-week IM, after the first retraining session (20-h retrain), and after 2 weeks of retraining (2-week retrain)
Ibuprofen treatment and protein supplementation
The included individuals were divided into 2 groups receiving either placebo (Plc, n = 10) or ibuprofen (Ibu, n = 8) in a randomized and double-blinded fashion. As previously described [11], the placebo group consists of 2 groups. The first group of 4 subjects has been included in a previous study [4] and received double-blinded placebo injections (placebo or growth hormone), whereas the remaining 6 subjects in the placebo group received placebo tablets in a double-blinded fashion (placebo or ibuprofen). All subjects completed the study intervention during the same time period.
The ibuprofen treatment started 2 days before the immobilization period to ensure that all subjects were fully treated from the onset of immobilization. The placebo tablets contained mainly potato starch and lactose monohydrate and were visually identical to the ibuprofen tablets. Moreover, all individuals were instructed to take their daily tablets (2 × 600 mg/day) at the same time every morning and evening together with a meal and to return the empty packages. Furthermore, subjects were not allowed to consume any cyclooxygenase-inhibiting drugs besides the tables provided during the study period.
The present study was carried out under free-living conditions, and all subjects were instructed to maintain their normal diets and to consume 2 × 20 g of whey protein (Lacprodan, Arla Foods Ingredients P/S, Viby J, Denmark) each day throughout the study period, as previously described [11]. This was done to ensure that eventual differences in myofibrillar FBR between groups would not be due to insufficient intake of protein and essential amino acids in one of the groups. Finally, both the ibuprofen treatment and the protein supplementation were initiated at baseline (day 70) and maintained for the rest of the study period.
Unilateral limb immobilization and retraining
The immobilization procedure has previously been described in detail and shown to induce substantial quadriceps muscle atrophy in both young and older individuals [4, 5]. Briefly, immobilization was accomplished by a lightweight fiber cast applied from the groin to just proximal to the malleoli (randomly selected limb). The cast was positioned at 50° of knee joint flexion to impede walking ability (0° corresponding to full extension). Furthermore, the subjects were carefully instructed to avoid any kind of quadriceps muscle contractions and to use crutches for locomotion. However, subjects were encouraged to remain physically active during the unilateral immobilization period, although it may cause them some difficulties. Moreover, all subjects were treated with acetylsalicylic acid (75 mg/day) during the 2-week immobilization period to reduce the potential risk of deep venous thrombosis. Even though acetylsalicylic acid is an NSAID, the anti-inflammatory effect of such a low dose was most likely negligible.
After removal of the cast, the subjects received supervised unilateral resistance training 3 times per week for 2 weeks (all individuals completed a total of 6 weeks of retraining, but only the 2 first weeks are included in the present investigation). After 5 min of warm-up on a stationary bike, the subjects performed knee extension and leg press in randomized order in training machines (Technogym, Gambettola, Italy). The training intensity and volume were 3–4 sets × 12 repetitions (15 repetitions maximum (RM)) in week 1 and 4 sets × 10 repetitions (12 RM) in week 2. The training load was adjusted each week by the use of 5 RM tests.
Measurements
At inclusion, the whole-body lean body mass (LBM) was determined by dual-energy X-ray absorptiometry scanning (Lunar DPX-IQ, GE Healthcare, Chalfont St. Giles, UK). During the scan, the individuals were wearing light clothing, no removable metal objects, and placed in a supine position. Thereafter (at day 0), subjects drank a total volume of 5.25-mL/kg LBM−1 of 99% 2H2O, which was diluted 1:1 with tap water and provided in 2–3 boluses over a 1-h period. Venous blood samples were taken right before and 2 h after the deuterated water bolus was consumed, and again at baseline (day 70), after immobilization (day 86), and after 2 weeks of retraining (day 100) in order to determine the deuterium enrichment of serum-free alanine. Blood samples were drawn from the antecubital vein into clot activator tubes, which were allowed to clot at room temperature for 30 min and followed by centrifugation (10 min at 3970 g at 4 °C). Serum was stored (− 80 °C) for analyses of 2H-alanine enrichment.
At baseline, after immobilization, and after 2 weeks of retraining, the subjects met at the lab, were placed in a supine position, and had a muscle biopsy taken from the vastus lateralis muscle using local anesthesia (lidocaine, 1%) and a 5-mm Bergström needle with suction. The muscle biopsies were cleared of external adipose tissue, connective tissue, and blood; frozen in liquid nitrogen; and stored at − 80 °C for subsequent analysis. The muscle biopsies were taken from the non-immobilized limb at baseline and from the immobilized limb after immobilization and 2 weeks of retraining. The biopsies from the immobilized limb were placed at least 3 cm apart to obtain samples unaffected by previous biopsies, and the order of location was randomized. Furthermore, these three biopsies were used for measurement of both muscle protein-bound abundances of 2H-alanine and muscle degradation signaling. The individuals refrained from strenuous physical activity 72 h prior to biopsy sampling at baseline (day 70), whereas prior retraining sessions were performed approximately 48 h before the biopsy obtained after 2 weeks of retraining (day 100). Additionally, a muscle biopsy was taken from the immobilized limb 20 h after the first retraining session (at day 87), with the purpose to measure the muscle degradation signaling response to acute retraining. At baseline and after immobilization, the biopsies were taken in the fasted state, whereas a light meal was ingested in the morning of the day of biopsy sampling after the first retraining session and after 2 weeks of retraining (ingested 2–3 h before the biopsy sampling). Finally, all muscle biopsies were collected at the same time of the day (± 1 h) to avoid effects of circadian variations on muscle degradation signaling.
Analyses
Serum-free alanine preparation
Serum 2H-alanine enrichment was measured in 200-μL serum. The samples were acidified with 1-mL 50% acidic acid and poured over resin columns (AG 50 W- × 8 resin; Bio-Rad Laboratories, Hercules, CA) preconditioned with 1-mL 50% acidic acid. After 5 washes with Millipore water, the purified amino acids were eluted with 2 × 1 mL of 2-M NH4OH. After being dried down under a stream of nitrogen, the purified amino acids were derivatized using MtBSTFA + 1% tBDMCS (Regis Technologies, Inc., Morton Grove, IL, USA) and acetonitrile with a volume relation of 1:1. The tBDMS derivatives of the amino acids were separated by gas chromatography (GC) on a 30-m CP-Sil 8 CB capillary column (ChromPack, Varian, Palo Alto, CA) using programmed temperature vaporization (PTV) mode and injecting approximately 1 μL. The alanine derivatives were analyzed by tandem mass spectrometry (MS) using a Thermo Scientific, TSQ Quantum GC-MS/MS (San Jose, CA, USA), operating in the electron ionization mode, as previously described [19].
Muscle myofibrillar protein-bound alanine preparation
Muscle specimens of 10–20-mg wet weight were homogenized (Fast-prep, 120A-230; Thermo Savant, Holbrook, NY, USA) for 2 × 45 s in 1.5 ml of ice cold Milli-Q saline water. After a spin (5 °C, 5.500 g, 10 min), the pellet was added 1 ml of homogenization buffer (0.02 M Tris, pH 7.4, 0.15 M NaCl, 2 mM EDTA, 0.5% Triton-X 100, and 0.25 M sucrose), homogenized for 2 × 45 s (Fast-prep), and left for 3 h at 5 °C. After a spin (5 °C, 800 g, 20 min), 1 ml of a homogenization buffer (0.02 M Tris, pH 7.4, 0.15-M NaCl, 2-mM EDTA, 0.5% Triton-X 100, and 0.25-M sucrose), homogenized for 45 s, and left for 30 min at 5 °C. After a spin (5 °C, 800 g, 20 min), the pellet was added 1.5 ml of high-salt solution (0.7-M KCl, 0.1-M pyrophosphate), homogenized (vortexed), and left overnight at 5 °C. After vortex and spinning (5 °C, 1600 g, 20 min), the supernatant (containing the myofibrillar fraction) was added 3.45-ml ethanol (99%), vortexed, and left for 2 h at 5 °C. After spinning (5 °C, 1600 g, 20 min), 1 ml of 70% ethanol was added to the pellet, which was vortexed and spun (5 °C, 1600 g, 20 min), where after the pellet was hydrolyzed overnight in 1 ml of 6 M HCl at 110 °C. The liberated amino acids were purified over resin columns (as described above for serum-free alanine) and N-acetyl-n-propyl (NAP)-derivatized. The 2H abundance in human myofibrillar proteins was analyzed on a gas chromatograph-pyrolysis-isotope ratio mass spectrometer (GC-P-IRMS) system (GC Combustion III, Delta Plus XL; Thermo Finnigan, Bremen, Germany). As previously described [18], the NAP-derivatized samples were injected in the PTV mode with the GC inlet initially set at 45 °C for 1 min, where after it was elevated to 280 °C with 20 °C/min. The GC column (30 m × 0.25-mm film thickness, 1 μm DB-1701; J&W Scientific) was ramped from 45 to 280 °C, and all GC column effluent passed into a high-temperature reduction reactor (1450 °C), where the organic compounds were reduced to hydrogen gas and carbon monoxide before entering the IRMS.
Calculation of muscle myofibrillar protein FBR
Paired samples were obtained, and we were able to determine the FBR of each individual’s muscle myofibrillar protein fraction. By log transformation of the measured enrichment values, the slope, kB, of the ln(E) versus time curve was determined as a gross mean between adjacent biopsy time points (16 days for immobilization and subsequently 14 days of retraining) [18].
Western blot analyses
Muscle samples of 10–25 mg were homogenized using a plastic pestle and fractionated into a cytosolic and nuclear fraction using a commercial fractionation kit (ProteoExtract Subcellular Proteome Extraction Kit, 539,790, Merck, Darmstadt, Germany) according to the manufacturer’s procedures. Subsequently, protein concentrations on the cytosolic and nuclear fractions were quantified using a commercial microplate kit (Bio-Rad DC protein microplate assay, 0113, 0114, 0115, Bio-Rad Laboratories, Hercules, CA, USA).
Equal amounts of protein were loaded per well (20–30 μg) and separated by 4–20% gradient Mini-PROTEAN TGX Stain-Free Precast protein gels (4,568,093, Bio-Rad Laboratories). Electrophoresis was performed under denaturized conditions for 40–45 min at 200 V in cold Tris/Glycine/SDS running buffer (161–0732, Bio-Rad Laboratories). After gel electrophoresis, proteins were transferred onto a PVDF-membrane at 100 V for 30 min (CriterionTM Blotter; Tris/Glycine buffer 161–0734, Bio-Rad Laboratories). Membranes were blocked at room temperature for 2 h in a TBS solution with 5% fat-free skimmed milk and 0.1% Tween 20 (TBS, 170–6435, Bio-Rad Laboratories; Tween-20, 437082Q, VWR International, Radnor, PA, USA; Skim milk, 1.15363.0500, Merck). Blocked membranes were incubated overnight at 4 °C with a primary antibody against ubiquitin (3933, Cell Signaling Technology, Danvers, MA, USA) diluted 1:1000. After incubation, membranes were washed and incubated at room temperature for 1 h with a secondary antibody diluted 1:3000 (7074, Cell Signaling Technology). After detection, membranes were stripped of primary and secondary antibodies using Restore Western Blot Stripping Buffer (21,059, Thermo Fisher Scientific, Rockford, IL, USA), blocked for 2 h at room temperature, and incubated at 4 °C overnight with antibodies against FoxO3a (2497, Cell Signaling Technology), NF-κB p65 (ab7970, Abcam, Cambridge, UK), and IκBα (ab32518, Abcam), diluted 1:1000, 1:500, and 1:400, respectively. Subsequently, membranes were incubated in secondary antibody (7074, Cell Signaling Technology). All antibodies were diluted in a 1% fat-free skimmed milk TBS solution added 0.1% Tween-20. Between stages, membranes were washed in 0.1% TBS-T. All samples were analyzed in duplicates, and bands were visualized using a HRP-detection system (Super Signal West Dura Extended Duration Substrate, 34,076, Thermo Fisher Scientific). Chemiluminescence was measured using a ChemiDoc MP System (Bio-Rad Laboratories), and band intensities were calculated with Image Lab (Bio-Rad Laboratories). The use of stain-free technology allowed normalization to tryptophan content in the membranes after transfer, as these gels have been added a 58-Da Trihalo compound that covalently binds to tryptophan residues in proteins when expressed to ultraviolet (UV) light.
Statistics
The sample size was calculated based on the hypothesis on the fractional breakdown rates. We estimated the relative difference in FBR between the two conditions as the following. In the immobilized and retraining states, we expected a reduction and increase of ~20%, respectively, from normal living/activity conditions. We used a value for normal living/activity conditions of 1.73 ± 0.49%/d (mean ± SD) [18], and hence, we estimated the sample size based on a power of 0.80, a significance level of 0.05, and a group difference of 0.7 ± 0.4%/d to a total of seven research participants in two independent groups.
Before statistical analysis, all data set were tested for normality. Data were analyzed using a two-way ANOVA with repeated measures and Student–Newman–Keuls post hoc tests were performed when significant overall effects were observed. Muscle protein signaling data were log transformed before the statistical analysis and presented as geometric means ± back-transformed SE. Data are reported as means ± standard error of mean (unless otherwise stated). Differences were considered significant when p < 0.05. The statistical software SigmaPlot v. 12.3 (Systat Software Inc., San Jose, CA, USA) was used for all statistical tests.
Results
Subject characteristic
Age, height, weight, and body mass index did not differ between groups. Body weight (kg) was 78 ± 3, 79 ± 3, and 79 ± 3 in the Plc group and 84 ± 3, 84 ± 4, and 84 ± 4 in the Ibu group at baseline, after immobilization, and after 2 weeks of retraining, respectively. For body weight, an interaction effect (p < 0.05) was observed, and the post hoc test indicated that body weight was higher (p < 0.05) after immobilization and 2 weeks of retraining in the Plc group, whereas it did not change in the Ibu group.
The subjects completed the immobilization and retraining periods without reporting any clinical problems. Subjects reported full compliance regarding the ibuprofen administration and protein intake, which was supported by measurements of blood ibuprofen concentration indicating that all subjects in the Ibu group took their medication regularly, as described previously [11]. Furthermore, the total training load and training intensity did not differ between the Ibu and the Plc group, as described previously [11].
Due to insufficient availability of muscle biopsy material, the myofibrillar FBR was measured in both the immobilization and retraining periods in 7 (of 8) and 6 (of 10) individuals in the Ibu and Plc groups, respectively. Further, at the time point of 20 h after the first retraining session, muscle degradation signaling could only be obtained in 6 (of 10) subjects in the Plc group and in 7 (of 8) subjects in the Ibu group. At all other time points, the muscle MPB signaling was measured in all the included individuals.
Serum 2H-alanine enrichment
An overall time effect (p < 0.001) was found for the serum-free 2H-alanine enrichment (Table 1). Two hours after ingestion of deuterated water (day 0), a marked 2H-alanine enrichment appeared in both groups (p < 0.001). At day 70 (start of immobilization period), 86 (end of immobilization), and 100 (end of retraining period), the 2H-alanine abundance had disappeared and was not different from zero (p > 0.05).
Muscle myofibrillar protein-bound 2H-alanine and fractional breakdown rate
An overall time effect (p < 0.01) was found for myofibrillar FBR (Fig. 2). The myofibrillar FBR was 1.61 ± 0.14%·d−1 during the immobilization period and 2.12 ± 0.34%·d−1 during the retraining period, in the Plc group. In the Ibu group, the myofibrillar protein FBR were 1.33 ± 0.23%·d−1 during the immobilization period and 2.27 ± 0.17%·d−1during the retraining period.
Muscle myofibrillar fractional breakdown rates (FBR) during 2 weeks of immobilization (• Immobilization) and 2 weeks of retraining (♦ Retraining). Data were analyzed with a two-way repeated measure ANOVA: A time effect (p < 0.01) was observed. Each dot represents the FBR of an individual, and connected dots are repeated measures during immobilization and retraining
Muscle protein breakdown signaling
Muscle protein concentrations of signaling targets related to MPB are shown in Figs. 3 and 4. The biopsies pre- and post-immobilization (Fig. 3) were taken during overnight fasting conditions, whereas a standardized meal was ingested in the morning before muscle biopsy sampling during retraining (Fig. 4). Thus, the protein expressions related to immobilization and retraining, respectively were analyzed in separate statistical tests.
a, b, c, d, e, and f. Muscle proteolysis signaling at baseline and after 2 weeks of immobilization (2-week IM). Data are expressed relative to baseline levels and presented as geometric means ± back-transformed SE. Log-transformed data were analyzed with a two-way repeated measure ANOVA. No group or interaction effects were observed (p > 0.05), but time effects appeared (p < 0.05). a Denote significant different from baseline (p < 0.05). Tendency (0.05 < p < 0.10) from ANOVA testing is shown within figure in upper right panel
a, b, c, d, e, and f. Muscle proteolysis signaling after the first retraining session (20-h retrain) and after 2 weeks of retraining (2-week retrain). Data are expressed relative to baseline levels and presented as geometric means ± back-transformed SE. Log-transformed data were analyzed with a two-way repeated measure ANOVA. Interaction effect was observed in f (p < 0.05), but no significant differences from subsequent post hoc testing were seen. No group effects were observed (p > 0.05), but time effects appeared (p < 0.05). b Denote significant different from 20-h retrain (p < 0.05). Tendency (0.05 < p < 0.10) from ANOVA testing is shown within the figure in upper right panel
Among all the investigated protein targets, no group effects were found (p > 0.05). An effect of group x time (interaction) was found for cytosolic ubiquitin expression (p < 0.05), but subsequent post hoc tests did not reveal any significant differences (Fig. 4f). However, overall time effects were identified (p < 0.05), revealing a difference during immobilization and retraining, respectively. Results from the post hoc tests are described in the following sections.
FoxO3a concentration
The protein expression of FoxO3a in the cytosolic subcellular fraction (Fig. 3a) decreased after immobilization. During retrain, no differences were seen between the first retraining session and after 2 weeks of retraining (Fig. 4a). The nuclear FoxO3a protein expression was unchanged after 2 weeks of immobilization (Fig. 3b) and was not affected by retraining (Fig. 4b).
NF-κB p65 concentration
The cytosolic NF-κB p65 protein expression was unchanged after immobilization (Fig. 3c), and not significantly affected during retraining (Fig. 4c), although a tendency toward an effect of time was seen in the latter condition (p = 0.074). After immobilization, the nuclear NF-κB p65 expression was unchanged (Fig. 3d), whereas after the first retraining session, nuclear NF-κB p65 expression was greater than after 2 weeks of retraining (Fig. 4d).
Cytosolic IκBα and ubiquitinated protein concentrations
The cytosolic IκBα protein expression remained unchanged throughout the study period (Fig. 3e and Fig. 4e). The cytosolic expression of ubiquitinated proteins was unchanged after immobilization (Fig. 3f). During retraining, an interaction effect was seen (Fig. 4f, p = 0.042). However, the post hoc tests could not reveal any significant differences.
Discussion
The primary finding was that the gross average myofibrillar breakdown rate was lower during 2 weeks of immobilization than during 2 weeks of subsequent retraining, whereas NSAID treatment had no impact on the myofibrillar breakdown rate. Moreover, muscle degradation signaling in the cytosolic and nuclear subcellular fractions differed between the measured time points, and correspondingly, the signaling was unaffected by NSAID treatment.
The present method of measuring muscle protein breakdown rate allowed us to determine a gross average over a 2-week period with the subjects staying in their usual living environments, hence reflecting real-life situations. Previously, the influence of physical inactivity on MPB has been measured in standardized laboratory setups [14, 42, 44]. However, to translate these acute “snap shot” measurements into the long-term changes taking place during periods of free-living muscle unloading the momentary measures of protein breakdown rates do not fully depict how changes in muscle protein breakdown rates underlines changes in muscle mass over time. The present method included both post-prandial and post-absorptive periods as well as periods of sleep, which makes it more representative of the actual myofibrillar breakdown rate during a given intervention and a better exploratory parameter for understanding the mechanisms underlying changes in muscle mass [11]. During the first 2 weeks of immobilization, fluctuations in the breakdown rate may have occurred [1, 44, 49]. A possible increase in MBP from the basal level during the initial days of muscle inactivity may likely have been counterbalanced by a later drop resulting in the reported average rate over the course of the 2-week measuring period [1]. Nevertheless, since no measurement of baseline MPB was included in the present study, it is not possible to say whether the mean MPB during immobilization actually differed from a normal free-living level.
Although it has been demonstrated that MPB increases in the hours/days of recovery following acute resistance exercise [3, 30], it is largely unknown if the mean MPB is similarly affected during longer periods of resistance training. In the present study, a limited number of subjects had the MPB measured (n = 7 and n = 6 in the Ibu and Plc groups, respectively), and therefore a risk of a type II error should be considered. However, in the two-way ANOVA test, the impact of NSAID appeared nonsignificant; hence, the condition comparison (immobilization versus retraining) becomes rather strong. Therefore, more power (N = 13) is present in that comparison, and the finding that the myofibrillar breakdown rate was higher during 2 weeks of unaccustomed rehabilitation resistance training compared to the immobilization period (Fig. 2) is rather strong. It was expected that the MPB would be higher during the retraining period compared to the muscle unloading period, because of the skeletal muscle remodeling processes taking place during early periods of resistance training [8, 9, 50]. Moreover, the present retraining period, following a period of muscle inactivity, could be expected to induce a more pronounced physiological impact than a 2-week period of resistance training applied to normally active muscles, due to the state of muscle atrophy combined with the abrupt and unaccustomed training sessions.
In the following section, we will discuss the findings during the immobilization and retraining periods, separately.
During immobilization, NSAID treatment did neither affect the loss of muscle mass [11], the MPB (Fig. 2), nor degradation signaling (Fig.3). These findings were in contrast to the study hypothesis. Previously, a muscle-protective effect of NSAIDs has been demonstrated in cancer patients and tumor-bearing mice challenged by muscle catabolism [10, 24, 26]. However, the muscle catabolism induced by cancer cachexia may likely differ from the muscle atrophy induced by simple limb immobilization in healthy, older humans. This may at least partly be due to differences in systemic levels of catabolic drivers, such as hypercortisolemia, hypercytokinemia, and insulin resistance that may directly stimulate MPB [1, 34]. Thus, inflammation and muscle degradation presumably plays a more prominent role in cancer-induced cachexia [28, 43], and thus, the underlying muscle-regulatory mechanisms and the effect of NSAIDs may not be directly comparable between cancer patients and healthy older individuals. Somehow in contrast though, dietary fish oil supplementation appears to reduce muscle degradation after 10 days of immobilization even in healthy adult rats [51]. This finding may be explained by the notion that muscle degradation contribute substantially more to the immobilization-induced muscle catabolism in rats than in humans [1, 32].
During retraining, the NSAID treatment did not significantly affect the muscle regrowth in the present study [11]. Previously, both fish oil and NSAID treatment have been shown to impair muscle recovery in rats and mice after periods of muscle inactivity [6, 52], which was associated with a decreased level of muscle inflammation [6]. However, even though muscle damage and infiltration of intramuscular inflammatory cells (e.g., macrophages or leucocytes) were not measured in the present study, it has been shown that NSAIDs do not influence the postexercise production of these inflammatory mediators in human muscle [29, 48]. Moreover, the present resistance training rehabilitation protocol is not comparable to the “normal ambulatory activity” used for muscle recovery in these animal studies [6, 52].
In the present study, protein expression of different signaling molecules involved in muscle protein degradation and inflammation was measured in both the cytosolic and nuclear subcellular fractions (Figs. 3 and 4). The exploration of both breakdown rates by tracer methodology and intramuscular signaling allows a better understanding of the processes and helps the interpretation of the obtained individual results. A general observation was that NSAIDs did not affect the measured signaling molecules, which is in line with the finding that the MPB rate was unaffected by NSAIDs.
Given that inactivity and bed rest have been shown to increase systemic inflammation [7, 22], we hypothesized that NF-kB signaling would increase after 2 weeks of immobilization and that this response would be attenuated by NSAID treatment. However, no changes in cytosolic or nuclear NF-kB p65 were observed in any of the groups, suggesting that local inflammation was not increased by the unilateral leg immobilization. The latter was supported by unchanged IKB-alpha levels. In the absence of an inflammatory response in the placebo group, it is not surprising that NF-kB signaling was unaffected by NSAID treatment. However, we cannot exclude the possibility that an inflammatory response occurred in the initial phase of the 2-week immobilization period.
FoxO3 has been shown to regulate the two major systems of protein breakdown in skeletal muscle, namely, the ubiquitin-proteasome system and the autophagy-lysosomal pathway, through transcription of genes such as MAFbx/atrogin-1, MuRF1, LC3B, and BNIP3 [23, 39]. In a situation of increased muscle protein breakdown, one might therefore expect increased translocation of FoxO3a [17], reflected by decreased cytosolic and increased nuclear levels. Indeed, cytosolic FoxO3a was reduced following 2 weeks of immobilization, but this did not coincide with increased nuclear levels (no change). Accordingly, the cytosolic reduction likely represented attenuated de novo synthesis of FoxO3a. The latter is supported by previous observations of reduced gene expression of FoxO3 following 4 days of immobilization in both young and old men [40]. Although the present reduction in cytosolic FoxO3a immediately after immobilization is somewhat in agreement with the lower myofibrillar breakdown rate during immobilization than during retraining, it should be noted that muscle protein signaling molecules only represent an instant picture of the muscle protein turnover rate at any given time point, which opposites the measured mean MPB rate. Therefore, the measured muscle protein signaling molecules may not necessarily be tightly coupled to the actual muscle protein turnover rate at a given time point.
Ubiquitination of cytosolic proteins, an estimate of the amount of proteins marked for degradation [37], was unchanged in both groups following immobilization. Previously, increased ubiquitination has been observed after 2, but not 14 days of disuse in young individuals [15]. Regardless, results for ubiquitination should be interpreted with care, considering that both the rate of protein ubiquitination and the rate of proteasomal degradation determine the net amount of ubiquitinated proteins at any given time point. Nevertheless, although it only represents an immediate “snap shot,” the measurement of ubiquitinated protein indicates that MPB was not increased from baseline levels after the immobilization period in the present study.
In untrained individuals, resistance exercise causes an inflammatory response [47], as well as increased MPB rates [31]. It has been shown previously that NSAIDs may inhibit training induced increases in gene expression of IL-6 and MuRF-1 in elderly [46]. Therefore, we investigated the effect of NSAIDS on pro-inflammatory signaling and markers of proteolysis in the early and late phase of retraining in the present study. Protein levels of nuclear NF-kB p65 were higher after the first, compared to the last retraining session, and a similar tendency was observed in the cytosolic fraction. This indicates a more pronounced inflammatory response early in the retraining period, when subjects are unfamiliar to the training stimulus. Nevertheless, no effect of NSAIDS was observed, neither on NF-kB, IKB alpha, ubiquitination, nor FoxO3a, in line with the observation that neither FBR nor hypertrophy was affected by the ibuprofen treatment.
Conclusion
The present study measured the average skeletal muscle myofibrillar breakdown rate in older individuals during daily life periods of immobilization and subsequent rehabilitation training using the deuterated (2H2O) water–based methodology. The myofibrillar breakdown was lower during immobilization than during retraining but was not affected by NSAID treatment in any of the periods. Moreover, the protein expression of different signaling molecules related to myofibrillar degradation in the cytosolic and nuclear subcellular fractions differed somewhat over the course of the intervention but was likewise unaffected by NSAID treatment.
References
Atherton PJ, Greenhaff PL, Phillips SM, Bodine SC, Adams CM, Lang CH (2016) Control of skeletal muscle atrophy in response to disuse: clinical/preclinical contentions and fallacies of evidence. Am J Physiol Endocrinol Metab 311:E594–E604
Bemelmans WJE, Lefrandt JD, Feskens EJM, Van Haelst P, Broer J, Meyboom-de Jong B, May JF, Tervaert JC, Smit AJ (2004) Increased α-linolenic acid intake lowers C-reactive protein, but has no effect on markers of atherosclerosis. Eur J Clin Nutr 58:1083–1089
Biolo G, Maggi S, Williams B, Tipton K, Wolfe R (1995) Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol Endocrinol Metab 268:E514–E520
Boesen AP, Dideriksen K, Couppé C, Magnusson SP, Schjerling P, Boesen M, Aagaard P, Kjaer M, Langberg H (2014) Effect of growth hormone on aging connective tissue in muscle and tendon: gene expression, morphology, and function following immobilization and rehabilitation. J Appl Physiol 116:192–203
Boesen AP, Dideriksen K, Couppé C, Magnusson SP, Schjerling P, Boesen M, Kjaer M, Langberg H (2013) Tendon and skeletal muscle matrix gene expression and functional responses to immobilisation and rehabilitation in young males: effect of growth hormone administration. J Physiol 23:6039–6052
Bondesen BA, Mills ST, Pavlath GK (2006) The COX-2 pathway regulates growth of atrophied muscle via multiple mechanisms. Am J Phys Cell Phys 290:C1651–C1659
Breen L, Stokes KA, Churchward-Venne T, Moore DR, Baker SK, Smith K, Atherton PJ, Phillips SM (2013) Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab 98:2604–2612
Brook MS, Wilkinson D, Mitchell WK, Lund JN, Szewczyk NJ, Greenhaff P, Smith K, Atherton PJ (2015) Skeletal muscle hypertrophy adaptations predominate in the early stages of resistance exercise training, matching deuterium oxide-derived measures of muscle protein synthesis and mechanistic target of rapamycin complex 1 signaling. FASEB J 29:4485–4496
Damas F, Phillips SM, Libardi CA, Vechin FC, Lixandrão ME, Jannig PR, Costa LAR, Bacurau AV, Snijders T, Parise G, Tricoli V, Roschel H, Ugrinowitsch C (2016) Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. J Physiol 58:7250–7257
Davis T, Zweifel B, O’Neal J, Heuvelman D, Abegg A, Hendrich T, Masferrer J (2004) Inhibition of cyclooxygenase-2 by celecoxib reverses tumor-induced wasting. J Pharmacol Exp Ther 308:929–934
Dideriksen K, Boesen AP, Kristiansen JF, Magnusson SP, Schjerling P, Holm L, Kjaer M (2016) Skeletal muscle adaptation to immobilization and subsequent retraining in elderly men: no effect of anti-inflammatory medication. Exp Gerontol 82:8–18
Dideriksen K, Boesen AP, Reitelseder S, Couppé C, Svensson R, Schjerling P, Magnusson SP, Holm L, Kjaer M (2017) Tendon collagen synthesis declines with immobilization in elderly humans: no effect of anti-inflammatory medication. J Appl Physiol 122:273–282
Drummond MJ, Dickinson JM, Fry CS, Walker DK, Gundermann DM, Reidy PT, Timmerman KL, Markofski MM, Paddon-Jones D, Rasmussen BB, Volpi E (2012) Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab 302:E1113–E1122
Ferrando A, Lane HW, Stuart CA, Davis-Street J, Wolfe RR (1996) Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Phys 270:E627–E633
Glover E, Yasuda N, Tarnopolsky MA, Abadi A, Phillips SM (2010) Little change in markers of protein breakdown and oxidative stress in humans in immobilization-induced skeletal muscle atrophy. Appl Physiol Nutr Metab 35:125–133
He K, Liu K, Daviglus ML, Jenny NS, Mayer-Davis E, Jiang R, Steffen L, Siscovick D, Tsai M, Herrington D (2009) Associations of dietary long-chain n-3 polyunsaturated fatty acids and fish with biomarkers of inflammation and endothelial activation (from the multi-ethnic study of atherosclerosis [MESA]). Am J Cardiol 103:1238–1243
van der Heide LP, Hoekman MFM, Smidt MP (2004) The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J 380:297–309
Holm L, O’Rourke B, Ebenstein D, Toth MJ, Bechshoeft R, Holstein-Rathlou N-H, Kjaer M, Matthews DE (2013) Determination of steady-state protein breakdown rate in vivo by the disappearance of protein-bound tracer-labeled amino acids: a method applicable in humans. Am J Physiol Endocrinol Metab 304:E895–E907
Holm L, Reitelseder S, Dideriksen K, Nielsen RH, Bülow J, Kjaer M (2014) The single-biopsy approach in determining protein synthesis in human slow-turning-over tissue: use of flood-primed, continuous infusion of amino acid tracers. Am J Physiol Endocrinol Metab 306:E1330–E1339
Hoppeler H (2016) Molecular networks in skeletal muscle plasticity. J Exp Biol 219:205–213
Jackman RW, Cornwell EW, Wu C-L, Kandarian SC (2013) Nuclear factor-κB signalling and transcriptional regulation in skeletal muscle atrophy. Exp Physiol 98:19–24
Jurdana M, Jenko-Pražnikar Z, Mohorko N, Petelin A, Jakus T, Šimunič B, Pišot R (2015) Impact of 14-day bed rest on serum adipokines and low-grade inflammation in younger and older adults. Age (Dordr) 37:116
Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M (2007) FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6:458–471
Mantovani G, Macciò A, Madeddu C, Serpe R, Antoni G, Massa E, Dessì M, Panzone F (2010) Phase II nonrandomized study of the efficacy and safety of COX-2 inhibitor celecoxib on patients with cancer cachexia. J Mol Med 88:85–92
Markworth JF, Vella L, Figueiredo VC, Cameron-Smith D (2014) Ibuprofen treatment blunts early translational signaling responses in human skeletal muscle following resistance exercise. J Appl Physiol 117:20–28
McCarthy DO, Whitney P, Hitt A, Al-Majid S (2004) Indomethacin and ibuprofen preserve gastrocnemius muscle mass in mice bearing the colon-26 adenocarcinoma. Res Nurs Health 27:174–184
McFarlane C, Plummer E, Thomas M, Hennebry A, Ashby M, Ling N, Smith H, Sharma M, Kambadur R (2006) Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism. J Cell Physiol 209:501–514
Op den Kamp CM, Langen RC, Snepvangers FJ, De TCC, Schellekens JM, Laugs F, Dingemans AC, Schols AM (2013) Nuclear transcription factor k B activation and protein turnover adaptations in skeletal muscle of patients with progressive stages of lung cancer cachexia. Am J Clin Nutr 98:738–748
Peterson JM, Trappe TA, Mylona E, White F, Lambert CP, Evans WJ, Pizza FX (2003) Ibuprofen and acetaminophen: effect on muscle inflammation after eccentric exercise. Med Sci Sports Exerc 35:892–896
Phillips SM, Tipton K, Aarsland A, Wolf SE, Wolfe RR (1997) Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab 273:E99–E107
Phillips SM, Tipton K, Ferrando A, Wolfe RR (1999) Resistance training reduces the acute exercise-induced increase in muscle protein turnover. [online]. Am J Phys 276:E118–E124 http://www.ncbi.nlm.nih.gov/pubmed/9886957
Rennie MJ, Selby A, Atherton PJ, Smith K, Kumar V, Glover E, Phillips SM (2010) Facts, noise and wishful thinking: muscle protein turnover in aging and human disuse atrophy. Scand J Med Sci Sports 20:5–9
Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Peyron MA, Combaret L, Dardevet D (2009) Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol 587:5483–5492
Rudrappa SS, Wilkinson D, Greenhaff PL, Smith K, Idris I, Atherton PJ (2016) Human skeletal muscle disuse atrophy: effects on muscle protein synthesis, breakdown, and insulin resistance—a qualitative review. Front Physiol 7:361
Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL (2004) IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab 287:E591–E601
Sandri M (2008) Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda) 23:160–170
Shaid S, Brandts CH, Serve H, Dikic I (2013) Ubiquitination and selective autophagy. Cell Death Differ 20:21–30
Standley RA, Liu SZ, Jemiolo B, Trappe SW, Trappe TA (2013) Prostaglandin E2 induces transcription of skeletal muscle mass regulators interleukin-6 and muscle RING finger-1 in humans. Prostaglandins Leukot Essent Fat Acids 88:361–364
Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14:395–403
Suetta C, Frandsen U, Jensen L, Jensen MM, Jespersen JG, Hvid LG, Bayer ML, Petersson SJ, Schrøder HD, Andersen J, Heinemeier KM, Aagaard P, Schjerling P, Kjaer M (2012) Aging affects the transcriptional regulation of human skeletal muscle disuse atrophy. PLoS One 7:e51238
Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P (2009) Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol 107:1172–1180
Symons T, Sheffield-Moore M, Chinkes DL, Ferrando A, Paddon-Jones D (2009) Artificial gravity maintains skeletal muscle protein synthesis during 21 days of simulated microgravity. J Appl Physiol 107:34–38
Tardif N, Klaude M, Lundell L, Thorell A, Rooyackers O (2013) Autophagic-lysosomal pathway is the main proteolytic system modified in the skeletal muscle of esophageal cancer patients1-3. Am J Clin Nutr 98:1485–1492
Tesch PA, von Walden F, Gustafsson T, Linnehan RM, Trappe TA (2008) Skeletal muscle proteolysis in response to short-term unloading in humans. J Appl Physiol 105:902–906
Trappe TA, Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Sullivan BE, Lee JD, Jemiolo B, Weinheimer EM, Hollon CJ (2011) Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Phys Regul Integr Comp Phys 300:R655–R662
Trappe TA, Standley RA, Jemiolo B, Carroll CC, Trappe SW (2013) Prostaglandin and myokine involvement in the cyclooxygenase-inhibiting drug enhancement of skeletal muscle adaptations to resistance exercise in older adults. Am J Phys Regul Integr Comp Phys 304:R198–R205
Vella L, Caldow MK, Larsen AE, Tassoni D, Della Gatta PA, Gran P, Russell AP, Cameron-Smith D (2012) Resistance exercise increases NF-κB activity in human skeletal muscle. Am J Phys Regul Integr Comp Phys 302:R667–R673
Vella L, Markworth JF, Peake J, Snow RJ, Cameron-Smith D, Russell AP (2014) Ibuprofen supplementation and its effects on NF-κB activation in skeletal muscle following resistance exercise. Phys Rep 2:1–11
Wall BT, Dirks M, van Loon LJC (2013) Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev 12:898–906
Wilkinson D, Franchi MV, Brook MS, Narici MV, Williams JP, Mitchell WK, Szewczyk NJ, Greenhaff P, Atherton PJ, Smith K (2014) A validation of the application of D2O stable isotope tracer techniques for monitoring day-to-day changes in muscle protein subfraction synthesis in humans. Am J Physiol Endocrinol Metab 306:E571–E579
You J-S, Park M-N, Song W, Lee Y-S (2010) Dietary fish oil alleviates soleus atrophy during immobilization in association with Akt signaling to p70s6k and E3 ubiquitin ligases in rats. Appl Physiol Nutr Metab 35:310–318
You J-S, Park MN, Lee YS (2010) Dietary fish oil inhibits the early stage of recovery of atrophied soleus muscle in rats via Akt-p70s6k signaling and PGF2α. J Nutr Biochem 21:929–934
Acknowledgments
We wish to express our gratitude to the subjects for their time and commitment to the study. Ann-Marie Sedstrøm and Ann-Christina Reimann are thanked for their expert technical assistance.
Funding
Funding is greatly acknowledged from Danish Dairy Research Foundation, Danish Council for Independent Research (09–073587), and Center for Healthy Aging (Nordea Foundation). Arla Foods Ingredients P/S provided the Lacprodan whey protein. The funding sources were not involved in the preparation or completion of the study or in the writing of the manuscript.
Author information
Authors and Affiliations
Contributions
K.D. and L.H. designed the study; K.D., A.P.B., and L.H. conducted the experimental work; K.D., S.R., J.A., S.N.A., T.R., and L.H. analyzed and interpreted the data; K.D. drafted the manuscript; K.D., S.R., J.A., A.P.B., S.N.A., T.R., and L.H. edited and revised the manuscript. All authors approved the final content of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the special issue on Exercise Physiology: future opportunities and challenges in Pflügers Archiv—European Journal of Physiology
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dideriksen, K., Reitelseder, S., Agergaard, J. et al. Muscle protein breakdown is impaired during immobilization compared to during a subsequent retraining period in older men: no effect of anti-inflammatory medication. Pflugers Arch - Eur J Physiol 472, 281–292 (2020). https://doi.org/10.1007/s00424-020-02353-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-020-02353-w