Abstract
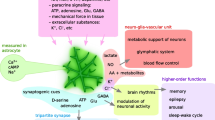
Gamma oscillations (30–100 Hz) represent a physiological fast brain rhythm that occurs in many cortex areas in awake mammals, including humans. They associate with sensory perception, voluntary movement, and memory formation and require precise synaptic transmission between excitatory glutamatergic neurons and inhibitory GABAergic interneurons such as parvalbumin-positive basket cells. Notably, gamma oscillations are exquisitely sensitive to shortage in glucose and oxygen supply (metabolic stress), with devastating consequences for higher cognitive functions. Herein, we explored the robustness of gamma oscillations against changes in the availability of alternative energy substrates and amino acids, which is partially regulated by glial cells such as astrocytes. We used organotypic slice cultures of the rat hippocampus expressing acetylcholine-induced persistent gamma oscillations under normoxic recording conditions (20% oxygen fraction). Our main findings are (1) partial substitution of glucose with pyruvate and the ketone body β-hydroxybutyrate increases the frequency of gamma oscillations, even at different stages of neuronal tissue development. (2) Supplementation with the astrocytic neurotransmitter precursor glutamine has no effect on the properties of gamma oscillations. (3) Supplementation with glycine increases power, frequency, and inner coherence of gamma oscillations in a dose-dependent manner. (4) During these treatments switches to other frequency bands or pathological network states such as neural burst firing or synchronized epileptic activity are absent. Our study indicates that cholinergic gamma oscillations show general robustness against these changes in nutrient and amino acid composition of the cerebrospinal fluid; however, modulation of their properties may impact on cortical information processing under physiological and pathophysiological conditions.






Similar content being viewed by others
References
Achanta LB, Rae CD (2017) β-Hydroxybutyrate in the brain: one molecule, multiple mechanisms. Neurochem Res 42:35–49. https://doi.org/10.1007/s11064-016-2099-2
Alberini CM, Cruz E, Descalzi G, Bessières B, Gao V (2017) Astrocyte glycogen and lactate: new insights into learning and memory mechanisms. Glia 66:1244–1262. https://doi.org/10.1002/glia.23250
Amaral AI (2013) Effects of hypoglycaemia on neuronal metabolism in the adult brain: role of alternative substrates to glucose. J Inherit Metab Dis 36:621–634. https://doi.org/10.1007/s10545-012-9553-3
An JH, Su Y, Radman T, Bikson M (2008) Effects of glucose and glutamine concentration in the formulation of the artificial cerebrospinal fluid (ACSF). Brain Res 1218:77–86. https://doi.org/10.1016/j.brainres.2008.04.007
Aroeira RI, Sebastião AM, Valente CA (2014) GlyT1 and GlyT2 in brain astrocytes: expression, distribution and function. Brain Struct Funct 219:817–830. https://doi.org/10.1007/s00429-013-0537-3
Bak LK, Schousboe A, Waagepetersen HS (2006) The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem 98:641–653
Barth A, Nguyen LB, Barth L, Newell DW (2005) Glycine-induced neurotoxicity in organotypic hippocampal slice cultures. Exp Brain Res 161:351–357
Bélanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14:724–738. https://doi.org/10.1016/j.cmet.2011.08.016
Betz H, Gomeza J, Armsen W, Scholze P, Eulenburg V (2006) Glycine transporters: essential regulators of synaptic transmission. Biochem Soc Trans 34:55–58
Brackmann M, Zhao C, Schmieden V, Braunewell KH (2004) Cellular and subcellular localization of the inhibitory glycine receptor in hippocampal neurons. Biochem Biophys Res Commun 324:1137–1142
Buzsáki G, Buhl DL, Harris KD, Csicsvari J, Czéh B, Morozov A (2003) Hippocampal network patterns of activity in the mouse. Neuroscience 116:201–211
Caeser M, Aertsen A (1991) Morphological organization of rat hippocampal slice cultures. J Comp Neurol 307:87–106
Chattipakorn SC, McMahon LL (2002) Pharmacological characterization of glycine-gated chloride currents recorded in rat hippocampal slices. J Neurophysiol 87:1515–1525
Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J (1995) Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron 15:711–720
Chen RQ, Wang SH, Yao W, Wang JJ, Ji F, Yan JZ, Ren SQ, Chen Z, Liu SY, Lu W (2011) Role of glycine receptors in glycine-induced LTD in hippocampal CA1 pyramidal neurons. Neuropsychopharmacology 36:1948–1958. https://doi.org/10.1038/npp.2011.86
Cunningham MO, Whittington MA, Bibbig A, Roopun A, LeBeau FE, Vogt A, Monyer H, Buhl EH, Traub RD (2004) A role for fast rhythmic bursting neurons in cortical gamma oscillations in vitro. Proc Natl Acad Sci U S A 101:7152–7157
Dalsgaard MK, Secher NH (2007) The brain at work: a cerebral metabolic manifestation of central fatigue? J Neurosci Res 85:3334–3339
De Simoni A, Griesinger CB, Edwards FA (2003) Development of rat CA1 neurones in acute versus organotypic slices: role of experience in synaptic morphology and activity. J Physiol 550:135–147
Dienel GA (2012) Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab 32:1107–1138. https://doi.org/10.1038/jcbfm.2011.175
Edmond J, Robbins RA, Bergstrom JD, Cole RA, de Vellis J (1987) Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res 18:551–561
Fisahn A, Pike FG, Buhl EH, Paulsen O (1998) Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature 394:186–189
Fish KN, Sweet RA, Lewis DA (2011) Differential distribution of proteins regulating GABA synthesis and reuptake in axon boutons of subpopulations of cortical interneurons. Cereb Cortex 21:2450–2460. https://doi.org/10.1093/cercor/bhr007
Galarreta M, Hestrin S (2002) Electrical and chemical synapses among parvalbumin fast-spiking GABAergic interneurons in adult mouse neocortex. Proc Natl Acad Sci U S A 99:12438–12443
Galeffi F, Foster KA, Sadgrove MP, Beaver CJ, Turner DA (2007) Lactate uptake contributes to the NAD(P)H biphasic response and tissue oxygen response during synaptic stimulation in area CA1 of rat hippocampal slices. J Neurochem 103:2449–2461
Galow LV, Schneider J, Lewen A, Ta TT, Papageorgiou IE, Kann O (2014) Energy substrates that fuel fast neuronal network oscillations. Front Neurosci 8:398. https://doi.org/10.3389/fnins.2014.00398
Geiger JR, Bischofberger J, Vida I, Fröbe U, Pfitzinger S, Weber HJ, Haverkampf K, Jonas P (2002) Patch-clamp recording in brain slices with improved slicer technology. Pflugers Arch 443:491–501
Gjessing LR, Gjesdahl P, Sjaastad O (1972) The free amino acids in human cerebrospinal fluid. J Neurochem 19:1807–1808
Gonzalez SV, Nguyen NH, Rise F, Hassel B (2005) Brain metabolism of exogenous pyruvate. J Neurochem 95:284–293
Gulyás AI, Szabó GG, Ulbert I, Holderith N, Monyer H, Erdélyi F, Szabó G, Freund TF, Hájos N (2010) Parvalbumin-containing fast-spiking basket cells generate the field potential oscillations induced by cholinergic receptor activation in the hippocampus. J Neurosci 30:15134–15145. https://doi.org/10.1523/JNEUROSCI.4104-10.2010
Hájos N, Mody I (2009) Establishing a physiological environment for visualized in vitro brain slice recordings by increasing oxygen supply and modifying aCSF content. J Neurosci Methods 183:107–113. https://doi.org/10.1016/j.jneumeth.2009.06.005
Harsing LG Jr, Matyus P (2013) Mechanisms of glycine release, which build up synaptic and extrasynaptic glycine levels: the role of synaptic and non-synaptic glycine transporters. Brain Res Bull 93:110–119. https://doi.org/10.1016/j.brainresbull.2012.12.002
Hayashi Y, Ishibashi H, Hashimoto K, Nakanishi H (2006) Potentiation of the NMDA receptor-mediated responses through the activation of the glycine site by microglia secreting soluble factors. Glia 53:660–668
Henneberger C, Papouin T, Oliet SH, Rusakov DA (2010) Long-term potentiation depends on release of D-serine from astrocytes. Nature 463:232–236. https://doi.org/10.1038/nature08673
Hertz L, Rothman DL (2016) Glucose, lactate, β-hydroxybutyrate, acetate, GABA, and succinate as substrates for synthesis of glutamate and GABA in the glutamine-glutamate/GABA cycle. Adv Neurobiol 13:9–42
Huchzermeyer C, Albus K, Gabriel HJ, Otáhal J, Taubenberger N, Heinemann U, Kovács R, Kann O (2008) Gamma oscillations and spontaneous network activity in the hippocampus are highly sensitive to decreases in pO2 and concomitant changes in mitochondrial redox state. J Neurosci 28:1153–1162. https://doi.org/10.1523/JNEUROSCI.4105-07.2008
Huchzermeyer C, Berndt N, Holzhütter HG, Kann O (2013) Oxygen consumption rates during three different neuronal activity states in the hippocampal CA3 network. J Cereb Blood Flow Metab 33:263–271. https://doi.org/10.1038/jcbfm.2012.165
Ivanov A, Zilberter Y (2011) Critical state of energy metabolism in brain slices: the principal role of oxygen delivery and energy substrates in shaping neuronal activity. Front Neuroenerg 3:9. https://doi.org/10.3389/fnene.2011.00009
Izumi Y, Ishii K, Katsuki H, Benz AM, Zorumski CF (1998) Beta-hydroxybutyrate fuels synaptic function during development. Histological and physiological evidence in rat hippocampal slices. J Clin Invest 101:1121–1132
Johnson JW, Ascher P (1987) Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325:529–531
Kam K, Nicoll R (2007) Excitatory synaptic transmission persists independently of the glutamate-glutamine cycle. J Neurosci 27:9192–9200
Kann O (2016) The interneuron energy hypothesis: implications for brain disease. Neurobiol Dis 90:75–85. https://doi.org/10.1016/j.nbd.2015.08.005
Kann O, Kovács R (2007) Mitochondria and neuronal activity. Am J Physiol Cell Physiol 292:C641–C657
Kann O, Schuchmann S, Buchheim K, Heinemann U (2003) Coupling of neuronal activity and mitochondrial metabolism as revealed by NAD(P)H fluorescence signals in organotypic hippocampal slice cultures of the rat. Neuroscience 119:87–100
Kann O, Huchzermeyer C, Kovács R, Wirtz S, Schuelke M (2011) Gamma oscillations in the hippocampus require high complex I gene expression and strong functional performance of mitochondria. Brain 134:345–358. https://doi.org/10.1093/brain/awq333
Kann O, Papageorgiou IE, Draguhn A (2014) Highly energized inhibitory interneurons are a central element for information processing in cortical networks. J Cereb Blood Flow Metab 34:1270–1282. https://doi.org/10.1038/jcbfm.2014.104
Kirischuk S, Héja L, Kardos J, Billups B (2016) Astrocyte sodium signaling and the regulation of neurotransmission. Glia 64:1655–1666. https://doi.org/10.1002/glia.22943
Künnecke B, Cerdan S, Seelig J (1993) Cerebral metabolism of [1,2-13C2]glucose and [U-13C4]3-hydroxybutyrate in rat brain as detected by 13C NMR spectroscopy. NMR Biomed 6:264–277
Lee HS, Ghetti A, Pinto-Duarte A, Wang X, Dziewczapolski G, Galimi F, Huitron-Resendiz S, Piña-Crespo JC, Roberts AJ, Verma IM, Sejnowski TJ, Heinemann SF (2014) Astrocytes contribute to gamma oscillations and recognition memory. Proc Natl Acad Sci U S A 111:E3343–E3352. https://doi.org/10.1073/pnas.1410893111
Lehre KP, Danbolt NC (1998) The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci 18:8751–8757
Li Y, Krupa B, Kang JS, Bolshakov VY, Liu G (2009) Glycine site of NMDA receptor serves as a spatiotemporal detector of synaptic activity patterns. J Neurophysiol 102:578–589. https://doi.org/10.1152/jn.91342.2008
Martina M, Gorfinkel Y, Halman S, Lowe JA, Periyalwar P, Schmidt CJ, Bergeron R (2004) Glycine transporter type 1 blockade changes NMDA receptor-mediated responses and LTP in hippocampal CA1 pyramidal cells by altering extracellular glycine levels. J Physiol 557:489–500
Marx MC, Billups D, Billups B (2015) Maintaining the presynaptic glutamate supply for excitatory neurotransmission. J Neurosci Res 93:1031–1044. https://doi.org/10.1002/jnr.23561
McIlwain H (1951) Metabolic response in vitro to electrical stimulation of sections of mammalian brain. Biochem J 49:382–393
McKenna MC (2012) Substrate competition studies demonstrate oxidative metabolism of glucose, glutamate, glutamine, lactate and 3-hydroxybutyrate in cortical astrocytes from rat brain. Neurochem Res 37:2613–2626. https://doi.org/10.1007/s11064-012-0901-3
McKenna MC, Stridh MH, McNair LF, Sonnewald U, Waagepetersen HS, Schousboe A (2016) Glutamate oxidation in astrocytes: roles of glutamate dehydrogenase and aminotransferases. J Neurosci Res 94:1561–1571. https://doi.org/10.1002/jnr.23908
Minota S, Miyazaki T, Wang MY, Read HL, Dun NJ (1989) Glycine potentiates NMDA responses in rat hippocampal CA1 neurons. Neurosci Lett 100:237–242
Nehlig A (2004) Brain uptake and metabolism of ketone bodies in animal models. Prostaglandins Leukot Essent Fatty Acids 70:265–275
Newell DW, Barth A, Ricciardi TN, Malouf AT (1997) Glycine causes increased excitability and neurotoxicity by activation of NMDA receptors in the hippocampus. Exp Neurol 145:235–244
Nishimura F, Nishihara M, Mori M, Torii K, Takahashi M (1995) Excitability of neurons in the ventromedial nucleus in rat hypothalamic slices: modulation by amino acids at cerebrospinal fluid levels. Brain Res 691:217–222
Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT, Salter MW (2003) Glycine binding primes NMDA receptor internalization. Nature 422:302–307
Papageorgiou IE, Gabriel S, Fetani AF, Kann O, Heinemann U (2011) Redistribution of astrocytic glutamine synthetase in the hippocampus of chronic epileptic rats. Glia 59:1706–1718. https://doi.org/10.1002/glia.21217
Papageorgiou IE, Lewen A, Galow LV, Cesetti T, Scheffel J, Regen T, Hanisch UK, Kann O (2016) TLR4-activated microglia require IFN-γ to induce severe neuronal dysfunction and death in situ. Proc Natl Acad Sci U S A 113:212–217. https://doi.org/10.1073/pnas.1513853113
Papp OI, Karlócai MR, Tóth IE, Freund TF, Hájos N (2013) Different input and output properties characterize parvalbumin-positive basket and axo-axonic cells in the hippocampal CA3 subfield. Hippocampus 23:903–918. https://doi.org/10.1002/hipo.22147
Penttonen M, Kamondi A, Acsády L, Buzsáki G (1998) Gamma frequency oscillation in the hippocampus of the rat: intracellular analysis in vivo. Eur J Neurosci 10:718–728
Pomper JK, Graulich J, Kovacs R, Hoffmann U, Gabriel S, Heinemann U (2001) High oxygen tension leads to acute cell death in organotypic hippocampal slice cultures. Brain Res Dev Brain Res 126:109–116
Roberts EL Jr (2007) The support of energy metabolism in the central nervous system with substrates other than glucose. In: Lajtha A, Gibson GE, Dienel GA (eds) Handbook of neurochemistry and molecular neurobiology. Brain energetics. Integration of molecular and cellular processes, 3rd edn. Springer, Berlin, pp 137–179
Rojas-Morales P, Tapia E, Pedraza-Chaverri J (2016) β-Hydroxybutyrate: a signaling metabolite in starvation response? Cell Signal 28:917–923. https://doi.org/10.1016/j.cellsig.2016.04.005
Schneider J, Lewen A, Ta TT, Galow LV, Isola R, Papageorgiou IE, Kann O (2015) A reliable model for gamma oscillations in hippocampal tissue. J Neurosci Res 93:1067–1078. https://doi.org/10.1002/jnr.23590
Schneider J, Berndt N, Papageorgiou IE, Maurer J, Bulik S, Both M, Draguhn A, Holzhütter HG, Kann O (2017) Local oxygen homeostasis during various neuronal network activity states in the mouse hippocampus. J Cereb Blood Flow Metab. https://doi.org/10.1177/0271678X17740091
Schurr A, West CA, Rigor BM (1988) Lactate-supported synaptic function in the rat hippocampal slice preparation. Science 240:1326–1328
Simpson IA, Carruthers A, Vannucci SJ (2007) Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27:1766–1791
Smith KE, Borden LA, Hartig PR, Branchek T, Weinshank RL (1992) Cloning and expression of a glycine transporter reveal colocalization with NMDA receptors. Neuron 8:927–935
Stenkamp K, Palva JM, Uusisaari M, Schuchmann S, Schmitz D, Heinemann U, Kaila K (2001) Enhanced temporal stability of cholinergic hippocampal gamma oscillations following respiratory alkalosis in vitro. J Neurophysiol 85:2063–2069
Traub RD, Bibbig A, Fisahn A, LeBeau FE, Whittington MA, Buhl EH (2000) A model of gamma-frequency network oscillations induced in the rat CA3 region by carbachol in vitro. Eur J Neurosci 12:4093–4106
Tsintsadze V, Minlebaev M, Suchkov D, Cunningham MO, Khazipov R (2015) Ontogeny of kainate-induced gamma oscillations in the rat CA3 hippocampus in vitro. Front Cell Neurosci 9:195. https://doi.org/10.3389/fncel.2015.00195
Uhlhaas PJ, Singer W (2010) Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 11:100–113. https://doi.org/10.1038/nrn2774
Vannucci SJ, Simpson IA (2003) Developmental switch in brain nutrient transporter expression in the rat. Am J Physiol Endocrinol Metab 285:E1127–E1134
Yamane K, Yokono K, Okada Y (2000) Anaerobic glycolysis is crucial for the maintenance of neural activity in guinea pig hippocampal slices. J Neurosci Methods 103:163–171
Zafra F, Aragón C, Olivares L, Danbolt NC, Giménez C, Storm-Mathisen J (1995) Glycine transporters are differentially expressed among CNS cells. J Neurosci 15:3952–3969
Zhang LH, Gong N, Fei D, Xu L, Xu TL (2008) Glycine uptake regulates hippocampal network activity via glycine receptor-mediated tonic inhibition. Neuropsychopharmacology 33:701–711
Acknowledgements
The authors thank Hasan Onur Dikmen for critical reading of the manuscript and helpful discussion.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft within the Collaborative Research Center 1134 (project B02).
Author information
Authors and Affiliations
Contributions
WV, JS, and OK designed the research; WV, JS, SE, JOH, and AL performed the research; WV, JS, SE, JOH, and AL analyzed the data; WV and OK wrote the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Vodovozov, W., Schneider, J., Elzoheiry, S. et al. Metabolic modulation of neuronal gamma-band oscillations. Pflugers Arch - Eur J Physiol 470, 1377–1389 (2018). https://doi.org/10.1007/s00424-018-2156-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2156-6