Abstract
Exercise provides a cornerstone in the prevention and treatment of several chronic diseases. The use of in vivo exercise models alone cannot fully establish the skeletal muscle-specific mechanisms involved in such health-promoting effects. As such, models that replicate exercise-like effects in vitro provide useful tools to allow investigations that are not otherwise possible in vivo. In this review, we provide an overview of experimental models currently used to induce exercise-like effects in skeletal muscle in vitro. In particular, the appropriateness of electrical pulse stimulation and several pharmacological compounds to resemble exercise, as well as important technical considerations, are addressed. Each model covered herein provides a useful tool to investigate different aspects of exercise with a level of abstraction not possible in vivo. That said, none of these models are perfect under all circumstances, and the choice of model (and terminology) used should be informed by the specific research question whilst accounting for the several inherent limitations of each model. Further work is required to develop and optimise the current experimental models used, such as combination with complementary techniques during treatment, and thereby improve their overall utility and impact within muscle biology research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vast evidence supports the role of physical activity and exercise in the prevention and treatment of many chronic diseases, such as cardiovascular disease and type 2 diabetes mellitus (T2DM) [20, 97]. Despite extensive research efforts, the exact underlying mechanisms responsible for these benefits remain to be determined. That said, skeletal muscle contraction per se likely contributes to some or many of the exercise-mediated health benefits, be that through direct effects within the contracting skeletal muscle cells (such as improved glucose uptake and insulin sensitivity [74, 102]) or via cross-talk with other organs mediated by factors liberated from the skeletal muscle (e.g. myokines, metabolites, microRNAs, exosomes) [95, 96]. Determining the key mechanisms through which exercise ameliorates disease-linked pathogenesis and signal transduction dysfunction could optimise the beneficial role of exercise in health promotion, minimise instances of impaired exercise adaptation and may facilitate the identification of novel exercise-responsive drug targets.
In vivo experiments with human volunteers provide the most integrated model with which to examine exercise-induced health-promoting responses. Skeletal muscle biopsies collected before and after acute exercise and/or exercise training programmes provide valuable observational insight into associations between in vivo health benefits and specific exercise-mediated changes in skeletal muscle gene expression, protein content and signal transduction pathways. For example, the glucoregulatory and insulin-sensitising effects of exercise have been associated with changes in skeletal muscle expression and activity of several proteins, such as glucose transporter 4 (GLUT4) [11, 49, 102]. However, to confirm the mechanistic importance of specific changes in skeletal muscle in the resultant exercise-mediated health benefits, further experiments (such as gain and loss of function) are required. The unethical nature of such experiments in human participants, as well as feasibility and practicality considerations, preclude the ability to study individual molecular mechanisms of exercise in isolation, in vivo in humans. Accordingly, a prime challenge for researchers working to understand the role of exercise in the prevention and treatment of disease is to develop and optimise experimental models that resemble elements of exercise physiology in a more reductionist approach. In vitro experimental models whereby skeletal muscle cells derived from immortalised cell lines (e.g. C2C12 and L6) or isolated from human or rodent skeletal muscle samples are exposed to various skeletal muscle-specific exercise-like treatments (shortened to ‘exercise-like treatments’ hereafter) such as electrical pulse stimulation (EPS) or pharmacological compounds have proved useful in this regard. Such ‘exercise in a dish’ approaches allow investigations into the cellular effects of individual aspects of exercise physiology, such as skeletal muscle contraction or activation of exercise-responsive signalling pathways, directly upon the skeletal muscle cells, as well as exercise-mediated cross-talk between different cell types. Accordingly, the purpose of this review is to provide the rationale, potential application and evaluation of several models that have been used to replicate and examine exercise-like effects in vitro.
Criteria of an in vitro skeletal muscle-specific exercise-like model
Models aiming to replicate skeletal muscle-specific aspects of exercise in vitro must be validated against the complex range of in vivo exercise-mediated changes. The exact mechanisms of exercise-induced responses are not fully understood and will vary depending on the exercise stimulus (modality, intensity, duration) and whether such stimulus is performed acutely or as part of a training regime [50]. That said, some aspects apply universally to skeletal muscle contraction, and these will be used for the purpose of evaluation of each exercise-like treatment discussed within this review. Specifically, exercise induces muscle contraction through increased intracellular calcium ion (Ca2+) concentrations. Subsequent contractile activity during exercise elicits multiple intracellular perturbations within the contracting muscle, including decreased adenosine triphosphate (ATP) and phosphocreatine (PCr) content, increased adenosine monophosphate (AMP) to ATP ratio and increased free radical production, as well as other metabolic and mechanical stresses [46]. Consequently, exercise in vivo induces a cascade of transcriptional (e.g. peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), GLUT4, enzyme and myokine mRNA expression), signalling (e.g. activation of AMP-activated protein kinase (AMPK) and Ca2+ activated kinases), proteomic (e.g. increased GLUT4 expression), metabolic (e.g. enhanced glucose uptake and GLUT4 translocation [104, 105]) and morphological (e.g. hypertrophy) changes within skeletal muscle (as reviewed previously [30]). Therefore, to be considered as an in vitro exercise-like treatment, an experimental model must demonstrate exercise-like effects with regard to the aforementioned transcriptional, signalling and metabolic changes observed in vivo. After such comparison, conclusions can be made regarding the extent to which a model can resemble exercise. In the following sections, we provide an outline of in vitro models that have been applied to skeletal muscle myotubes in conventional 2D cell culture models within the context of exercise, including a comparison of the effects of each in vitro exercise-like treatment to several exercise-induced responses common to multiple in vivo exercise stimuli. See Table 1 and Fig. 1 for summaries of this narrative. Please note that for reasons further discussed within the limitations section below, we have not directly compared each model to specific types of exercise (e.g. endurance vs. resistance).
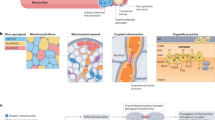
Proposed experimental models to examine skeletal muscle-specific exercise-like effects in vitro. Exercise in vivo (highlighted orange) potently activates a range of changes within skeletal muscle that contribute to the well-documented improvement of human health. Exercise-like treatments (highlighted red) elicit several intracellular perturbations (highlighted purple) and activate several exercise-responsive signalling pathways (highlighted green), and as discussed within the text, consequently induce different exercise-like functional and metabolic responses (highlighted blue) in skeletal muscle myotubes. References are provided within the text of the relevant section. ✓ indicates the same direction of change induced by exercise-like treatment compared to in vivo exercise; × indicates different direction of change induced by exercise-like treatment compared to in vivo exercise; ✓× indicates contradictory findings; ? indicates evidence lacking regarding the effect of exercise-like treatment
Electrical pulse stimulation
Background
Skeletal muscle contraction that occurs during exercise induces multiple disturbances in intracellular homeostasis and subsequently activates multiple contraction-mediated signalling pathways. Therefore, models that induce skeletal muscle contraction, and subsequent activation of contraction-mediated signalling pathways, provide perhaps the most obvious option when trying to resemble exercise in vitro. Electrical pulse stimulation (EPS) is an in vitro model of skeletal muscle contraction that has been used for this purpose. The application of EPS to skeletal muscle myotubes derived from cell lines (e.g. C2C12 and L6 cells) and cultured primary skeletal muscle cells has proved to be a major tool for studying contraction-induced adaptations, and a comprehensive overview has recently been published by Nikolić and colleagues [86].
Applications
While the use of EPS dates back to the 1970s, its specific use as an exercise-like treatment in vitro is a more recent development. In particular, EPS has been applied to study contraction-mediated changes in skeletal muscle signal transduction [37, 83], metabolism [67, 85], myokine regulation/release [99, 100, 112] and morphology [119]. Additionally, contraction-mediated cross-talk between skeletal muscle cells and other cell types (e.g. pancreatic beta cells [5, 18], hepatocytes [33], monocytes [78], endothelial cells [126]) and donor-specific adaptations [15, 34, 48] have been addressed using EPS.
Comparison to in vivo exercise
Many studies have now demonstrated that EPS induces visible contraction and elicits several exercise-like effects. Similar to in vivo exercise, EPS-induced responses depend upon the EPS stimulus (as discussed by Nikolić et al. [86]). For example, short-term, high-frequency stimulation has been proposed as a model of acute high-intensity exercise as it induces exercise-like upregulation of glucose uptake [2, 85] and lactate production, decreased ATP and PCr content [85] and the activation of contraction-related signalling pathways [75]. In contrast, chronic low-frequency stimulation has been used to resemble changes seen following exercise training given its exercise-like effects upon activation of exercise-mediated signal transduction (e.g. PGC-1α mRNA expression [99], AMPK [37, 67, 83], mitogen-activated protein kinase (MAPK), Ca2+ transients [37, 120]), stimulation of beneficial metabolic effects (e.g. enhanced glucose uptake and oxidation, and insulin sensitivity [67, 85] and increased mitochondrial content [85]), myokine expression and release [7, 67, 99, 100, 112, 123] and morphological changes (e.g. sarcomeric structure formation [37, 67] and myotube hypertrophy [119]). Such EPS-induced sarcomeric structure formation reportedly relies upon calpain-mediated proteolysis [37]. EPS has also been demonstrated to increase lactate production, reduce intracellular glycogen content [68] and protect against lipid-induced insulin resistance [84]. However, some key changes seen following exercise in vivo have not been reproduced in vitro. For example, GLUT4 mRNA and protein expression and fatty acid oxidation are increased following exercise in vivo, but these effects have not been consistently demonstrated following EPS. Additionally, whilst GLUT4 translocation, a key process which contributes to the glucoregulatory effects of exercise in vivo, has been demonstrated in C2C12 myotubes [52, 68, 83], such effects have not yet been reported in human primary muscle cell-based EPS models. Additionally, whilst C2C12 cells are responsive to EPS in regard to contractility, primary human myotubes reportedly vary in their contractility depending on culture conditions and cell donor meaning that the degree to which EPS induces exercise-like contraction can be variable. The aforementioned discrepancies in results are likely, at least in part, attributable to variability in EPS parameters used (as detailed previously [86]).
Technical considerations
While EPS provides a useful tool with which several key aspects of in vivo exercise can be induced in vitro, several key technical considerations must be acknowledged. Firstly, as discussed above, EPS-induced contraction activates multiple signalling pathways. Therefore, if the aim is to focus on a specific signalling pathway, alternative methods (such as pharmacological compounds discussed below) are required. Secondly, EPS can be applied using commercial (e.g. C-Pace IonOptix generators) or home-made systems. Commercially available systems are perhaps the more convenient option, but lack of tracking capabilities mean it is difficult to determine/confirm whether EPS parameters (e.g. frequency and voltage) are being consistently applied during the experiment. Conversely, while tracking is possible with home-made systems, such setups likely require expertise in electrical engineering [86].
Regarding protocol selection, whilst experimental data supports the use of two distinct protocols to resemble acute (i.e. short-term high-frequency stimulation) vs. chronic (i.e. long-term low-frequency stimulation) exercise-like stimulation [85, 86], direct comparison of specific EPS protocols to different in vivo exercise programmes (e.g. aerobic vs. resistance vs. interval exercise) is more difficult. By altering the EPS parameters (e.g. duration, frequency, pulse trains vs. single pulses, etc.), attempts have been made to resemble these different types of exercise in vitro. Whilst different EPS models do evoke different responses that may align a particular EPS protocol with a particular type of exercise, any single EPS model can induce both similarities and differences to both endurance and resistance exercise (e.g. [18]). Therefore, it is perhaps best to consider EPS as a generalised skeletal muscle contraction model that can be applied acutely or chronically, rather than any specific type of exercise.
One consequence of the aforementioned attempts to resemble different types of exercise is the clear inconsistency in EPS parameters (duration, voltage, pulse duration and frequency) used in published studies. Inevitably, discrepancies in EPS-induced effects across studies are also evident. This is particularly true in studies performed on C2C12 and other rodent models, with protocols rarely used in more than 1–2 publications, resulting in 40+ different protocol combinations (as summarised by Nikolić et al. [86]). That said, some consistency can be seen with recent human EPS models with several studies setting their EPS parameters as follows: 1 Hz, 2 ms, 11.5 V for 2–24 h [39,40,41, 58, 67, 100, 101]. This protocol has also recently been applied to C2C12 cells [33].
As discussed by Nikolić and colleagues [86], correct care and cleaning of carbon electrodes after each use are vital to prevent chemicals leaching from the electrodes into the media and inducing effects unrelated to the stimulus itself and/or compromising cell viability. This is particularly important when studying conditioned media-mediated effects within cross-talk experiments. To do so, the following procedure can be used: (1) rinse electrodes with distilled water, (2) soak electrodes in distilled water for 2 days (refreshing water after 24 h), (3) disinfect electrodes with 70% ethanol, and (4) position electrodes under UV lamp in tissue culture hood. Following these procedures after each use should prevent any issues developing as a result of incorrect care of the EPS electrodes. Nevertheless, measurement of lactate dehydrogenase (LDH) and/or creatine kinase activity in the supernatant should be completed to confirm that cell viability is not being compromised by incorrect electrode cleaning procedures and/or an unsuitable EPS protocol [67, 83, 85]. Similarly, in all applications, the no EPS (control) condition should involve placement of electrodes as in the EPS condition, but without power. The addition of no cell/no EPS and no cell/EPS control conditions are worthwhile additions to exclude any non-specific effects that may result from factors leaching from the EPS electrodes rather than factors secreted by skeletal muscle myotubes [86].
Pharmacological compounds
Several compounds have been used in vitro to investigate the molecular regulation of exercise-induced skeletal muscle adaptations. Such compounds are primarily used based on their ability to activate specific exercise-like signalling or metabolic conditions that occur within exercising skeletal muscle. These compounds include 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR; an AMPK activator), caffeine (a Ca2+ ionophore), ionomycin (a Ca2+ ionophore) and forskolin (a protein kinase A (PKA) activator), and on occasion a combination/cocktail of these. Below, we provide the rationale behind the use of each of these compounds, with the specific protocol parameters used in the cited studies summarised in Table 2.
AICAR
Background
AMPK, a heterotrimeric protein complex, is a master regulator of energy metabolism in skeletal muscle [45]. Exercise potently activates the AMPK pathway within skeletal muscle due to contraction-mediated increases in the intracellular AMP/ATP and adenosine diphosphate (ADP)/ATP ratios [45, 47]. This exercise-induced activation of AMPK contributes to several exercise-mediated changes in skeletal muscle metabolism, such as enhanced substrate uptake and oxidation [47, 62]. Therefore, compounds that activate AMPK in skeletal muscle myotubes have been proposed as tools to study the importance of exercise-like activation of AMPK in the subsequent regulation of skeletal muscle metabolism. In particular, AICAR, which activates AMPK through its role as an AMP mimetic, has frequently been used for this purpose. Once transported into cells, AICAR is converted to 5-aminoimidazole-4-carboxamide-1-β-d-ribosyl monophosphate (ZMP; an AMP analogue), and subsequently results in AMPK activation [21].
Applications
Whilst AICAR is not always specifically used as an ‘exercise-like treatment’, AICAR has commonly been used as an AMPK activator within the context of exercise physiology. For example, AICAR applied to C2C12 and L6 myotubes to study signalling pathways [8, 27, 31, 56, 79, 88, 111], metabolism [17, 51, 117], myokine release [114] and protection against palmitate [108, 124] that may be regulated by exercise-like AMPK activation. That said, studies have also used AICAR with the stated intention to induce ‘exercise signals’ [79], to serve as an ‘exercise-mimetic precursor’ [90, 110] and to generate ‘trained’ cells [94] to study exercise-related signalling [90, 91], myokine release [110] and cross-talk [94]. AICAR has also been applied to primary human myotubes to investigate donor-specific responses to AMPK activation, such as lean vs. obese [10], sedentary vs. obese vs. T2DM vs. athletes [22] and healthy vs. T2DM [61, 82]. For example, different responses between donors have been shown, with AICAR increasing AMPK activation in lean but not T2DM myotubes [61]. Conversely, similar effects between donors have also been reported with regard to the AICAR-induced enhancement of glucose uptake [77].
Comparison to in vivo exercise
Whilst AICAR does not induce skeletal muscle contraction, AICAR administration to skeletal muscle myotubes induces several exercise-like effects in vitro. In C2C12 or L6 cells, AICAR has been shown to increase mRNA expression levels of PGC-1α [69, 88, 111] and interleukin (IL)-6 [121]. Unsurprisingly, AICAR also robustly activates the AMPK-related pathway in C2C12 [31, 56, 76, 94, 109, 110, 115, 117, 121], L6 [43, 60, 90, 91, 98] and primary human myotubes [22, 61], with only one study lacking this effect [98]. Similarly, acetyl-CoA carboxylase (ACC) activation is a common finding across all cell types following AICAR treatment (C2C12, [51, 76]; L6, [43, 60, 98]; human [36]). Other exercise-like changes reported following AICAR treatment include increased GLUT4 protein content [91, 107], glucose uptake or oxidation [17, 66, 77, 98, 107], fatty acid uptake or oxidation [60, 66, 82, 98], insulin responsiveness [10, 124], GLUT4 translocation [43, 107], mitochondrial content [22] and IL-6 secretion [110]. However, in contrast to some of these above-described findings, other studies demonstrate a lack of effect of AICAR upon PGC-1α mRNA expression [6, 110], pyruvate dehydrogenase kinase 4 (PDK4) mRNA expression [6, 64], FNDC5 (gene that encodes for irisin) mRNA expression [110], as well as lack of effect upon protein kinase B (Akt) protein content [56] and activation [31, 43], as well as Akt substrate 160 (AS160) activation [31, 43]. Findings regarding MAPK activation are currently inconsistent, with increased activation [121] and no effect [51] reported. Additionally, during skeletal muscle contraction, intracellular ATP, PCr and glycogen levels decline. However, reports have demonstrated that ATP content remains unchanged following AICAR exposure in C2C12 [117] and L6 [43] myotubes, with no evidence of the effects upon PCr or glycogen content. Similarly, in contrast to in vivo exercise, AICAR treatment has been shown to have no effect upon myotube lactate production [51] and appears to inhibit hypertrophy [31] and mitochondrial function [117]. The wide range of AICAR protocol parameters used (as detailed in Table 2), including duration (e.g. 2 mins up to 8 days), concentration (e.g. 0.05 mM up to 10 mM) and single vs. repeated/intermittent exposure, likely contribute to the aforementioned discrepant findings and therefore should be considered when interpreting results.
Caffeine
Background
Skeletal muscle contraction results from sustained elevations in intracellular Ca2+concentration, inducing several downstream effects including activation of the Ca2+/calmodulin-dependent protein kinase II (CaMKII) pathway. Ca2+ ionophores are compounds which increase intracellular Ca2+ concentrations and thus have been used to study the effects of exercise-like CaMKII pathway activation in skeletal muscle myotubes. Caffeine triggers Ca2+ release from the sarcoplasmic reticulum and increases intracellular Ca2+ concentration in skeletal muscle [89] and is one compound that has been used for this purpose.
Applications
Caffeine treatment has been applied to skeletal muscle myotubes as a Ca2+ ionophore, as well as with the specific intent to provide an ‘exercise-like treatment’ [79], mimic exercise activation [64, 87] or exercise signals [91] and trigger exercise-like Ca2+ changes [90]. In this context, caffeine has been used to examine exercise-regulated changes in signal transduction and metabolism in L6 [6, 28, 89,90,91] and C2C12 cells [79, 113]. To date, caffeine has been less readily applied to primary human myotubes than other compounds discussed in this review, and in the studies that have, healthy donor myotubes are primarily used [64, 87].
Comparison to in vivo exercise
Caffeine has been shown to stimulate several exercise-like effects within skeletal muscle myotubes. Unsurprisingly, caffeine consistently induces Ca2+ transients and thus can induce contraction [35, 89, 90, 115]. Caffeine has been shown to increase mRNA expression of markers of mitochondrial biogenesis and oxidative metabolism, such as PGC-1α [6, 87, 113], GLUT4 [87], PDK4 [6, 64, 87] and citrate synthase (CS) [6]. Protein abundance of GLUT4 [91, 113] and mitochondrial enzyme [89] proteins, as well as fatty acid uptake/oxidation [90] and mitochondrial function [113], have been shown to be increased following caffeine treatment, similar to exercise in vivo. Conversely, some inconsistent effects have also been reported. For example, AMPK activation has been shown to be increased [113, 115] and unchanged [90] following caffeine treatment. Similarly, increased [113] and inhibited [35] glucose uptake or oxidation and increased [89, 90] and unchanged [113] mitochondrial content have also been reported following caffeine treatment. Caffeine administration to primary human myotubes has been shown to induce several responses different to those seen following in vivo exercise, such as reduced FNDC5 expression [87] and insulin responsiveness [35], with the latter being a stark contrast to the insulin-sensitising effect of exercise in vivo. Additionally, limited data exist regarding the effects of caffeine on other exercise-responsive signalling pathways, such as ACC, Akt, AS160 and MAPK activation, intracellular content of high-energy phosphates (e.g. ATP and PCr) and aspects of skeletal muscle metabolism (e.g. GLUT4 translocation), morphology and myokine secretion. Similar to EPS and AICAR models, variation in the exact protocols used within caffeine-based models (as detailed in Table 2) with regard to duration (e.g. 2 mins up to 48 h), concentration (e.g. 0.05 mM up to 20 mM) and use of single vs. repeated/intermittent pulses likely contribute to inconsistent results between studies.
Palmitate, forskolin and ionomycin (PFI)
Background
Exercise enhances in vivo fat oxidation whilst stimulating both Ca2+ and adrenergic (cyclic AMP (cAMP) and PKA) signalling pathways within skeletal muscle. As such, compounds that increase lipid oxidation in skeletal muscle in vitro and activate one or both of these pathways provide tools which can be used to delineate the role of the specific pathway in exercise-mediated changes within skeletal muscle. Palmitate (in low concentrations, increases lipid oxidative capacity of skeletal muscle myotubes without impairing insulin sensitivity [118]), forskolin (raises intracellular cAMP and activates PKA) and ionomycin (a Ca2+ ionophore) have been used alone or in combination for this purpose.
Applications
Of the compounds discussed within this review, ionomycin/forskolin/PFI have the highest proportion of articles where it has been applied with the specific intention of providing an exercise-like treatment in vitro, with terminology used including ‘exercise mimetic signalling compounds’ [9], ‘exercise mimetic stimulation’ [24], ‘in vitro exercise model’ [25] or ‘exercise mimicking cocktail’ [65]. Ionomycin or forskolin alone have been applied to C2C12 or L6 cells to investigate skeletal muscle signalling [6, 76, 90], metabolism [55, 90] or myokine release and expression [114]. Primary applications of ionomycin or forskolin alone to primary human myotubes include investigations into intracellular signalling and myokine release or expression [9, 14, 59, 87], with myotubes primarily established from healthy donors. These compounds have also frequently been used in combination with palmitate as part of a lipolytic cocktail (palmitate, forskolin, ionomycin or PFI). Within this context, PFI has almost solely been applied to primary human myotubes to investigate exercise-related signalling [4, 22, 65], metabolism [22, 118] and myokine release/regulation [65]. Additionally, most of these studies have worked to determine if donor-specific responses exist, including sedentary vs. active vs. obese vs. T2DM [22], healthy vs. T2DM [4, 65] and sedentary vs. active vs. T2DM [24] comparisons. For example, lipid-droplet protein perilipin 3 (PLIN3) content, which is associated with lipid metabolism in vivo, increases after both exercise and PFI treatment [23]. Interestingly, the PFI-induced increase in PLIN3 protein was greatest in sedentary donor myotubes, with gradual increases in T2DM donor myotubes and minimal changes in active donor myotubes which almost exclusively favour the expression of another perilipin protein, PLIN5, following PFI treatment [24]. Additionally, differential myokine expression has been reported following PFI treatment, with IL-8 increased vs. decreased in myotubes from sedentary vs. active donors following PFI, respectively [25].
Comparison to in vivo exercise
Forskolin alone demonstrates few responses similar to exercise in vivo. Whilst forskolin-induced upregulation of PGC-1α mRNA expression [87] is similar to exercise in vivo, negative effect upon IL-6 and FNDC5 mRNA expression [9] and a lack of effect upon AMPK and ACC activation [76] suggest that forskolin alone does not satisfactorily resemble enough aspects of in vivo exercise. In contrast, ionomycin application to skeletal muscle myotubes has been shown to increase PGC-1α [6, 87], IL-6 [9, 59] and CS [6] mRNA expression, Ca2+ transients [59, 90], AS160 activation [28], glucose uptake and oxidation [55] and GLUT4 translocation [28]. That said, evidence from ionomycin only experiments have shown a lack of effect upon ATP and PCr levels [90] and PDK4 mRNA expression levels [6]. PFI has been primarily applied to primary human myotubes, and several exercise-like effects have been shown. Firstly, PFI treatment reportedly stimulates contraction of myotubes [118], as well as increases in PGC-1α [65] and IL-6 [25] mRNA expression, AMPK activation [22], insulin responsiveness (at the level of glycogen storage [118]), fatty acid uptake and oxidation [118] and mitochondrial content [22, 118]. However, unlike exercise, PFI has been shown to have no effect upon CS mRNA expression [118], glucose uptake [118] and lactate production [118], as well as inducing a reduction in FNDC5 mRNA expression [65]. The apparent greater consistency of results between PFI-based studies (compared to relative inconsistency within the other models discussed) is likely attributable, at least in part, to the lower number of protocol variations used across studies (summarised in Table 2). That said, to date, the effects of PFI upon several exercise-like responses, including GLUT4 mRNA expression, protein content and translocation, PDK4 mRNA expression, Ca2+ transients, intracellular ATP, PCr and glycogen content as well as exercise-responsive signalling cascades (such as activation of ACC, Akt, AS160 and MAPK), have not been fully characterised.
Other pharmacological compounds
Theoretically, any compound that can stimulate exercise-like responses at transcriptional, signalling and metabolic levels may be a viable tool to investigate exercise-related adaptations. A23187, a Ca2+ ionophore also known as calcimycin, has been applied to C2C12 or L6 myotubes to increase intracellular Ca2+ content [3, 29, 44, 72, 115], as well as with a stated intention to serve as an exercise-like treatment [38, 54]. A23187 has been shown to increase IL-6 mRNA expression [3], myosin heavy chain (MHC)-I expression [29], activate AMPK [115], increase hexokinase II expression [44], LDH activity [54] and increase p38 phosphorylation [72]. However, further studies establishing the effects of A23187 on primary human myotubes are required.
Improved glucose uptake and fatty acid oxidation have been reported in L6 myotubes following treatment with ex229 (an AMPK activator) [66]. Similarly, AMPK activator, R419, reportedly increased glucose uptake and GLUT4 promotor activity in C2C12 myotubes [57]. SRT170 provides another compound with which AMPK activation and mitochondrial biogenesis can be upregulated in C2C12 myotubes [92]. Peroxisome proliferator-activated receptor (PPAR) β/δ levels increase in skeletal muscle during exercise and are central to exercise-mediated metabolic regulation. PPAR agonists, such as GW501516, have also been used to study the effects of exercise-like activation of PPAR pathways in primary human myotubes. GW501516 elicits AMPK and ACC activation, enhanced glucose uptake, fatty acid uptake and oxidation, as well as upregulation of lipid metabolism-related genes (e.g. PDK4) [63, 122].
To induce exercise-like reactive oxygen species (ROS) production, hydrogen peroxide (H2O2) has been applied to C2C12 and L6 myotubes, demonstrating exercise-like effects with regard to increased mRNA expression of PGC-1α, CS and PDK4 [6] and genes involved in ribosome biogenesis [79]. Finally, nitrate (NaNO3) treatment of C2C12 myotubes has been shown to increase mRNA expression of PGC-1α, PPARδ, MYH7 and MYH2, as well as induce the release of suspected muscle-derived factors into conditioned media (e.g. irisin and beta-aminoisobutyric acid). Such observations have led authors to conclude that NaNO3 induces responses that mimic exercise [103].
All of the above-described approaches to induce exercise-like effects in vitro have their advantages. However, several science gaps urgently warrant exploration. Namely, the effects of these different modalities on functional measures like glucose uptake, myokine secretion and oxygen consumption should be determined in primary human myotubes. Such future work would better characterise the response to each treatment and allow for a thorough comparison to corresponding in vivo measurements of the same functional outcomes.
Limitations and future developments applicable to all approaches
Each of the experimental models discussed has their own specific limitations. Additionally, there are several limitations that apply to most or all of the aforementioned models. These are discussed below along with suggestions for improving methodological clarity and improving the physiological nature of such models. See Fig. 2 for a summary of this discussion.
Summary of limitations and possible future improvements applicable to all in vitro skeletal muscle-specific exercise-like models. Several limitations inherent to all models discussed have been identified from existing literature (highlighted red), and possible improvements have been suggested (highlighted green). See text for thorough discussion of each limitation/improvement
The absence of aspects of in vivo exercise physiology
A myriad of physiological changes occur during exercise in vivo, including increased blood flow and alterations in circulating substrates and molecules (e.g. hormones), microenvironment (e.g. local hypoxia, temperature) and autonomic nervous system [46]. The absence of these exercise-mediated stimuli as well as a lack of cell-extracellular matrix interaction and mechanical load [55] should be acknowledged when making conclusions using data derived from these in vitro models. The lack of flow across skeletal muscle cells during culture/exercise-like treatment can result in a static accumulation of secreted factors, which is particularly relevant within cross-talk experiments where final concentrations of secreted factors can become supraphysiological. Additionally, substrate uptake is regulated by a balance of delivery, transport and metabolism [105], and during exercise in vivo the balance between carbohydrate and fatty acid oxidation shifts depending on exercise duration and intensity. However, the lack of flow across skeletal muscle cells (i.e. no change in substrate delivery) and the use of only one energy source in most studies during exercise-like treatment mean current models do not account for these important aspects of in vivo exercise physiology. Introduction of a perifusion system whereby culture media can be continuously circulated would prevent static accumulation of secreted factors and allow alterations in the presence of exercise-responsive hormones and/or fuel source provision during treatment to be made. Inclusion of mechanical stretching to resemble exercise-like mechanical load may be a useful future addition because it has been shown to activate AMPK [81, 106], Akt [106, 116], CaMKII [55, 106], and p70S6 kinase [80, 81]-related signalling pathways, and to increase glucose uptake [55], GLUT4 translocation [106] and myotube hypertrophy [116]. It should be noted that mechanical stretching is not discussed fully in this review because it requires flexible and patterned culture membranes within stretch chambers that are not applicable to conventional 2D culture, the focus of this review. Finally, it should be noted that adaptations to repeated exercise during a period of training result from transient activation followed by inactivation of exercise-related transcriptional and signalling responses within skeletal muscle [30]. In contrast, exercise-like treatment models where the stimulus is often applied chronically over many hours or days may not accurately reflect the episodic nature of in vivo exercise and thus may explain some of the discordances in effects seen following in vivo exercise vs. exercise-like treatment in vitro. That said, efforts to resemble the episodic nature of in vivo exercise within exercise-like treatment models have been made, with EPS (e.g. [16]), AICAR [60, 91], caffeine [89,90,91] and PFI [22,23,24,25, 65, 118] applied through daily pulses interspersed by periods of ‘rest’. Overall, a combination of these exercise-like treatments with other techniques, such as hypoxic cell culture conditions (which has recently been combined with EPS [39]), mechanical stretching, co-culturing with other cell types and perifusion systems, could make each model more representative of in vivo exercise physiology.
Difficulty comparing between and replicating studies
Differences in routine cell culture procedures and ‘exercise treatment’ protocols used can make direct comparisons between and replication of studies difficult. For example, media constituents (e.g. serum and glucose concentration), days of differentiation, cell seeding densities, the presence of plate coating, media change frequency, cell passage and sample harvesting time could all feasibly influence how cells respond to each exercise-like treatment. Equally, differences in parameters used in EPS-based (duration, frequency, pulse duration; as detailed previously [86]) and compound-based (duration, concentration, single vs. repeated/intermittent pulses; as summarised in Table 2) experiments all likely contribute to different responses. Whilst such differences in protocols across studies is not a limitation of the models per se, these details are often omitted and/or vary greatly across studies, highlighting the need for caution before making direct comparisons across studies. That said, there is one exception: protocol parameter selection in the existing literature that has used PFI as a stimulus is admirably consistent. Additionally, the selection of the specific protocol parameters used in published work, such as concentration (compound-based methods), duration (all methods), frequency and voltage (EPS), have very likely been informed by dose-response and/or time course pilot experiments. The results of such pilot experiments, along with specific protocol information and video footage demonstrating myotube contractility, should be presented in publications or at the very least within the supplementary material. This would improve consistency and reduce systematic error in repeated work and would facilitate fairer between-study comparisons and therefore improve the quality of data interpretations.
Lack of a time course of the post-treatment changes
As determined by serial skeletal muscle biopsies following exercise cessation, the evidence demonstrates a time-dependent effect upon exercise-regulated signalling events within skeletal muscle in vivo (e.g. [19, 30, 70, 71]). It is likely that time-dependent activation also likely occurs following cessation of exercise-like treatment in vitro. Indeed, serial sample harvests have demonstrated that PFI-induced changes in IL-6 mRNA expression [25], and EPS-induced GLUT4 recycling [83], IL-6 mRNA expression [7], PPARδ mRNA expression [93] and myotube hypertrophy [119] exhibit significant time effects over the period following treatment cessation. However, most studies harvest samples at a single time point following treatment cessation, which can vary across studies, meaning the time-course for changes following each of these treatments is not well characterised. Therefore, time-course studies incorporating serial sample harvests following treatment cessation are required to ensure changes with different kinetics (e.g. rapid, transient or delayed) are not missed and thus allow full characterisation of responses to each model.
The exercise-like effect of each model is not fully characterised
As addressed in each section above and clearly demonstrated by the question marks in Table 1, several functional and molecular responses to in vivo exercise have not been similarly examined nor characterised in each in vitro model. For example, whilst EPS- and AICAR-based models are fairly well characterised, large gaps remain regarding the effects of the other pharmacological compounds on key exercise-responsive intracellular signalling and metabolic changes. Without such information, a full comparison of each in vitro model to in vivo exercise is not possible. Therefore, to fully characterise the extent to which each model resembles in vivo exercise, future studies should incorporate relevant exercise-responsive measures (referred to as positive controls in the next section) to address the gaps highlighted in Table 1.
Experiments conducted under abnormal/disease-like conditions without a normal/healthy comparator condition
While cell lines offer consistent, robust and predictable cell growth patterns, excessive culture durations and handling make cell lines prone to deviate from normal function. Additionally, donor characteristics (which are to an extent retained in cultured myotubes [1]), biopsy site (which can influence cell proliferation and differentiation rates [12] and fibre type heterogeneity/distribution) and reason for biopsy sample collection (including surgical biopsies, which can introduce confounding effects of non-specified chronic disease) could all influence how primary human myotubes respond to each treatment. Additionally, culture media glucose concentration prior to and during these exercise-like treatments varies from 5 to 25 mM. Since exposure to high glucose concentrations (i.e. 25 mM) can induce insulin resistance in skeletal muscle myotubes [42, 53, 125], studies that have cultured myotubes in hyperglycaemic conditions prior to and during the respective exercise-like treatment (for example, EPS-based [7, 26, 52, 75, 93, 126], AICAR-based [88, 94, 110] and caffeine-based [113, 115] models) may have been conducted under disease-inducing conditions. Therefore, acknowledging whether the effects of these exercise-like treatments have been investigated in the context of health (e.g. insulin responsive) or disease (e.g. insulin resistant) is important for increasing the accuracy of data interpretation and subsequent impact.
Choice of terminology
As mentioned in each section, the choice of terminology used when describing each treatment varies greatly across studies and may not always be fully appropriate. For example, terms such as exercise mimetic or exercise treatment inherently imply that the tool copies all aspects of exercise, which, as discussed throughout, is not the case. Equally, whilst altering the specific protocol parameters (EPS—duration, frequency, pulse duration; pharmacological compound—duration, concentration, single vs. repeated/intermittent pulses) can evoke different responses that may align a particular protocol with a particular type of exercise, any one protocol can induce both similarities and differences to both endurance and resistance exercise. Moreover, an apparent focus of compound-based literature (particularly caffeine and PFI) upon endurance exercise-like responses, and a relative dearth of information addressing the potential effects upon resistance exercise-like responses preclude the ability to exclude the possibility that a single protocol does indeed evoke a hybrid of endurance-like and resistance-like effects in vitro. Therefore, whilst the notion of designing distinct endurance exercise-like or resistance exercise-like treatments is an appealing one, current evidence does not permit researchers to confidently state that protocol A fully resembles endurance exercise, and protocol B fully resembles resistance exercise. Such considerations strongly influenced our decision to not directly compare each experimental model to specific types of exercise and clearly highlight the need for careful choice of terminology when referring to each treatment. For example, terminology such as in vitro skeletal muscle-specific exercise-like treatment (or shortened to ‘exercise-like treatment’ as throughout this review) may provide a more accurate and conservative description of this set of models in general. Better still, stating whether the model is being used to target skeletal muscle contraction or specific exercise-responsive signalling pathways would improve consistency between studies, as well as enhance the quality of data interpretation.
Model selection and practical considerations
In vitro, muscle-specific exercise-like treatments enable us to probe specific molecular mechanisms of exercise adaptations. This is not otherwise possible using human exercise studies alone. Each of these in vitro models induces several exercise-like changes in skeletal muscle cells. This includes the enhancement of glucose metabolism and activation of several exercise-responsive signalling pathways. Nevertheless, each model has different advantages and careful consideration is required before selecting a single model.
Research aim
Firstly, the specific research aim should form the basis of this decision. For example, if the aim is to investigate skeletal muscle contraction per se and all signalling pathways that contraction regulates, EPS may be preferable. In contrast, if the aim is to target a single, specific exercise-responsive signalling pathway (e.g. AMPK, cAMP/PKA, CaMKII, etc.) to investigate downstream targets/metabolic outcomes, then utilising the appropriate pharmacological agonist would likely be more appropriate. The main target for each application is summarised in Fig. 1. That said, as discussed above, these compounds often activate multiple pathways and have additional off-target effects. This must be considered when designing protocols and interpreting results.
Equipment available
EPS requires specialised equipment that can be expensive and difficult to maintain, whereas the use of pharmacological agonist compounds simply requires the addition of such compounds to cell culture media. Therefore, a cost-benefit analysis of logistics, equipment, and finances available must also be considered.
Controls
When investigating the effects of each of these exercise-like treatments, it is important to include measurements that can be used to confirm the treatment has induced some exercise-like effect. For example, assessment of muscle contraction, glucose metabolism, exercise-related signalling and myokine secretion provide appropriate positive controls to determine the extent to which a model is able to resemble aspects of in vivo exercise. In cross-talk experiments, additional controls whereby the exercise-like treatment is applied to media without cells are also important. Implementation of such controls in EPS-based models is recommended by Nikolić et al. [86] and is discussed above. Within compound-based experiments, such controls would involve incubating the target cell in fresh (i.e. non-conditioned) compound-containing media to investigate whether the compound alone impacts the target cell. Such controls have been implemented previously (e.g. [94]) and are vital to exclude the possibility that observed effects result from the compound that remains in the skeletal muscle cell conditioned media rather than factors secreted from skeletal muscle cells.
Future applications
As discussed throughout, each model has been used to answer different exercise-related questions. Because many research questions remain, in vitro models will likely form a large component of future research in this field. For example, application of these models, where possible, to primary human skeletal muscle myotubes as a more physiologically and genetically relevant model of human health and disease [1] would enhance the impact of findings gained. In particular, the evidence demonstrates the preservation of metabolic phenotype in myotubes established from different donors (e.g. healthy, obese, diabetic [1]), as well as following acute/chronic exercise in vivo (e.g. improved lipid metabolism and glucose metabolism) [13, 73] and surgery [48]. Therefore, application of these in vitro exercise-like treatments to myotubes derived from human donors of different disease states and/or exercise statuses may prove most useful in the identification of new molecular targets to prevent/treat disease. Similarly, application of these models to myotubes isolated from individuals showing different adaptations to the same exercise stimulus (i.e. responders vs. non-responders) will advance our understanding of inter-individual variability in the response to exercise. Such an approach would contribute to reducing the occurrence of a non-response to exercise.
Conclusion
The application of EPS or various pharmacological compounds to skeletal muscle myotubes provide distinct experimental models able to induce some exercise-like effects in vitro and therefore provide valuable high-throughput tools to study the skeletal muscle cell biology of exercise-mediated health benefits. By targeting muscle contraction (e.g. using EPS) or exercise-related signalling pathways (e.g. AMPK or CaMKII using pharmacological compounds), specific aspects of muscle function and metabolism can be isolated using in vitro approaches. Future optimisation to enhance the physiological nature of these current in vitro models will further enhance their impact within muscle biology research and thus contribute to addressing key challenges in this field, such as maximising the therapeutic potential of exercise and facilitating the identification of novel exercise-responsive drug targets.
References
Aas V, Bakke SS, Feng YZ, Kase ET, Jensen J, Bajpeyi S, Thoresen GH, Rustan AC (2013) Are cultured human myotubes far from home? Cell Tissue Res 354:671–682. https://doi.org/10.1007/s00441-013-1655-1
Aas V, Torbla S, Andersen MH, Jensen J, Rustan AC (2002) Electrical stimulation improves insulin responses in a human skeletal muscle cell model of hyperglycemia. Ann N Y Acad Sci 967:506–515. https://doi.org/10.1111/j.1749-6632.2002.tb04309.x
Allen DL, Uyenishi JJ, Cleary AS, Mehan RS, Lindsay SF, Reed JM (2010) Calcineurin activates interleukin-6 transcription in mouse skeletal muscle in vivo and in C2C12 myotubes in vitro. Am J Physiol Regul Integr Comp Physiol 298:R198–R210. https://doi.org/10.1152/ajpregu.00325.2009
Bajpeyi S, Covington JD, Taylor EM, Stewart LK, Galgani JE, Henagan TM (2017) Skeletal muscle PGC1α - 1 nucleosome position and - 260 ntdnamethylationdetermine exercise response and prevent ectopic lipid accumulation in men. Endocrinology 158:2190–2199. https://doi.org/10.1210/en.2017-00051
Barlow J, Carter S, Solomon T (2018) Probing the effect of physiological concentrations of IL-6 on insulin secretion by INS-1 832/3 insulinoma cells under diabetic-like conditions. Int J Mol Sci 19:1924. https://doi.org/10.3390/ijms19071924
Barrès R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, Caidahl K, Krook A, O’Gorman DJ, Zierath JR (2012) Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab 15:405–411. https://doi.org/10.1016/j.cmet.2012.01.001
Beiter T, Hudemann J, Burgstahler C, Nieß AM, Munz B (2018) Effects of extracellular orotic acid on acute contraction-induced adaptation patterns in C2C12 cells. Mol Cell Biochem 1–13. https://doi.org/10.1007/s11010-018-3330-z
Benziane B, Bjornholm M, Pirkmajer S, Austin RL, Kotova O, Viollet B, Zierath JR, Chibalin AV (2012) Activation of AMP-activated protein kinase stimulates Na+,K+-ATPase activity in skeletal muscle cells. J Biol Chem 287:23451–23463. https://doi.org/10.1074/jbc.M111.331926
Besse-Patin A, Montastier E, Vinel C, Castan-Laurell I, Louche K, Dray C, Daviaud D, Mir L, Marques MA, Thalamas C, Valet P, Langin D, Moro C, Viguerie N (2014) Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. Int J Obes 38:707–713. https://doi.org/10.1038/ijo.2013.158
Bikman BT, Zheng D, Reed MA, Hickner RC, Houmard JA, Dohm GL (2010) Lipid-induced insulin resistance is prevented in lean and obese myotubes by AICAR treatment. Am J Physiol Regul Integr Comp Physiol 298:R1692–R1699. https://doi.org/10.1152/ajpregu.00190.2009
Bird SR, Hawley JA (2017) Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med 2:e000143. https://doi.org/10.1136/bmjsem-2016-000143
Bonavaud S, Thibert P, Gherardi RK, Barlovatz-Meimon G (1997) Primary human muscle satellite cell culture: variations of cell yield, proliferation and differentiation rates according to age and sex of donors, site of muscle biopsy, and delay before processing. Biol Cell 89:233–240. https://doi.org/10.1016/S0248-4900(97)80039-1
Bourlier V, Saint-Laurent C, Louche K, Badin PM, Thalamas C, De GI, Langin D, Sengenes C, Moro C (2013) Enhanced glucose metabolism is preserved in cultured primary Myotubes from obese donors in response to exercise training. J Clin Endocrinol Metab 98:3739–3747. https://doi.org/10.1210/jc.2013-1727
Broholm C, Mortensen OH, Nielsen S, Akerstrom T, Zankari A, Dahl B, Pedersen BK (2008) Exercise induces expression of leukaemia inhibitory factor in human skeletal muscle. J Physiol 586:2195–2201. https://doi.org/10.1113/jphysiol.2007.149781
Brown AE, Jones DE, Walker M, Newton JL (2015) Abnormalities of AMPK activation and glucose uptake in cultured skeletal muscle cells from individuals with chronic fatigue syndrome. PLoS One 10:1–14. https://doi.org/10.1371/journal.pone.0122982
Burch N, Arnold AS, Item F, Summermatter S, Santos GBS, Christe M, Boutellier U, Toigo M, Handschin C (2010) Electric pulse stimulation of cultured murine muscle cells reproduces gene expression changes of trained mouse muscle. PLoS One 5:e10970. https://doi.org/10.1371/journal.pone.0010970
Chen HC, Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert M, Farese RV, Farese RV (2002) Activation of the ERK pathway and atypical protein kinase C isoforms in exercise- and aminoimidazole-4-carboxamide- 1-β-d-riboside (AICAR)-stimulated glucose transport. J Biol Chem 277:23554–23562. https://doi.org/10.1074/jbc.M201152200
Christensen CS, Christensen DP, Lundh M, Dahllöf MS, Haase TN, Velasquez JM, Laye MJ, Mandrup-Poulsen T, Solomon TPJ (2015) Skeletal muscle to pancreatic β-cell cross-talk: the effect of humoral mediators liberated by muscle contraction and acute exercise on β-cell apoptosis. J Clin Endocrinol Metab 100:E1289–E1298. https://doi.org/10.1210/jc.2014-4506
Coffey VG (2005) Early signaling responses to divergent exercise stimuli in skeletal muscle from well-trained humans. FASEB J 19:1–20. https://doi.org/10.1096/fj.05-4809fje
Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, Horton ES, Castorino K, Tate DF (2016) Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care 39:2065–2079. https://doi.org/10.2337/dc16-1728
Corton JM, Gillespie JG, Hawley SA, Hardie DG (1995) 5-aminoimidazole-4-carboxamide ribonucleoside—a specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem 229:558–565
Costford SR, Bajpeyi S, Pasarica M, Albarado DC, Thomas SC, Xie H, Church TS, Jubrias SA, Conley KE, Smith SR (2010) Skeletal muscle NAMPT is induced by exercise in humans. Am J Physiol Endocrinol Metab 298:E117–E126. https://doi.org/10.1152/ajpendo.00318.2009
Covington JD, Galgani JE, Moro C, LaGrange JM, Zhang Z, Rustan AC, Ravussin E, Bajpeyi S (2014) Skeletal muscle perilipin 3 and coatomer proteins are increased following exercise and are associated with fat oxidation. PLoS One 9:1–8. https://doi.org/10.1371/journal.pone.0091675
Covington JD, Noland RC, Hebert RC, Masinter BS, Smith SR, Rustan AC, Ravussin E, Bajpeyi S (2015) Perilipin 3 differentially regulates skeletal muscle lipid oxidation in active, sedentary, and type 2 diabetic males. J Clin Endocrinol Metab 100:3683–3692. https://doi.org/10.1210/JC.2014-4125
Covington JD, Tam CS, Bajpeyi S, Galgani JE, Noland RC, Smith SR, Redman LM, Ravussin E (2016) Myokine expression in muscle and myotubes in response to exercise stimulation. Med Sci Sports Exerc 48:384–390. https://doi.org/10.1249/MSS.0000000000000787
Danilov K, Sidorenko S, Milovanova K, Klimanova E, Kapilevich LV, Orlov SN (2017) Electrical pulse stimulation decreases electrochemical Na+ and K+ gradients in C2C12 myotubes. Biochem Biophys Res Commun 493:875–878. https://doi.org/10.1016/j.bbrc.2017.09.133
Das AK, Yang QY, Fu X, Liang JF, Duarte MS, Zhu MJ, Trobridge GD, Du M (2012) AMP-activated protein kinase stimulates myostatin expression in C2C12 cells. Biochem Biophys Res Commun 427:36–40. https://doi.org/10.1016/j.bbrc.2012.08.138
Deng B, Zhu X, Zhao Y, Zhang D, Pannu A, Chen L, Niu W (2017) PKC and Rab13 mediate Ca2+ signal-regulated GLUT4 traffic. Biochem Biophys Res Commun 495:1956–1963. https://doi.org/10.1016/j.bbrc.2017.12.064
Drenning JA, Lira VA, Simmons CG, Soltow QA, Sellman JE, Criswell DS (2008) Nitric oxide facilitates NFAT-dependent transcription in mouse myotubes. Am J Physiol Cell Physiol 294:C1088–C1095. https://doi.org/10.1152/ajpcell.00523.2007
Egan B, Zierath JR (2013) Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17:162–184. https://doi.org/10.1016/j.cmet.2012.12.012
Egawa T, Ohno Y, Goto A, Ikuta A, Suzuki M, Ohira T, Yokoyama S, Sugiura T, Ohira Y, Yoshioka T, Goto K (2014) AICAR-induced activation of AMPK negatively regulates myotube hypertrophy through the HSP72-mediated pathway in C2C12 skeletal muscle cells. Am J Physiol Endocrinol Metab 306:E344–E354. https://doi.org/10.1152/ajpendo.00495.2013
Erickson KA, Smith ME, Anthonymuthu TS, Evanson MJ, Brassfield ES, Hodson AE, Bressler MA, Tucker BJ, Thatcher MO, Prince JT, Hancock CR, Bikman BT (2012) AICAR inhibits ceramide biosynthesis in skeletal muscle. Diabetol Metab Syndr 4:45. https://doi.org/10.1186/1758-5996-4-45
Evers-Van Gogh IJA, Alex S, Stienstra R, Brenkman AB, Kersten S, Kalkhoven E (2015) Electric pulse stimulation of myotubes as an in vitro exercise model: cell-mediated and non-cell-mediated effects. Sci Rep 5:1–11. https://doi.org/10.1038/srep10944
Feng YZ, Nikolić N, Bakke SS, Kase ET, Guderud K, Hjelmesæth J, Aas V, Rustan AC, Thoresen GH (2015) Myotubes from lean and severely obese subjects with and without type 2 diabetes respond differently to an in vitro model of exercise. Am J Physiol Cell Physiol 308:C548–C556. https://doi.org/10.1152/ajpcell.00314.2014
Freymond D, Guignet R, Lhote P, Passaquin AC, Rüegg UT (2002) Calcium homeostasis and glucose uptake of murine myotubes exposed to insulin, caffeine and 4-chloro-m-cresol. Acta Physiol Scand 176:283–292. https://doi.org/10.1046/j.1365-201X.2002.01039.x
Fritzen AM, Madsen AB, Kleinert M, Treebak JT, Lundsgaard AM, Jensen TE, Richter EA, Wojtaszewski J, Kiens B, Frøsig C (2016) Regulation of autophagy in human skeletal muscle: effects of exercise, exercise training and insulin stimulation. J Physiol 594:745–761. https://doi.org/10.1113/JP271405
Fujita H, Nedachi T, Kanzaki M (2007) Accelerated de novo sarcomere assembly by electric pulse stimulation in C2C12 myotubes. Exp Cell Res 313:1853–1865. https://doi.org/10.1016/j.yexcr.2007.03.002
Goode JM, Pearen MA, Tuong ZK, Wang SCM, Oh TG, Shao EX, Muscat GEO (2016) The nuclear receptor, Nor-1, induces the physiological responses associated with exercise. Mol Endocrinol 30:660–676. https://doi.org/10.1210/me.2015-1300
Görgens SW, Benninghoff T, Eckardt K, Springer C, Chadt A, Melior A, Wefers J, Cramer A, Jensen J, Birkeland KI, Drevon CA, Al-Hasani H, Eckel J (2017) Hypoxia in combination with muscle contraction improves insulin action and glucose metabolism in human skeletal muscle via the HIF-1α pathway. Diabetes 66:2800–2807. https://doi.org/10.2337/db16-1488
Görgens SW, Hjorth M, Eckardt K, Wichert S, Norheim F, Holen T, Lee S, Langleite T, Birkeland KI, Stadheim HK, Kolnes KJ, Tangen DS, Kolnes AJ, Jensen J, Drevon CA, Eckel J (2016) The exercise-regulated myokine chitinase-3-like protein 1 stimulates human myocyte proliferation. Acta Physiol 216:330–345. https://doi.org/10.1111/apha.12579
Görgens SW, Raschke S, Holven KB, Jensen J, Eckardt K, Eckel J (2013) Regulation of follistatin-like protein 1 expression and secretion in primary human skeletal muscle cells. Arch Physiol Biochem 119:75–80. https://doi.org/10.3109/13813455.2013.768270
Green CJ, Henriksen TI, Pedersen BK, Solomon TPJ (2012) Glucagon like peptide-1-induced glucose metabolism in differentiated human muscle satellite cells is attenuated by hyperglycemia. PLoS One 7:e44284. https://doi.org/10.1371/journal.pone.0044284
Habegger KM, Hoffman NJ, Ridenour CM, Brozinick JT, Elmendorf JS (2012) AMPK enhances insulin-stimulated GLUT4 regulation via lowering membrane cholesterol. Endocrinology 153:2130–2141. https://doi.org/10.1210/en.2011-2099
Halseth AE, O’Doherty RM, Printz RL, Bracy DP, Granner DK, Wasserman DH (2000) Role of Ca2+ fluctuations in L6 myotubes in the regulation of the hexokinase II gene. J Appl Physiol 88:669–673. https://doi.org/10.1152/jappl.2000.88.2.669
Hardie DG, Ross FA, Hawley SA (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13:251–262. https://doi.org/10.1038/nrm3311
Hawley JA, Hargreaves M, Joyner MJ, Zierath JR (2014) Integrative biology of exercise. Cell 159:738–749. https://doi.org/10.1016/j.cell.2014.10.029
Hawley JA, Lessard SJ (2008) Exercise training-induced improvements in insulin action. Acta Physiol 192:127–135. https://doi.org/10.1111/j.1748-1716.2007.01783.x
Hinkley JM, Zou K, Park S, Turner K, Zheng D, Houmard JA (2017) Roux-en-Y gastric bypass surgery enhances contraction-mediated glucose metabolism in primary human myotubes. Am J Physiol Endocrinol Metab 313:E195–E202. https://doi.org/10.1152/ajpendo.00413.2016
Holloszy JO (2005) Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol 99:338–343. https://doi.org/10.1152/japplphysiol.00123.2005
Hoppeler H (2016) Molecular networks in skeletal muscle plasticity. J Exp Biol 219:205–213. https://doi.org/10.1242/jeb.128207
Hsu CG, Burkholder TJ (2015) Activation of p38 in C2C12 myotubes following ATP depletion depends on extracellular glucose. J Physiol Biochem 71:253–265. https://doi.org/10.1007/s13105-015-0406-z
Hu F, Li N, Li Z, Zhang C, Yue Y, Liu Q, Chen L, Bilan PJ, Niu W (2018) Electrical pulse stimulation induces GLUT4 translocation in a Rac-Akt-dependent manner in C2C12 myotubes. FEBS Lett 592:644–654. https://doi.org/10.1002/1873-3468.12982
Huang C, Somwar R, Patel N, Niu W, Torok D, Klip A (2002) Sustained exposure of L6 myotubes to high glucose and insulin decreases insulin-stimulated GLUT4 translocation but upregulates GLUT4 activity. Diabetes 51:2090–2098. https://doi.org/10.2337/diabetes.51.7.2090
Hurst RD, Wells RW, Hurst SM, McGhie TK, Cooney JM, Jensen DJ (2010) Blueberry fruit polyphenolics suppress oxidative stress-induced skeletal muscle cell damage in vitro. Mol Nutr Food Res 54:353–363. https://doi.org/10.1002/mnfr.200900094
Iwata M, Hayakawa K, Murakami T, Naruse K, Kawakami K, Inoue-Miyazu M, Yuge L, Suzuki S (2007) Uniaxial cyclic stretch-stimulated glucose transport is mediated by a Ca2+-dependent mechanism in cultured skeletal muscle cells. Pathobiology 74:159–168. https://doi.org/10.1159/000103375
Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE (2001) 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 Myotubes in response to 5-aminoimidazole-4-carboxamide Riboside. J Biol Chem 276:46912–46916. https://doi.org/10.1074/jbc.C100483200
Jenkins Y, Sun TQ, Markovtsov V, Foretz M, Li W, Nguyen H, Li Y, Pan A, Uy G, Gross L, Baltgalvis K, Yung SL, Gururaja T, Kinoshita T, Owyang A, Smith IJ, McCaughey K, White K, Godinez G, Alcantara R, Choy C, Ren H, Basile R, Sweeny DJ, Xu X, Issakani SD, Carroll DC, Goff DA, Shaw SJ, Singh R, Boros LG, Laplante MA, Marcotte B, Kohen R, Viollet B, Marette A, Payan DG, Kinsella TM, Hitoshi Y (2013) AMPK activation through mitochondrial regulation results in increased substrate oxidation and improved metabolic parameters in models of diabetes. PLoS One 8:1–19. https://doi.org/10.1371/journal.pone.0081870
Kanzleiter T, Rath M, Görgens SW, Jensen J, Tangen DS, Kolnes AJ, Kolnes KJ, Lee S, Eckel J, Schürmann A, Eckardt K (2014) The myokine decorin is regulated by contraction and involved in muscle hypertrophy. Biochem Biophys Res Commun 450:1089–1094. https://doi.org/10.1016/j.bbrc.2014.06.123
Keller C, Hellsten Y, Steensberg A, Pedersen BK (2006) Differential regulation of IL-6 and TNF-α via calcineurin in human skeletal muscle cells. Cytokine 36:141–147. https://doi.org/10.1016/j.cyto.2006.10.014
Kelly KR, Abbott MJ, Turcotte LP (2010) Short-term AMP-regulated protein kinase activation enhances insulin-sensitive fatty acid uptake and increases the effects of insulin on fatty acid oxidation in L6 muscle cells. Exp Biol Med 235:514–521. https://doi.org/10.1258/ebm.2009.009228
Kitzmann M, Lantier L, Hébrard S, Mercier J, Foretz M, Aguer C (2011) Abnormal metabolism flexibility in response to high palmitate concentrations in myotubes derived from obese type 2 diabetic patients. Biochim Biophys Acta 1812:423–430. https://doi.org/10.1016/j.bbadis.2010.12.007
Kjøbsted R, Hingst JR, Fentz J, Foretz M, Sanz MN, Pehmøller C, Shum M, Marette A, Mounier R, Treebak JT, Wojtaszewski JFP, Viollet B, Lantier L (2018) AMPK in skeletal muscle function and metabolism. FASEB J 32:1741–1777. https://doi.org/10.1096/fj.201700442R
Krämer DK, Al-Khalili L, Guigas B, Leng Y, Garcia-Roves PM, Krook A (2007) Role of AMP kinase and PPARδ in the regulation of lipid and glucose metabolism in human skeletal muscle. J Biol Chem 282:19313–19320. https://doi.org/10.1074/jbc.M702329200
Kulkarni SS, Salehzadeh F, Fritz T, Zierath JR, Krook A, Osler ME (2012) Mitochondrial regulators of fatty acid metabolism reflect metabolic dysfunction in type 2 diabetes mellitus. Metabolism 61:175–185. https://doi.org/10.1016/j.metabol.2011.06.014
Kurdiova T, Balaz M, Mayer A, Maderova D, Belan V, Wolfrum C, Ukropec J, Ukropcova B (2014) Exercise-mimicking treatment fails to increase Fndc5 mRNA & irisin secretion in primary human myotubes. Peptides 56:1–7. https://doi.org/10.1016/j.peptides.2014.03.003
Lai YC, Kviklyte S, Vertommen D, Lantier L, Foretz M, Viollet B, Hallén S, Rider MH (2014) A small-molecule benzimidazole derivative that potently activates AMPK to increase glucose transport in skeletal muscle: comparison with effects of contraction and other AMPK activators. Biochem J 460:363–375. https://doi.org/10.1042/BJ20131673
Lambernd S, Taube A, Schober A, Platzbecker B, Görgens SW, Schlich R, Jeruschke K, Weiss J, Eckardt K, Eckel J (2012) Contractile activity of human skeletal muscle cells prevents insulin resistance by inhibiting pro-inflammatory signalling pathways. Diabetologia 55:1128–1139. https://doi.org/10.1007/s00125-012-2454-z
Li Z, Yue Y, Hu F, Zhang C, Ma X, Li N, Qiu L, Fu M, Chen L, Yao Z, Bilan PJ, Klip A, Niu W (2018) Electrical pulse stimulation induces GLUT4 glucose transporter translocation in C2C12 myotubes that depends on Rab8A, Rab13 and Rab14. Am J Physiol Endocrinol Metab 314:E478–E493. https://doi.org/10.1152/ajpendo.00103.2017
Lira VA, Soltow QA, Long JHD, Betters JL, Sellman JE, Criswell DS (2007) Nitric oxide increases GLUT4 expression and regulates AMPK signaling in skeletal muscle. Am J Physiol Endocrinol Metab 32611:1062–1068. https://doi.org/10.1152/ajpendo.00045.2007
Little JP, Safdar A, Bishop D, Tarnopolsky MA, Gibala MJ (2011) An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1α and activates mitochondrial biogenesis in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 300:R1303–R1310. https://doi.org/10.1152/ajpregu.00538.2010
Louis E, Raue U, Yang Y, Jemiolo B, Trappe S (2007) Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol 47306:1744–1751. https://doi.org/10.1152/japplphysiol.00679.2007
Ludlow AT, Lima LCJ, Wang J, Hanson ED, Guth LM, Spangenburg EE, Roth SM (2012) Exercise alters mRNA expression of telomere-repeat binding factor 1 in skeletal muscle via p38 MAPK. J Appl Physiol 113:1737–1746. https://doi.org/10.1152/japplphysiol.00200.2012
Lund J, Rustan AC, Løvsletten NG, Mudry JM, Langleite TM, Feng YZ, Stensrud C, Brubak MG, Drevon CA, Birkeland KI, Kolnes KJ, Johansen EI, Tangen DS, Stadheim HK, Gulseth HL, Krook A, Kase ET, Jensen J, Thoresen GH (2017) Exercise in vivo marks human myotubes in vitro: training-induced increase in lipid metabolism. PLoS One 12:1–24. https://doi.org/10.1371/journal.pone.0175441
Maarbjerg SJ, Sylow L, Richter EA (2011) Current understanding of increased insulin sensitivity after exercise - emerging candidates. Acta Physiol (Oxford) 202:323–335. https://doi.org/10.1111/j.1748-1716.2011.02267.x
Manabe Y, Miyatake S, Takagi M, Nakamura M, Okeda A, Nakano T, Hirshman MF, Goodyear LJ, Fujii NL (2012) Characterization of an acute muscle contraction model using cultured C2C12 myotubes. PLoS One 7:e52592. https://doi.org/10.1371/journal.pone.0052592
Mason RR, Meex RCR, Lee-Young R, Canny BJ, Watt MJ (2012) Phosphorylation of adipose triglyceride lipase Ser 404 is not related to 5′-AMPK activation during moderate-intensity exercise in humans. Am J Physiol Endocrinol Metab 303:E534–E541. https://doi.org/10.1152/ajpendo.00082.2012
McIntyre EA, Halse R, Yeaman SJ, Walker M (2004) Cultured muscle cells from insulin-resistant type 2 diabetes patients have impaired insulin, but normal 5-amino-4-imidazolecarboxamide riboside-stimulated, glucose uptake. J Clin Endocrinol Metab 89:3440–3448. https://doi.org/10.1210/jc.2003-031919
Miyatake S, Bilan PJ, Pillon NJ, Klip A (2015) Contracting C2C12 myotubes release CCL2 in an NF-κB-dependent manner to induce monocyte chemoattraction. Am J Physiol Endocrinol Metab 310:E160–E170. https://doi.org/10.1152/ajpendo.00325.2015
Mobley CB, Fox CD, Thompson RM, Healy JC, Santucci V, Kephart WC, McCloskey AE, Kim M, Pascoe DD, Martin JS, Moon JR, Young KC, Roberts MD (2016) Comparative effects of whey protein versus L-leucine on skeletal muscle protein synthesis and markers of ribosome biogenesis following resistance exercise. Amino Acids 48:733–750. https://doi.org/10.1007/s00726-015-2121-z
Nakai N, Kawano F, Murakami T, Nakata K, Higashida K (2018) Leucine supplementation after mechanical stimulation activates protein synthesis via L-type amino acid transporter 1 in vitro. J Cell Biochem 119:2094–2101. https://doi.org/10.1002/jcb.26371
Nakai N, Kawano F, Nakata K (2015) Mechanical stretch activates mammalian target of rapamycin and AMP-activated protein kinase pathways in skeletal muscle cells. Mol Cell Biochem 406:285–292. https://doi.org/10.1007/s11010-015-2446-7
Nascimento EB, Osler ME, Zierath JR (2013) Sestrin 3 regulation in type 2 diabetic patients and its influence on metabolism and differentiation in skeletal muscle. Am J Physiol Endocrinol Metab 305:E1408–E1414. https://doi.org/10.1152/ajpendo.00212.2013
Nedachi T, Fujita H, Kanzaki M (2008) Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. Am J Physiol Endocrinol Metab 295:E1191–E1204. https://doi.org/10.1152/ajpendo.90280.2008
Nieuwoudt S, Mulya A, Fealy CE, Martelli E, Dasarathy S, Naga Prasad SV, Kirwan JP (2017) In vitro contraction protects against palmitate-induced insulin resistance in C2C12 myotubes. Am J Physiol Cell Physiol 313:C575–C583. https://doi.org/10.1152/ajpcell.00123.2017
Nikolić N, Bakke SS, Kase ET, Rudberg I, Flo Halle I, Rustan AC, Thoresen GH, Aas V (2012) Electrical pulse stimulation of cultured human skeletal muscle cells as an in vitro model of exercise. PLoS One 7:1–10. https://doi.org/10.1371/journal.pone.0033203
Nikolić N, Görgens SW, Thoresen GH, Aas V, Eckel J, Eckardt K (2017) Electrical pulse stimulation of cultured skeletal muscle cells as a model for in vitro exercise—possibilities and limitations. Acta Physiol 220:310–331. https://doi.org/10.1111/apha.12830
Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, Gulseth HL, Birkeland KI, Jensen J, Drevon CA (2014) The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J 281:739–749. https://doi.org/10.1111/febs.12619
Nsiah-Sefaa A, Brown EL, Russell AP, Foletta VC (2014) New gene targets of PGC-1α and ERRα co-regulation in C2C12 myotubes. Mol Biol Rep 41:8009–8017. https://doi.org/10.1007/s11033-014-3698-0
Ojuka EO, Jones TE, Han DH, Chen M, Holloszy JO (2003) Raising Ca2+ in L6 myotubes mimics effects of exercise on mitochondrial biogenesis in muscle. FASEB J 17:675–681. https://doi.org/10.1096/fj.02-0951com
Ojuka EO, Jones TE, Han DH, Chen M, Wamhoff BR, Sturek M, Holloszy JO (2002) Intermittent increases in cytosolic Ca2+ stimulate mitochondrial biogenesis in muscle cells. Am J Physiol Endocrinol Metab 283:E1040–E1045. https://doi.org/10.1152/ajpendo.00242.2002
Ojuka EO, Jones TE, Nolte LA, Chen M, Wamhoff BR, Sturek M, Holloszy JO (2002) Regulation of GLUT4 biogenesis in muscle: evidence for involvement of AMPK and Ca2+. Am J Physiol Endocrinol Metab 282:E1008–E1013. https://doi.org/10.1152/ajpendo.00512.2001
Park SJ, Ahmad F, Um JH, Brown AL, Xu X, Kang H, Ke H, Feng X, Ryall J, Philp A, Schenk S, Kim MK, Sartorelli V, Chung JH (2017) Specific Sirt1 activator-mediated improvement in glucose homeostasis requires Sirt1-independent activation of AMPK. EBioMedicine 18:128–138. https://doi.org/10.1016/j.ebiom.2017.03.019
Pattamaprapanont P, Garde C, Fabre O, Barrès R (2016) Muscle contraction induces acute hydroxymethylation of the exercise-responsive gene Nr4a3. Front Endocrinol (Lausanne) 7:1–9. https://doi.org/10.3389/fendo.2016.00165
Paula FMM, Leite NC, Vanzela EC, Kurauti MA, Freitas-Dias R, Carneiro EM, Boschero AC, Zoppi CC (2015) Exercise increases pancreatic β-cell viability in a model of type 1 diabetes through IL-6 signaling. FASEB J 29:1805–1816. https://doi.org/10.1096/fj.14-264820
Pedersen BK, Febbraio MA (2008) Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88:1379–1406. https://doi.org/10.1152/physrev.90100.2007
Pedersen BK, Febbraio MA (2012) Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 8:457–465. https://doi.org/10.1038/nrendo.2012.49
Pedersen BK, Saltin B (2015) Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 25:1–72. https://doi.org/10.1111/sms.12581
Pirkmajer S, Kulkarni SS, Tom RZ, Ross FA, Hawley SA, Hardie DG, Zierath JR, Chibalin AV (2015) Methotrexate promotes glucose uptake and lipid oxidation in skeletal muscle via AMPK activation. Diabetes 64:360–369. https://doi.org/10.2337/db14-0508
Pourteymour S, Eckardt K, Holen T, Langleite T, Lee S, Jensen J, Birkeland KI, Drevon CA, Hjorth M (2017) Global mRNA sequencing of human skeletal muscle: search for novel exercise-regulated myokines. Mol Metab 6:352–365. https://doi.org/10.1016/j.molmet.2017.01.007
Raschke S, Eckardt K, Bjørklund Holven K, Jensen J, Eckel J (2013) Identification and validation of novel contraction-regulated myokines released from primary human skeletal muscle cells. PLoS One 8:e62008. https://doi.org/10.1371/journal.pone.0062008
Raschke S, Elsen M, Gassenhuber H, Sommerfeld M, Schwahn U, Brockmann B, Jung R, Wisløff U, Tjønna AE, Raastad T, Hallén J, Norheim F, Drevon CA, Romacho T, Eckardt K, Eckel J (2013) Evidence against a beneficial effect of irisin in humans. PLoS One 8:1–12. https://doi.org/10.1371/journal.pone.0073680
Richter EA, Hargreaves M (2013) Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 93:993–1017. https://doi.org/10.1152/physrev.00038.2012
Roberts LD, Ashmore T, McNally BD, Murfitt SA, Fernandez BO, Feelisch M, Lindsay R, Siervo M, Williams EA, Murray AJ, Griffin JL (2017) Inorganic nitrate mimics exercise-stimulated muscular fiber-type switching and myokine and γ-aminobutyric acid release. Diabetes 66:674–688. https://doi.org/10.2337/db16-0843
Rose AJ, Jeppesen J, Kiens B, Richter EA (2009) Effects of contraction on localization of GLUT4 and v-SNARE isoforms in rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol 297:R1228–R1237. https://doi.org/10.1152/ajpregu.00258.2009
Rose AJ, Richter EA (2005) Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology (Bethesda) 20:260–270. https://doi.org/10.1152/physiol.00012.2005
Saito T, Okada S, Shimoda Y, Tagaya Y, Osaki A, Yamada E, Shibusawa R, Nakajima Y, Ozawa A, Satoh T, Mori M, Yamada M (2016) APPL1 promotes glucose uptake in response to mechanical stretch via the PKCζ-non-muscle myosin IIa pathway in C2C12 myotubes. Cell Signal 28:1694–1702. https://doi.org/10.1016/j.cellsig.2016.07.010
Sajan MP, Bandyopadhyay G, Miura A, Standaert ML, Nimal S, Longnus SL, Van Obberghen E, Hainault I, Foufelle F, Kahn R, Braun U, Leitges M, Farese RV (2010) AICAR and metformin, but not exercise, increase muscle glucose transport through AMPK-, ERK-, and PDK1-dependent activation of atypical PKC. Am J Physiol Endocrinol Metab 298:E179–E192. https://doi.org/10.1152/ajpendo.00392.2009
Salvadó L, Coll T, Gómez-Foix AM, Salmerón E, Barroso E, Palomer X, Vázquez-Carrera M (2013) Oleate prevents saturated-fatty-acid-induced ER stress, inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia 56:1372–1382. https://doi.org/10.1007/s00125-013-2867-3
Sanchez AMJ, Candau R, Bernardi H (2018) AMP-activated protein kinase stabilizes FOXO3 in primary myotubes. Biochem Biophys Res Commun 499:493–498. https://doi.org/10.1016/j.bbrc.2018.03.176
Sánchez J, Nozhenko Y, Palou A, Rodríguez AM (2013) Free fatty acid effects on myokine production in combination with exercise mimetics. Mol Nutr Food Res 57:1456–1467. https://doi.org/10.1002/mnfr.201300126
Sasaki T, Nakata R, Inoue H, Shimizu M, Inoue J, Sato R (2014) Role of AMPK and PPARγ 1 in exercise-induced lipoprotein lipase in skeletal muscle. Am J Physiol Endocrinol Metab 306:E1085–E1092. https://doi.org/10.1152/ajpendo.00691.2013
Scheler M, Irmler M, Lehr S, Hartwig S, Staiger H, Al-Hasani H, Beckers J, De Angelis MH, Haring HU, Weigert C (2013) Cytokine response of primary human myotubes in an in vitro exercise model. Am J Physiol Cell Physiol 305:C877–C886. https://doi.org/10.1152/ajpcell.00043.2013
Schnuck JK, Gould LM, Parry HA, Johnson MA, Gannon NP, Sunderland KL, Vaughan RA (2017) Metabolic effects of physiological levels of caffeine in myotubes. J Physiol Biochem 74:1–11. https://doi.org/10.1007/s13105-017-0601-1
Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW (2012) Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem 287:11968–11980. https://doi.org/10.1074/jbc.M111.336834
Shaw A, Jeromson S, Watterson KR, Pediani JD, Gallagher IJ, Whalley T, Dreczkowski G, Brooks N, Galloway SD, Hamilton DL (2017) Multiple AMPK activators inhibit L-carnitine uptake in C2C12 skeletal muscle myotubes. Am J Physiol Cell Physiol 312:C689–C696. https://doi.org/10.1152/ajpcell.00026.2016
Soltow QA, Zeanah EH, Lira VA, Criswell DS (2013) Cessation of cyclic stretch induces atrophy of C2C12 myotubes. Biochem Biophys Res Commun 434:316–321. https://doi.org/10.1016/j.bbrc.2013.03.048
Spangenburg EE, Jackson KC, Schuh RA (2013) AICAR inhibits oxygen consumption by intact skeletal muscle cells in culture. J Physiol Biochem 69:909–917. https://doi.org/10.1007/s13105-013-0269-0
Sparks LM, Moro C, Ukropcova B, Bajpeyi S, Civitarese AE, Hulver MW, Thoresen GH, Rustan AC, Smith SR (2011) Remodeling lipid metabolism and improving insulin responsiveness in human primary myotubes. PLoS One 6:1–10. https://doi.org/10.1371/journal.pone.0021068
Tarum J, Folkesson M, Atherton PJ, Kadi F (2017) Electrical pulse stimulation: an in vitro exercise model for the induction of human skeletal muscle cell hypertrophy. A proof-of-concept study. Exp Physiol 102:1405–1413. https://doi.org/10.1113/EP086581
Valdés JA, Gaggero E, Hidalgo J, Leal N, Jaimovich E, Carrasco MA (2008) NFAT activation by membrane potential follows a calcium pathway distinct from other activity-related transcription factors in skeletal muscle cells. Am J Physiol Cell Physiol 294:C715–C725. https://doi.org/10.1152/ajpcell.00195.2007
Weigert C, Dufer M, Simon P, Debre E, Runge H, Brodbeck K, Haring HU, Schleicher ED (2007) Upregulation of IL-6 mRNA by IL-6 in skeletal muscle cells: role of IL-6 mRNA stabilization and Ca2+-dependent mechanisms. Am J Physiol Cell Physiol 293:C1139–C1147. https://doi.org/10.1152/ajpcell.00142.2007
Wensaas AJ, Rustan AC, Lövstedt K, Kull B, Wikström S, Drevon CA, Hallén S (2007) Cell-based multiwell assays for the detection of substrate accumulation and oxidation. J Lipid Res 48:961–967. https://doi.org/10.1194/jlr.D600047-JLR200
Whitham M, Chan MHS, Pal M, Matthews VB, Prelovsek O, Lunke S, El-Osta A, Broenneke H, Alber J, Brüning JC, Wunderlich FT, Lancaster GI, Febbraio MA (2012) Contraction-induced interleukin-6 gene transcription in skeletal muscle is regulated by c-Jun terminal kinase/activator protein-1. J Biol Chem 287:10771–10779. https://doi.org/10.1074/jbc.M111.310581
Yuan H, Wang T, Niu Y, Liu X, Fu L (2017) AMP-activated protein kinase-mediated expression of heat shock protein beta 1 enhanced insulin sensitivity in the skeletal muscle. FEBS Lett 591:97–108. https://doi.org/10.1002/1873-3468.12516
Zhang W, Liu J, Tian L, Liu Q, Fu Y, Garvey WT (2013) TRIB3 mediates glucose-induced insulin resistance via a mechanism that requires the hexosamine biosynthetic pathway. Diabetes 62:4192–4200. https://doi.org/10.2337/db13-0312
Zhao Y, Li N, Li Z, Zhang D, Chen L, Yao Z, Niu W (2018) Conditioned medium from contracting skeletal muscle cells reverses insulin resistance and dysfunction of endothelial cells. Metabolism 82:36–46. https://doi.org/10.1016/j.metabol.2017.12.008
Acknowledgements
At the time of writing, TPJS was funded by a Marie Skłodowska-Curie Individual Fellowship awarded by the European Commission and was in receipt of research project grants from the European Foundation for the Study of Diabetes/Astra Zeneca and the Physiological Society.
Author information
Authors and Affiliations
Contributions
Both authors contributed to the writing and edited the final draft of the manuscript. TPJS takes responsibility for the integrity of its content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This article is part of the special issue on Exercise Physiology: future opportunities and challenges in Pflügers Archiv – European Journal of Physiology.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Carter, S., Solomon, T.P.J. In vitro experimental models for examining the skeletal muscle cell biology of exercise: the possibilities, challenges and future developments. Pflugers Arch - Eur J Physiol 471, 413–429 (2019). https://doi.org/10.1007/s00424-018-2210-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2210-4