Abstract
Purpose
Regular resistance exercise training and a balanced diet may counteract the age-related muscular decline on a molecular level. The aim of this study was to investigate the influence of elastic band resistance training and nutritional supplementation on circulating muscle growth and degradation factors, physical performance and muscle quality (MQ) of institutionalized elderly.
Methods
Within the Vienna Active Ageing Study, 91 women aged 83.6 (65.0–92.2) years were randomly assigned to one of the three intervention groups (RT, resistance training; RTS, resistance training plus nutritional supplementation; CT, cognitive training). Circulating levels of myostatin, activin A, follistatin, IGF-1 and GDF-15, as well as MQ and functional parameters were tested at baseline as well as after 3 and 6 months of intervention.
Results
MQ of lower extremities significantly increased in the RT group (+14 %) and RTS group (+12 %) after 6 months. Performance improved in the RT and RTS groups for chair stand test (RT: +18 %; RTS: +15 %). Follistatin increased only in the RT group (+18 %) in the latter phase of the intervention, accompanied by a decrease in the activin A-to-follistatin ratio (−7 %). IGF-1, myostatin and GDF-15 levels were not affected by the intervention.
Conclusion
Our data confirm that strength training improves physical performance and MQ even in very old institutionalized women. This amelioration appears to be mediated by blocking muscle degradation pathways via follistatin rather than inducing muscle growth through the IGF-1 pathway. As plasma levels of biomarkers reflect an overall status of various organ systems, future studies of tissue levels are suggested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Muscle weakness induced by old age is associated with a higher risk for functional impairment and loss of independence (Rantanen et al. 2002), falls (Scott et al. 2014) and even mortality (Metter et al. 2002; Rantanen et al. 2003). Although skeletal muscle mass correlates with skeletal muscle strength (Chen et al. 2013), strength and power decline more rapidly than muscle mass (Goodpaster et al. 2006). Therefore, determination of both muscle mass and strength are necessary for the assessment of sarcopenia (Cruz-Jentoft et al. 2010). More recently, the term muscle quality (MQ) has been suggested in the clinical setting to describe muscle strength or power per unit of muscle mass (Barbat-Artigas et al. 2012b). In this respect, MQ can be regarded as a marker of muscle efficiency. Kennis et al. (2014) showed that although the quantity of muscle even increased in middle-aged men within a period of 9.5 years, their strength- and power-generating capacity strongly declined resulting in a loss of MQ of 1.5–2.4 % per year. Several studies reveal that strength training programmes improve MQ even in elderly (Fragala et al. 2014; Radaelli et al. 2014; Ring-Dimitriou et al. 2009), but general knowledge about the association of MQ with biochemical markers of muscle growth and degradation is scarce.
Skeletal muscle is a highly malleable tissue, whereby muscle mass is determined by a fine-tuned network of muscle growth and degradation pathways. While the activation of the phosphoinositide 3-kinase (PI3K)/Akt pathway by insulin-like growth factor-1 (IGF-1) induces muscle hypertrophy, its inhibition by myostatin, a member of the transforming growth factor-β (TGF-β) family, generally leads to muscle atrophy and inhibits muscle differentiation (Glass 2010). Also other TGF-β family members such as activin A and growth differentiation factor-15 (GDF-15) seem to have a negative impact on skeletal muscle growth (Bloch et al. 2015; Han et al. 2013).
With these aspects in mind it is not surprising that many of these molecules are suggested as blood-based biomarkers for the clinical determination of sarcopenia (Kalinkovich and Livshits 2015). In older women, IGF-1 correlates negatively with age and positively with muscle mass while GDF-15 is positively associated with age and negatively with muscle mass (Hofmann et al. 2015). Contrasting results have been detected for myostatin levels which are negatively correlated to muscle mass in male patients with chronic obstructive pulmonary disease (Ju and Chen 2012) and to handgrip strength in hemodialysis patients (Han et al. 2011). For healthy individuals data are still inconsistent as some studies did not detect any association with lean body mass in old men (Lakshman et al. 2009) and women (Hofmann et al. 2015), while others have found an inverse correlation of circulating myostatin with fat free mass and muscle mass (Yarasheski et al. 2002). As blocking myostatin using antibodies has been shown to beneficially affect muscle mass and grip strength in mice (Whittemore et al. 2003) and lean body mass and some functional parameters in old weak persons (Becker et al. 2015) it is not surprising that myostatin is consistently included in any suggested set of biomarkers for sarcopenia (Kalinkovich and Livshits 2015).
Results of the Vienna Active Ageing Study (VAAS) previously demonstrated that resistance training using elastic bands for 6 months led to an increase in functional performance of lower and upper extremities, and improved genome stability and resistance against DNA damage of very old adults (Franzke et al. 2015a; Oesen et al. 2015). However, the supplementation of a drink rich in proteins, vitamin D, B2, and B12 had no additional effect on functional performance (Oesen et al. 2015), but reduced chromosomal damage (Franzke et al. 2015b).
Using these findings as a starting point, the aim of the current study was to investigate whether this type of training and nutritional supplementation affects MQ as well as serum markers involved in muscle growth and degradation in elderly women (IGF-1, myostatin, follistatin, GDF-15, and activin A).
Methods
Study design
VAAS was a randomized, controlled intervention study which was designed to test whether 6 months of a supervised, progressive resistance exercise training using elastic bands with and without nutrient supplementation were able to influence physical fitness of institutionalized elderly men and women (Oesen et al. 2015). Eligible participants were randomly, but stratified by gender, assigned to one of the three intervention groups: resistance exercise training (RT), RT plus nutritional supplementation (RTS), or cognitive training (CT), the latter serving as control group. The interventions started immediately following the baseline tests (T1), which were repeated after 3 (T2) and 6 months (T3).
Subjects
Inclusion and exclusion criteria have been described previously (Oesen et al. 2015). Briefly, the participants were untrained, over 65 years old and free of any medical condition which would impair their participation in a resistance training class. Mini-mental state scores were ≥22 (Folstein et al. 1975). Written informed consent was obtained from all participants. The present study was conducted in accordance to the Austrian laws (including Doctors Act, CISA, Data Protection Act), the Declaration of Helsinki (as revised in Edinburgh 2000), and in analogous accordance with ICH-GCP guidelines. The study has been approved by the ethics committee of the City of Vienna (EK-11-151-0811) and registered at ClinicalTrials.gov (NCT01775111).
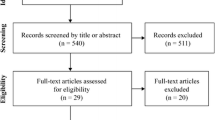
From a total of 117 participants of the VAAS, only those were initially included in the current study where data on MQ and serum markers were available (n = 104). Due to the low sample size of men (n = 13) these were additionally excluded from the presented analyses. Blood samples were available from all women (n = 91) at baseline (T1), from 76 women after 3 months (T2), and from 70 women after 6 months (T3). Additionally, MQ of both upper and lower extremities could be assessed from 77 women at T1, from 68 women at T2, and from 59 women at T3 (Fig. 1).
Interventions
All intervention groups met twice a week for about 60 min in small groups of not more than 10 people. The groups were supervised by experienced sports scientists. The resistance exercise programme was designed to train all major muscle groups based on guidelines provided by the American College of Sports Medicine (ACSM) for older adults (Nelson et al. 2007). Following an adaptation phase of 4 weeks using low external resistance (1 set of 15 repetitions per exercise, yellow Thera-Band® (The Hygenic Corporation, Akron, OH, USA), exercise intensity was progressively increased by adapting the resistance of the elastic band (based on the Thera-Band® force–elongation table) (Page and Ellenbecker 2011) from yellow to red and further to black. The exercise volume was further enhanced by increasing the number of sets from one to two. The RTS group followed the same training protocol as the RT group. Additionally, a nutrient supplement drink (FortiFit, NUTRICIA GmbH, Vienna, Austria) was provided every morning after breakfast, as well as immediately after each training session. Each drink supplied a total energy of 150 kcal and contained 20.7 g protein (3 g leucine, >10 g essential amino acids), 9.3 g carbohydrates, 3 g fat, vitamins (800 IU vitamin D, 2.9 mg vitamin B6, 3 μg vitamin B12) and minerals. A research dietician distributed the supplements and monitored adherence. Participants were instructed to maintain their regular food intake. The CT group served as control participating in activities including cognitive tasks (memory training) and coordinative tasks (such as manual dexterity) twice weekly to provide a timely effort which was equal to those of the RT and RTS group (Oesen et al. 2015).
Anthropometrical measurements
Standing height was measured without shoes to the nearest 0.5 cm using a commercial stadiometer (Seca, Hamburg, Germany) with the shoulders kept in a relaxed position and arms allowed to hang freely. Body mass was evaluated with a digital scale (BWB 700, Tanita, Amsterdam, Netherlands) to the nearest 0.1 kg with subjects lightly dressed and barefoot. Body mass index (BMI) was calculated as the ratio between the weight (kg) and height squared (m). To determine body composition bioelectric impedance analysis (BIA) was used, which has been shown to provide reliable data of body composition in comparison to dual-energy X-ray absorptiometry (DXA) (Roubenoff et al. 1997). BIA was performed in the morning after an overnight fast using a BIA Analyzer 2000-S (Data-Input GmbH, Darmstadt, Germany). Skeletal muscle mass was determined using the equation of Janssen et al. (2000): Skeletal muscle mass (kg) = [height2/R × 0.401) + (gender × 3.825)–(age × 0.071)] + 5.102, where height is measured in centimeters; R is BIA resistance in ohms; for gender, men = 1 and women = 0; and age is in years.
Physical performance
Chair stand test
Chair stand performance is influenced by strength and power of the lower extremities. For this test the maximum number of completed cycles of unsupported chair rises (from a seated to a fully erected position (hip and knees straightened) completed within 30 s was counted. A straight-back chair with a seat height of 46 cm was placed against a wall for support and safety purposes. Participants performed a 2–3 repetition practice trial to familiarize with the technique. They were instructed to keep their arms crossed at the wrists and held them against the chest and place their feet flat on the floor approximately shoulder-width apart. The number of stands executed correctly within 30 s were counted by the tester and used for data analyses (Oesen et al. 2015; Rikli and Jones 2013).
Handgrip strength
Handgrip strength of the right hand was measured to the nearest kilogram (kg) using a Jamar hand dynamometer (Sammsons Preston, Inc. Bolingbrook IL, USA). Subjects were seated with their elbow unsupported and bent at an angle of 90°. Prior to data collection, the width of the dynamometer handle was adjusted to the individual hand size and participants performed two sub maximal trials to get acquainted with the instrument and measurement procedure. Finally, participants were encouraged to perform a maximal contraction within approximately 4–5 s. After a rest of 60 s, participants were asked to perform a second trial. The highest score of maximum voluntary contraction was used for data analyses (Mijnarends et al. 2013; Oesen et al. 2015).
Muscle quality
MQ was determined as the ratio of muscle strength or power and muscle mass as suggested by Barbat-Artigas et al. (2012b). The MQ score of upper extremities was calculated based on handgrip strength measured by hand dynamometer (kg) divided by muscle mass calculated using the BIA equation of Janssen et al. (2000). To calculate power for MQ of lower extremities the equation for peak power (W) [−715.218 + 13.915 × body weight (kg) + 33.425 × stand in 20 s] of Smith et al. (2010) predicting lower-body muscle power in older adults using 30-s chair stand test was used. The calculated power was then divided by muscle mass.
Serum parameters
Venous blood samples were taken in the morning between 06:30 and 08:00 after an overnight fast. Z Serum Sep Clot Activator collection tubes (Vacuette, Kremsmünster, Austria) were used to obtain about 8 ml of venous blood. Between 30 and 90 min after collection, the tubes were centrifuged (10 min, 3000×g) and the obtained serum was stored in aliquots at −80 °C until further analysis. Commercial enzyme-linked immunosorbent assays for myostatin (Immundiagnostik, Bensheim, Germany, K1012), follistatin (R&D Systems, Abingdon, UK, DFN00), activin A (R&D Systems, Abingdon, UK, DY338), GDF-15 (R&D Systems, Abingdon, UK, DY957) and IGF-1 (Mediagnost, Reutlingen, Germany, E20) were performed. The analyses were carried out according to the manufacturers’ protocols and on a 1420 Multilabel Counter (Victor3, Perkin Elmer, Waltham, MA, USA).
Statistical analysis
Statistical analyses were performed with IBM SPSS Statistics 22 (IBM Corporation, New York, USA). The number of participants chosen for the VAAS was based on power estimation (G*Power 3.1.0), which estimated the sample size to be 86 using isokinetic peak torque as primary endpoint (Faul et al. 2007; Oesen et al. 2015). Although men were excluded for this secondary analysis, a total of 91 women participated in the current study still representing a higher sample size as anticipated.
For all secondary endpoints as included into the current analyses Shapiro–Wilk test was used to check for normal distribution which was violated for most of the blood-based parameters. Therefore, differences between groups at baseline were determined by Kruskal–Wallis test followed by Bonferroni-corrected Mann–Whitney tests for post hoc analyses. To analyse for time effects, Friedman tests were applied and if significant followed by Wilcoxon tests with Bonferroni corrections. Effect sizes (r) were calculated by dividing the standardized test statistics (z score) by the square root of the total observations. Potential correlations between functional parameters and blood-based biomarkers were assessed using Spearman’s rank correlation coefficient. Data are shown as median (minimum–maximum) and a p value of less than 0.05 was considered significant.
Results
Baseline characteristics
The women (n = 91) which were included in the current analysis were distributed nearly equally between intervention groups (CT: 33 %, RT: 36 %, RTS: 31 %). At baseline the intervention groups did not differ in age, weight, BMI, muscle mass, fat mass and MMST (p > 0.05). Comparisons between groups revealed differences for MQ of upper extremities [H(2) = 6.81, p = 0.033] and IGF-1 [H(2) = 6.71, p = 0.035] at baseline. However, pairwise comparisons with adjusted p values have confirmed a difference just for MQ of upper extremities which was slightly higher in the RT group as compared to the CT group (+24 %, p = 0.029, r = 0.326) (Table 1).
Intervention effects
Physical function and skeletal muscle mass
Strength of lower extremities as assessed by chair stand test increased significantly over time in both strength training groups [RT: χ 2(2) = 10.634, p = 0.005; RTS: χ 2(2) = 9.973, p = 0.007] while performance in chair stand test was unchanged in CT [χ 2(2) = 0.237, p = 0.888]. To follow up these findings Wilcoxon tests with Bonferroni corrections were used. Significant improvements were detected between T1 and T2 (RT: +17 %, r = −0.357, p = 0.026) and T1 and T3 (RT: +18 %, r = −0.407, p = 0.010; RTS: +15 %, r = −0.464, p = 0.008). These changes in functional performance were paralleled by ameliorations in power as calculated from chair stand test using the formula by Smith et al. (2010) [CT: χ 2(2) = 1.059, p = 0.915; RT: χ 2(2) = 6.000, p = 0.050; RTS: χ 2(2) = 10.000, p = 0.007]. However, significant changes in post hoc analyses were detected only for the RTS group between T1 and T3 (+ 18 %, r = −0.488, p = 0.005) (data not shown).
Skeletal muscle mass [CT: χ 2(2) = 1.059, p = 0.589; RT: χ 2(2) = 0.900, p = 0.638; RTS: χ 2(2) = 4.000, p = 0.135] and handgrip strength [CT: χ 2(2) = 0.295, p = 0.863; RT: χ 2(2) = 3.455, p = 0.178; RTS: χ 2(2) = 0.295, p = 0.824] did not change over 6 months of intervention in any of the intervention groups (data not shown).
Muscle quality
MQ of lower extremities changed significantly over time in the RT group [χ 2(2) = 10.300, p = 0.006] and the RTS group [χ 2(2) = 8.444, p = 0.015] but not in CT [χ 2(2) = 0.118, p = 0.943]. Bonferroni-corrected post hoc analyses revealed a significant increase from T1 to T3 in RT (+14 %, r = −0.435, p = 0.005), whereas differences in the RTS group were detected only between T1 and T2 (+12 %, r = −0.377, p = 0.023). MQ of upper extremities was not influenced by any of the interventions [CT: χ 2(2) = 4.308, p = 0.116; RT: χ 2(2) = 1.600, p = 0.449; RTS: χ 2(2) = 2.333, p = 0.311] (Table 2).
Blood-based parameters
Overall analyses for positive regulators of muscle mass revealed significant time effects for follistatin in the RT group [χ 2(2) = 9.750, p = 0.008] while in the other intervention groups follistatin was unaffected [CT: χ 2(2) = 4.957, p = 0.084; RTS: χ 2(2) = 0.667, p = 0.717]. Post hoc analysis for the RT group observed that follistatin increased in the latter phase of intervention between T2 and T3 (+18 %, r = −0.420, p = 0.007). Another positive regulator of muscle mass, IGF-1, was not altered in any of the intervention groups [CT: χ 2(2) = 0.087, p = 0.957; RT: χ 2(2) = 0.750, p = 0.687; RTS: χ 2(2) = 3.524, p = 0.172] (Table 2).
With respect to the negative regulators of muscle mass, activin A levels were not affected by strength training alone or combined with the nutritional supplement [RT: χ 2(2) = 0.628, p = 0.731; RTS: χ 2(2) = 3.818, p = 0.148]. Surprisingly, activin A levels decreased in the CT group [χ 2(2) = 8.205, p = 0.017]. Post hoc analyses revealed differences between T1 and T3 (−7 %, r = 0.402, p = 0.019). In contrast, GDF-15 [CT: χ 2(2) = 1.130, p = 0.568; RT: χ 2(2) = 1.750, p = 0.417; RTS: χ 2(2) = 4.667, p = 0.097] and myostatin [CT: χ 2(2) = 1.130, p = 0.568; RT: χ 2(2) = 3.739, p = 0.154; RTS: χ 2(2) = 3.660, p = 0.165] levels were not affected by any of the interventions (Table 2).
As follistatin can inhibit the function of both, activin A and myostatin, the activin A-to-follistatin and myostatin-to-follistatin ratios were calculated. Interestingly, the activin A-to-follistatin ratio was significantly altered in the RT group [χ 2(2) = 6.750, p = 0.034] while it was unaffected in the RTS and CT groups [RTS: χ 2(2) = 4.095, p = 0.129; CT: χ 2(2) = 1.652, p = 0.438]. Detailed analyses showed that the activin A-to-follistatin ratio decreased in the RT group between T2 and T3 (−7 %, r = 0.360, p = 0.028). Differently to these findings, the myostatin-to-follistatin ratio was not affected in any of the groups [CT: χ 2(2) = 5.304, p = 5.304; RT: χ 2(2) = 1.130, p = 0.568; RTS: χ 2(2) = 3.700, p = 0.157].
Association between serum markers and MQ or physical function
As partially shown previously (Hofmann et al. 2015), baseline levels of GDF-15 correlated negatively with fat mass (ρ = −0.258; p = 0.016), skeletal muscle mass (ρ = −0.234; p = 0.032), MQ of upper extremities (ρ = −0.259; p = 0.023), and handgrip strength (ρ = −0.381; p < 0.001). Activin A levels were negatively associated with MQ of lower extremities (ρ = −0.282; p = 0.009). IGF-1 correlated positively with muscle mass (ρ = 0.313; p = 0.004) and handgrip strength (ρ = 0.224; p = 0.046) but not with MQ of either upper or lower extremities.
Interestingly, lower levels of myostatin but higher levels of activin A as well as higher activin A-to-follistatin ratios at baseline T1 were associated with a smaller increase in muscle mass between T1 and T3 or even muscle loss (myostatin: ρ = 0.336; p = 0.010 Fig. 2a, activin A: ρ = −0.268 Fig. 2b; p = 0.042, activin A-to-follistatin ratio: ρ = −0.345; p = 0.008). On the other hand, performance in chair stand test at T1 correlated negatively with differences in GDF-15 between T and T3 (ρ = −0.243; p = 0.043) (Fig. 3).
Associations between changes of T3-T1 in muscle mass and serum levels of myostatin (a), activin A (b) and follistatin (c) at baseline (T1). Correlations between pre and post intervention differences in muscle mass and changes in myostatin (d), activin A (e) and follistatin (f). Different symbols represent the assignment to the intervention groups (asterisk, CT cognitive training; filled triangle, RT resistance training; filled circle, RTS resistance training and supplementation) Linear fitting lines are shown for significant correlations (ρ Spearman rho correlation coefficient)
Association between difference of T3-T1 in GDF-15 serum levels and repetitions of chair stand test. Different symbols represent the assignment to the intervention groups (Asterisk, CT cognitive training; filled triangle, RT resistance training; filled circle, RTS resistance training and supplementation); ρ Spearman rho correlation coefficient; GDF-15 growth and differentiation factor-15
As valuable blood-based biomarkers should be sensitive to changes in skeletal muscle mass or function, associations between the respective differences between T1 and T3 were calculated. In this respect, changes in follistatin correlated positively with changes in muscle mass (ρ = 0.367; p = 0.005) while changes in myostatin or activin A were not associated with skeletal muscle mass alterations (Fig. 2d–f). We did not detect any correlations between changes in blood-based biomarkers and changes in functional parameters or MQ.
Discussion
To our knowledge this is the first study investigating the influence of a strength training intervention—either alone or in combination with a nutritional supplement—on blood-based biomarkers of skeletal muscle degradation and growth as well as on MQ. Our results confirm that in women the MQ of lower extremities can be increased with elastic band resistance training even at an older age, whereas an additional supplementation with proteins and vitamins seems to be ineffective in exerting an additive effect. These changes were associated with an increase in follistatin and a decrease of activin A-to-follistatin ratio in the RT group while activin A levels were decreased only in the CT group.
Intervention effects on muscle quality
In this study the MQ of lower, but not that of upper extremities increased in both training groups (RT and RTS). As whole body skeletal muscle mass was used for the determination of both the MQ of upper and lower extremities, this observation might indirectly reflect the amelioration in chair stand test while handgrip strength was not affected by the strength training programme. Interestingly, the elastic band resistance training that we have used in our study seems to have more pronounced effects on functional performance than on strength as the arm lifting test and chair stand test showed improvements while handgrip strength and isokinetic peak torque measurements of knee extensors and flexors were not affected (Oesen et al. 2015). Furthermore, it is noteworthy that leg muscles suffer greater losses in strength and MQ than arm muscles with ageing (Frontera et al. 2000; Lynch et al. 1999). If we assume that our strength training programme was designed to challenge upper and lower extremities in a similar way, the adaptation capacity of the lower extremities to strength training could have been higher leading to more pronounced effects, although this is in contrast to another study showing that strength gains are higher for upper extremities as the muscles of the lower limbs are elicited more frequently and therefore, have a smaller potential to gain strength at older age (Sousa et al. 2011). Another factor that influences the determination of MQ is the lack of a uniform consensus which parameters to include in the calculation of MQ. While some studies including ours used whole body skeletal mass determined by BIA or DXA (Barbat-Artigas et al. 2012a; Schroeder et al. 2012), others determined skeletal muscle mass separately for upper and lower body by means of DXA or ultrasound (Kennis et al. 2014; Radaelli et al. 2014; Straight et al. 2015). In addition, several formulas are used to determine muscle mass by BIA, whereby it is recommended to use equations derived from a similar age group, which is what we ensured by using the formula of Janssen et al. (2000). This formula has been developed in a multiethnic sample of 388 men and women, aged 18–86 years and includes age and gender as parameters. Furthermore, we checked the quality of BIA measurements by a parallel assessment of muscle mass by DXA in a smaller subgroup of the participants (n = 45). Statistical analyses revealed a high correlation between DXA and BIA (ρ = 0.847, p < 0.001). The situation is even more complex for assessing strength or power as a variety of methods is available. Therefore, we decided to use chair stand test and handgrip test as suggested by Barbat-Artigas et al. (2012b), but future studies evaluating the impact of these different methods on the assessment of MQ and on its influence on clinically relevant outcomes are highly recommended.
Intervention effects on blood-based parameters
Although performance parameters and MQ were increased in both training groups (RT and RTS), follistatin and the activin A-to-follistatin ratio were altered in the RT group only between T2 and T3 hinting to an adaptive delay in the response to training. Follistatin antagonizes myostatin and activin A and as such it is regarded as a positive regulator of muscle mass. Animal studies have shown that acute and chronic endurance exercises increase follistatin mRNA in the liver as well as in skeletal muscles (Hansen et al. 2011; Ziaaldini et al. 2015). Furthermore, serum levels of follistatin increased transiently during an ultra-marathon (Kerschan-Schindl et al. 2015). One of the rare studies investigating the influence of a comparable training setting (endurance or strength training) on follistatin levels in blood and muscle biopsies of young men has been published by Diel et al. (2010). Similar to our study, follistatin remained unchanged in blood and biopsy samples after 3 months (Diel et al. 2010), but the authors did not investigate long-term effects of training making a conclusion difficult. Interestingly, follistatin was not altered in the RTS group. As the strength training programme was the same in both groups, this hints to some direct or indirect effect of the nutritional supplement containing proteins and vitamins on serum follistatin levels. It has been shown that circulating follistatin levels increase in response to a fasting period while activin A levels decrease (Vamvini et al. 2011). We have collected the blood after an overnight fast which was the same in all groups. The nutritional intake was assessed at T1 and T3. In addition, the nutritional supplementation resulted in an increased uptake of vitamin D and folic acid but not in protein being between 0.8 and 1.0 g/kg/day in the RTS group (Franzke et al. 2015a). Furthermore, plasma levels of vitamin B12 and folic acid in erythrocytes were enhanced due to supplementation (Franzke et al. 2015b). There is an ongoing discussion of whether antioxidant supplementation may blunt an exercise-induced training effect (Peternelj and Coombes 2011). With respect to functional performance we did not observe any differences between the RT and RTS group while we did in follistatin levels. In vitro studies have shown that there could be a direct effect of vitamin D administration on follistatin levels as 1α,25-dihydroxyvitamin D3 decreased follistatin in osteoblasts (Woeckel et al. 2013) but increased follistatin in myoblasts (Garcia et al. 2011) showing the complex situation in different organ systems. As circulating levels of follistatin represent an overall measure of all follistatin-generating tissues further studies are needed to elucidate these complex interacting networks.
Follistatin regulates both, activin A and myostatin (Lee et al. 2010; Vamvini et al. 2013). Elevated expression of activins promotes muscle wasting and cachexia, whereas blocking of activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy (Chen et al. 2014; Lach-Trifilieff et al. 2014). In addition, Chen et al. (2014) showed that increasing circulating activin A in mice not only promoted the reduction of body weight and muscle mass in a dose-dependent manner, but also reduced muscle function highlighting the therapeutic potential of activin A inhibitors. Therefore, we would have expected a decrease of activin A in the RT and RTS group but instead we detected a small decrease in activin A in CT. However, concerning age-related changes it is still not clear whether activin A levels are influenced by age itself. While some studies have revealed increased circulating activin A levels with age (Baccarelli et al. 2001; Loria et al. 1998), our working group and others did not find any differences between young and old women (Hofmann et al. 2015; Klein et al. 2004). Taking a closer look at the data we found that most of the activin A levels at baseline were below 1 ng/ml while only few subjects (n = 12) displayed higher levels up to 6 ng/ml. Interestingly, higher levels of activin A at baseline were associated with decreases of skeletal muscle mass between T1 and T3 irrespective of the intervention group hinting to a more catabolic situation in these individuals at the beginning of the intervention and potentially making it more difficult for them to increase muscle mass (Fig. 2b). As activin A function can be inhibited by binding of follistatin to activin A, the activin A-to-follistatin ratio was determined. We observed that the ratio was lowered in the RT group shifting the plasma environment to a more follistatin-dominated one while it was unaffected in the CT group despite lower levels of activin A in this group. This highlights the importance of observing networks of biomarkers, such as the follistatin/activin A/myostatin-axis rather than single ones.
Both representatives of the TGF-β superfamily, myostatin (also known as GDF-8) and GDF-15 were found to be unaffected by the intervention. Because of its distinct impact on fat and muscle mass, several studies have dealt with muscular myostatin expression in context with acute strength exercise (Hulmi et al. 2008; Jensky et al. 2010, 2007) and long-term training (Brooks et al. 2010; Diel et al. 2010; Suetta et al. 2013) in young as well as aged women and men. One interesting though unexpected finding of our study was that myostatin levels at baseline correlated positively with changes in muscle mass. Studies investigating circulating levels of myostatin in response to training interventions are still contradictory which weakens final statements on the role of circulating myostatin in adaptations to resistance training. In this respect it has been shown that 10 weeks of high-intensity resistance exercise in young healthy men leads to a decrease in circulating myostatin (Walker et al. 2004). On the other hand serum myostatin propeptide is not altered in young and healthy men performing strength training for 3 months (Diel et al. 2010), and serum myostatin even increases after a 6-month lifestyle intervention programme in obese children (Ehehalt et al. 2011). Differences in measurement methods, age, training loads and training durations may cause these conflicting results. Another hypothesis was provided by Willboughby (2004) who suggested that increases in serum levels of the follistatin-like related gene and the concomitant down-regulation of the activin IIb receptor would counteract even increases in myostatin observed after heavy resistance training. Having these aspects in mind we conclude that the beneficial effects of strength training observed for MQ and functional parameters in our study might be due to blocking of activin A and myostatin by enhanced levels of follistatin rather than by lower circulating levels of activin A and myostatin.
Similar to myostatin we could not find any changes in circulating GDF-15 neither in one of the training groups nor in CT over the time of intervention, but GDF-15 levels were negatively associated with MQ of upper extremities at baseline. Additionally, better performance in the chair stand test was negatively associated with changes in muscle mass between T1 and T3 confirming the negative effects of GDF-15 on skeletal muscle (Fig. 3). This is similar to a study of patients undergoing cardiac surgery in which elevated GDF-15 levels were associated with quadriceps muscle atrophy and were elevated after the surgery (Bloch et al. 2013). Traditionally, this biomarker is suggested to reflect the status of cardiac muscle (Sinning et al. 2015) or lung tissue (Mutlu et al. 2015) and we suggest further investigation is needed to elucidate its role in skeletal muscle.
IGF-1 is considered as an important positive regulator of muscle mass which did not change in response to resistance training with or without supplementation. Our data confirm a previous study in older men and women showing positive effects of a 12-week elastic band exercise programme on body composition and physical fitness without improving IGF-1 levels (So et al. 2013). Similarly, IGF-1 was unaffected by low-load resistance exercise with blood flow restriction in older men (Patterson et al. 2013). However, age seems to be a main determinant in the IGF-1 response to exercise training. We have shown that IGF-1 level differ between young and old women (Hofmann et al. 2015), and young men responded to a strength training programme with increased levels of IGF-1 (Takano et al. 2005). There is growing evidence that a chronic inflammatory state suppresses the GH/IGF1 axis (Andreassen et al. 2012; O’Connor et al. 2008; Pass et al. 2009; Strle et al. 2007). According to the ‘inflammageing theory’ elevated levels of proinflammatory cytokines are found with ageing (Schmidt et al. 2011). As we have shown that hs-CRP concentrations in our study population are higher than in young women (Halper et al. 2015), we hypothesize that increased levels of proinflammatory mediators could have blunted an increase in IGF-1 levels.
Conclusions
Based on our data, we confirm that strength training improves physical performance and MQ even in very old women, whereas nutritional supplementation seems to be ineffective in exerting additive effects. With respect to blood-based biomarkers, our main finding was that strength training alone enhanced follistatin levels leading to a decreased activin A-to-follistatin ratio. Therefore, we conclude that the positive effects of strength training in the elderly women is mediated by blocking muscle degradation pathways via follistatin rather than inducing muscle growth by the IGF-1 pathway. However, plasma levels of biomarkers are influenced by various organ systems involved in the adaptation to exercise such as skeletal muscle, fat, liver tissue and others. Therefore, these biomarkers may reflect an overall status rather than the status of skeletal muscle making future studies of tissue levels very important. Finally, it has to be mentioned that the current study measured resting values of blood-based parameters. Therefore, it would be interesting for future studies to investigate the influence of acute changes of these biomarkers following adaptations to exercise training.
Abbreviations
- BIA:
-
Bioelectric impedance analysis
- BMI:
-
Body mass index
- CT:
-
Cognitive training
- DXA:
-
Dual-energy X-ray absorptiometry
- FLRG:
-
Follistatin-related gene
- GDF-15:
-
Growth and differentiation factor-15
- IGF-1:
-
Insulin-like growth factor-1
- MQ:
-
Muscle quality
- RT:
-
Resistance training
- RTS:
-
Resistance training and supplementation
- TGF-β:
-
Transforming growth factor-β
- VAAS:
-
Vienna Active Ageing Study
References
Andreassen M, Frystyk J, Faber J, Kristensen LO (2012) GH activity and markers of inflammation: a crossover study in healthy volunteers treated with GH and a GH receptor antagonist. Eur J Endocrinol/Eur Fed Endocr Soc 166:811–819. doi:10.1530/EJE-11-1009
Baccarelli A et al (2001) Activin A serum levels and aging of the pituitary-gonadal axis: a cross-sectional study in middle-aged and elderly healthy subjects. Exp Gerontol 36:1403–1412. doi:10.1016/S0531-5565(01)00117-6
Barbat-Artigas S, Filion ME, Plouffe S, Aubertin-Leheudre M (2012a) Muscle quality as a potential explanation of the metabolically healthy but obese and sarcopenic obese paradoxes. Metab Syndr Relat Disord 10:117–122. doi:10.1089/met.2011.0092
Barbat-Artigas S, Rolland Y, Zamboni M, Aubertin-Leheudre M (2012b) How to assess functional status: a new muscle quality index. J Nutr Health Aging 16:67–77
Becker C et al (2015) Myostatin antibody (LY2495655) in older weak fallers: a proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. doi:10.1016/S2213-8587(15)00298-3
Bloch SA, Lee JY, Wort SJ, Polkey MI, Kemp PR, Griffiths MJ (2013) Sustained elevation of circulating growth and differentiation factor-15 and a dynamic imbalance in mediators of muscle homeostasis are associated with the development of acute muscle wasting following cardiac surgery. Crit Care Med 41:982–989. doi:10.1097/CCM.0b013e318274671b
Bloch SA, Lee JY, Syburra T, Rosendahl U, Griffiths MJ, Kemp PR, Polkey MI (2015) Increased expression of GDF-15 may mediate ICU-acquired weakness by down-regulating muscle microRNAs. Thorax 70:219–228. doi:10.1136/thoraxjnl-2014-206225
Brooks NE et al (2010) Effects of resistance exercise combined with essential amino acid supplementation and energy deficit on markers of skeletal muscle atrophy and regeneration during bed rest and active recovery. Muscle Nerve 42:927–935. doi:10.1002/mus.21780
Chen L, Nelson DR, Zhao Y, Cui Z, Johnston JA (2013) Relationship between muscle mass and muscle strength, and the impact of comorbidities: a population-based, cross-sectional study of older adults in the United States. BMC Geriatr 13:74. doi:10.1186/1471-2318-13-74
Chen JL et al (2014) Elevated expression of activins promotes muscle wasting and cachexia. FASEB J 28:1711–1723. doi:10.1096/fj.13-245894
Cruz-Jentoft AJ et al (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing 39:412–423. doi:10.1093/ageing/afq034
Diel P et al (2010) Analysis of the effects of androgens and training on myostatin propeptide and follistatin concentrations in blood and skeletal muscle using highly sensitive immuno PCR. Mol Cell Endocrinol 330:1–9. doi:10.1016/j.mce.2010.08.015
Ehehalt S et al (2011) Investigation of myostatin serum levels before and after a 6-month lifestyle intervention program in obese children. Exp Clin Endocrinol Diabetes: Off J Ger Soc Endocrinol Ger Diabetes Assoc 119:238–242. doi:10.1055/s-0030-1267964
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191
Folstein MF, Folstein SE, McHugh PR (1975) ‘‘Mini-mental state’’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Fragala MS et al (2014) Muscle quality index improves with resistance exercise training in older adults. Exp Gerontol 53:1–6. doi:10.1016/j.exger.2014.01.027
Franzke B et al (2015a) The impact of 6 months strength training, nutritional supplementation or cognitive training on DNA damage in institutionalised elderly. Mutagenesis 30:147–153. doi:10.1093/mutage/geu074
Franzke B et al (2015b) The effect of 6 months of elastic band resistance training, nutritional supplementation or cognitive training on chromosomal damage in institutionalized elderly. Exp Gerontol 65:16–22. doi:10.1016/j.exger.2015.03.001
Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R (2000) Aging of skeletal muscle: a 12-year longitudinal study. J Appl Physiol 88:1321–1326
Garcia LA, King KK, Ferrini MG, Norris KC, Artaza JN (2011) 1,25(OH)2vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology 152:2976–2986. doi:10.1210/en.2011-0159
Glass DJ (2010) PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr Top Microbiol Immunol 346:267–278. doi:10.1007/82_2010_78
Goodpaster BH et al (2006) The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61:1059–1064. doi:10.1093/gerona/61.10.1059
Halper B et al (2015) Influence of age and physical fitness on miRNA-21, TGF-beta and its receptors in leukocytes of healthy women. Exerc Immunol Rev 21:154–163
Han DS, Chen YM, Lin SY, Chang HH, Huang TM, Chi YC, Yang WS (2011) Serum myostatin levels and grip strength in normal subjects and patients on maintenance haemodialysis. Clin Endocrinol 75:857–863. doi:10.1111/j.1365-2265.2011.04120.x
Han HQ, Zhou X, Mitch WE, Goldberg AL (2013) Myostatin/activin pathway antagonism: molecular basis and therapeutic potential. Int J Biochem Cell Biol 45:2333–2347. doi:10.1016/j.biocel.2013.05.019
Hansen J et al (2011) Exercise induces a marked increase in plasma follistatin: evidence that follistatin is a contraction-induced hepatokine. Endocrinology 152:164–171. doi:10.1210/en.2010-0868
Hofmann M et al (2015) Serum concentrations of insulin-like growth factor-1, members of the TGF-beta superfamily and follistatin do not reflect different stages of dynapenia and sarcopenia in elderly women. Exp Gerontol 64:35–45. doi:10.1016/j.exger.2015.02.008
Hulmi JJ, Kovanen V, Lisko I, Selanne H, Mero AA (2008) The effects of whey protein on myostatin and cell cycle-related gene expression responses to a single heavy resistance exercise bout in trained older men. Eur J Appl Physiol 102:205–213. doi:10.1007/s00421-007-0579-4
Janssen I, Heymsfield SB, Baumgartner RN, Ross R (2000) Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol 89:465–471
Jensky NE, Sims JK, Rice JC, Dreyer HC, Schroeder ET (2007) The influence of eccentric exercise on mRNA expression of skeletal muscle regulators. Eur J Appl Physiol 101:473–480. doi:10.1007/s00421-007-0521-9
Jensky NE, Sims JK, Dieli-Conwright CM, Sattler FR, Rice JC, Schroeder ET (2010) Exercise does not influence myostatin and follistatin messenger RNA expression in young women. J Strength Cond Res 24:522–530. doi:10.1519/JSC.0b013e3181c8664f
Ju CR, Chen RC (2012) Serum myostatin levels and skeletal muscle wasting in chronic obstructive pulmonary disease. Respir Med 106:102–108. doi:10.1016/j.rmed.2011.07.016
Kalinkovich A, Livshits G (2015) Sarcopenia–The search for emerging biomarkers. Ageing Res Rev 22:58–71. doi:10.1016/j.arr.2015.05.001
Kennis E, Verschueren S, Van Roie E, Thomis M, Lefevre J, Delecluse C (2014) Longitudinal impact of aging on muscle quality in middle-aged men. Age 36:9689. doi:10.1007/s11357-014-9689-1
Kerschan-Schindl K et al (2015) Changes in serum levels of Myokines and Wnt-Antagonists after an Ultramarathon Race. PloS One 10:e0132478. doi:10.1371/journal.pone.0132478
Klein NA, Houmard BS, Hansen KR, Woodruff TK, Sluss PM, Bremner WJ, Soules MR (2004) Age-related analysis of inhibin A, inhibin B, and activin a relative to the intercycle monotropic follicle-stimulating hormone rise in normal ovulatory women. J Clin Endocrinol Metab 89:2977–2981. doi:10.1210/jc.2003-031515
Lach-Trifilieff E et al (2014) An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy. Mol Cell Biol 34:606–618. doi:10.1128/MCB.01307-13
Lakshman KM et al (2009) Measurement of myostatin concentrations in human serum: circulating concentrations in young and older men and effects of testosterone administration. Mol Cell Endocrinol 302:26–32. doi:10.1016/j.mce.2008.12.019
Lee SJ et al (2010) Regulation of muscle mass by follistatin and activins. Mol Endocrinol 24:1998–2008. doi:10.1210/me.2010-0127
Loria P et al (1998) Influence of age and sex on serum concentrations of total dimeric activin A. Eur J Endocrinol/Eur Fed Endocr Soc 139:487–492
Lynch NA et al (1999) Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol 86:188–194
Metter EJ, Talbot LA, Schrager M, Conwit R (2002) Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci 57:B359–B365
Mijnarends DM et al (2013) Validity and reliability of tools to measure muscle mass, strength, and physical performance in community-dwelling older people: a systematic review. J Am Med Dir Assoc 14:170–178. doi:10.1016/j.jamda.2012.10.009
Mutlu LC et al (2015) Growth differentiation factor-15 is a novel biomarker predicting acute exacerbation of chronic obstructive pulmonary disease. Inflammation 38:1805–1813. doi:10.1007/s10753-015-0158-5
Nelson ME et al (2007) Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 39:1435–1445. doi:10.1249/mss.0b013e3180616aa2
O’Connor JC, McCusker RH, Strle K, Johnson RW, Dantzer R, Kelley KW (2008) Regulation of IGF-I function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cell Immunol 252:91–110. doi:10.1016/j.cellimm.2007.09.010
Oesen S et al (2015) Effects of elastic band resistance training and nutritional supplementation on physical performance of institutionalised elderly—a randomized controlled trial. Exp Gerontol. doi:10.1016/j.exger.2015.08.013
Page P, Ellenbecker TS (2011) Strength band training. Human Kinetics, Leeds
Pass C, MacRae VE, Ahmed SF, Farquharson C (2009) Inflammatory cytokines and the GH/IGF-I axis: novel actions on bone growth. Cell Biochem Funct 27:119–127. doi:10.1002/cbf.1551
Patterson SD, Leggate M, Nimmo MA, Ferguson RA (2013) Circulating hormone and cytokine response to low-load resistance training with blood flow restriction in older men. Eur J Appl Physiol 113:713–719. doi:10.1007/s00421-012-2479-5
Peternelj TT, Coombes JS (2011) Antioxidant supplementation during exercise training: beneficial or detrimental? Sports Med 41:1043–1069. doi:10.2165/11594400-000000000-00000
Radaelli R, Wilhelm EN, Botton CE, Rech A, Bottaro M, Brown LE, Pinto RS (2014) Effects of single vs. multiple-set short-term strength training in elderly women. Age 36:9720. doi:10.1007/s11357-014-9720-6
Rantanen T, Avlund K, Suominen H, Schroll M, Frandin K, Pertti E (2002) Muscle strength as a predictor of onset of ADL dependence in people aged 75 years. Aging Clin Exp Res 14:10–15
Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM (2003) Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc 51:636–641
Rikli RE, Jones CJ (2013) Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist 53:255–267. doi:10.1093/geront/gns071
Ring-Dimitriou S, Steinbacher P, von Duvillard SP, Kaessmann H, Muller E, Sanger AM (2009) Exercise modality and physical fitness in perimenopausal women. Eur J Appl Physiol 105:739–747. doi:10.1007/s00421-008-0956-7
Roubenoff R et al (1997) Application of bioelectrical impedance analysis to elderly populations. J Gerontol A Biol Sci Med Sci 52:M129–M136
Schmidt D, Kwetkat A, Gogol M (2011) Chronic inflammation and biomarkers. Is ageing an expression of chronic inflammation? Z Gerontol Geriatr 44:153–157. doi:10.1007/s00391-011-0198-x
Schroeder ET et al (2012) Value of measuring muscle performance to assess changes in lean mass with testosterone and growth hormone supplementation. Eur J Appl Physiol 112:1123–1131. doi:10.1007/s00421-011-2077-y
Scott D, Hayes A, Sanders KM, Aitken D, Ebeling PR, Jones G (2014) Operational definitions of sarcopenia and their associations with 5-year changes in falls risk in community-dwelling middle-aged and older adults. Osteoporos Int 25:187–193. doi:10.1007/s00198-013-2431-5
Sinning JM et al (2015) Risk scores and biomarkers for the prediction of 1-year outcome after transcatheter aortic valve replacement. Am Heart J 170:821–829. doi:10.1016/j.ahj.2015.07.003
Smith WN, Del Rossi G, Adams JB, Abderlarahman KZ, Asfour SA, Roos BA, Signorile JF (2010) Simple equations to predict concentric lower-body muscle power in older adults using the 30-second chair-rise test: a pilot study. Clin Interv Aging 5:173–180
So WY, Song M, Park YH, Cho BL, Lim JY, Kim SH, Song W (2013) Body composition, fitness level, anabolic hormones, and inflammatory cytokines in the elderly: a randomized controlled trial. Aging Clin Exp Res 25:167–174. doi:10.1007/s40520-013-0032-y
Sousa N, Mendes R, Abrantes C, Sampaio J (2011) Differences in maximum upper and lower limb strength in older adults after a 12 week intense resistance training program. J Hum Kinet 30:183–188. doi:10.2478/v10078-011-0086-x
Straight CR, Brady AO, Evans EM (2015) Muscle quality and relative adiposity are the strongest predictors of lower-extremity physical function in older women. Maturitas 80:95–99. doi:10.1016/j.maturitas.2014.10.006
Strle K et al (2007) Novel activity of an anti-inflammatory cytokine: IL-10 prevents TNFalpha-induced resistance to IGF-I in myoblasts. J Neuroimmunol 188:48–55. doi:10.1016/j.jneuroim.2007.05.003
Suetta C et al (2013) Ageing is associated with diminished muscle re-growth and myogenic precursor cell expansion early after immobility-induced atrophy in human skeletal muscle. J Physiol 591:3789–3804. doi:10.1113/jphysiol.2013.257121
Takano H et al (2005) Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur J Appl Physiol 95:65–73. doi:10.1007/s00421-005-1389-1
Vamvini MT, Aronis KN, Chamberland JP, Mantzoros CS (2011) Energy deprivation alters in a leptin- and cortisol-independent manner circulating levels of activin A and follistatin but not myostatin in healthy males. J Clin Endocrinol Metab 96:3416–3423. doi:10.1210/jc.2011-1665
Vamvini MT et al (2013) Irisin mRNA and circulating levels in relation to other myokines in healthy and morbidly obese humans. Eur J Endocrinol 169:829–834. doi:10.1530/EJE-13-0276
Walker KS, Kambadur R, Sharma M, Smith HK (2004) Resistance training alters plasma myostatin but not IGF-1 in healthy men. Med Sci Sports Exerc 36:787–793
Whittemore LA et al (2003) Inhibition of myostatin in adult mice increases skeletal muscle mass and strength. Biochem Biophys Res Commun 300:965–971
Willoughby DS (2004) Effects of heavy resistance training on myostatin mRNA and protein expression. Med Sci Sports Exerc 36:574–582
Woeckel VJ, van der Eerden BC, Schreuders-Koedam M, Eijken M, Van Leeuwen JP (2013) 1alpha,25-dihydroxyvitamin D3 stimulates activin A production to fine-tune osteoblast-induced mineralization. J Cell Physiol 228:2167–2174. doi:10.1002/jcp.24388
Yarasheski KE, Bhasin S, Sinha-Hikim I, Pak-Loduca J, Gonzalez-Cadavid NF (2002) Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle wasting. J Nut Health Aging 6:343–348
Ziaaldini MM, Koltai E, Csende Z, Goto S, Boldogh I, Taylor AW, Radak Z (2015) Exercise training increases anabolic and attenuates catabolic and apoptotic processes in aged skeletal muscle of male rats. Exp Gerontol 67:9–14. doi:10.1016/j.exger.2015.04.008
Acknowledgments
The authors thank the Curatorship of Viennese retirement homes (KWP) and its residents for taking part in the study. Financial support was provided by the University of Vienna by funding the Research Platform Active Ageing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Communicated by William J. Kraemer.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hofmann, M., Schober-Halper, B., Oesen, S. et al. Effects of elastic band resistance training and nutritional supplementation on muscle quality and circulating muscle growth and degradation factors of institutionalized elderly women: the Vienna Active Ageing Study (VAAS). Eur J Appl Physiol 116, 885–897 (2016). https://doi.org/10.1007/s00421-016-3344-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-016-3344-8