Abstract
The aim of the present study was to test the hypothesis that a relationship exists between respiratory-induced blood pressure variability (BPV) and transductal shunting in premature infants with respiratory distress. Ten premature infants (27–32 weeks gestation) with respiratory distress ventilated in the synchronised, positive-pressure mode were examined. The interrelations between blood pressure and transthoracic impedance were described using time and frequency domain analysis. Haemodynamic effects of left-to-right transductal shunting were assessed using Doppler echocardiography (ratio of diastolic flow to systolic flow in the subdiaphragmatic aorta). The dependence of blood pressure fluctuations on the respiratory cycle was seen consistently in both time-domain and cross-spectral analysis. The amplitude of these fluctuations varied between infants. In the time domain, the beat-to-beat pressure difference was 0.69–4.1 mmHg for diastolic and 0.99–5.24 mmHg for systolic blood pressure. There was a positive linear relationship between the respiratory-related BPV indicators and the extent of left-to-right transductal shunting (r=0.86 for diastolic and 0.80 for systolic pressure, P<0.01). Respiratory-related BPV was not correlated to the indicators of left ventricle preload. It is concluded that respiratory related BPV involves both diastolic and systolic blood pressure and is correlated to the magnitude of left-to-right transductal shunting in the population studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although a certain variability in blood pressure (BP) and heart rate is associated with the normal autonomic nervous system control of the cardiovascular system, both low and high variability have been associated with increased morbidity [9, 14, 25].
Respiratory-induced blood pressure variability (BPV) is dependent mainly on cardiac loading conditions and cardio-respiratory interactions [4, 8, 20]. It has been correlated with echocardiographic estimates of left ventricle preload [6] and has been shown to be a sensitive indicator of hypovolaemia in ventilated animals and in adult humans [13, 16]. During the neonatal period, respiratory-related BPV is influenced by the severity of the respiratory distress syndrome and the mode of ventilation [2, 14]. In these situations, large fluctuations in BP and a large patent ductus arteriosus are noted frequently and both have been implicated in the development of severe intra- or periventricular haemorrhage [9, 11, 15].
We hypothesised that a patent ductus arteriosus, which has an influence on cardiac loading conditions and cardio-respiratory interactions, may have an effect on beat-to-beat arterial BPV. The aim of this study was to verify and to quantify the association between BPV and respiration in premature infants ventilated in the synchronised, intermittent-positive-pressure mode and to test our hypothesis of a relationship between this variability and the Doppler echocardiographic index of transductal left-to-right shunting.
BPV was analysed using standardised statistical methods of short-term variability measurement. Both spectral analysis and time-domain methods were used because they complement each other in describing biological variation [17, 25].
Materials and methods
Subjects
Data were obtained from ten consecutive premature infants admitted to the neonatal intensive care unit at the university hospital of Rennes. Inclusion criteria were a gestational age of 27–32 weeks, a postnatal age less than 12 h and a respiratory distress syndrome requiring mechanical ventilation with FIO2×P aw/PaO2>6 (where FIO2 is the fractional concentration of O2 in the inspired air, P aw the mean airway pressure and PaO2 the arterial PO2). Exclusion criteria were the absence of an umbilical arterial catheter or malformation. The study was approved by the local ethics committee (Ref.: 96/22-129). Parents were informed and consent obtained.
Study conditions
The ten premature infants included were studied prospectively. The median postnatal age was 3 h 32 min (range: 1 h 46 min–6 h 20 min). None were sedated or paralysed, nor had they received any drug known to have an effect on the cardiovascular system. The volume of infusion was standardised (80 ml/kg per day 10% dextrose). In all cases, the premature infants were ventilated in the synchronised intermittent-positive-pressure mode (Babylog 8000, Draeger, Lubeck, Germany). Ventilator settings for all the babies were: peak inspiratory pressure 20 cmH20, positive end-expiratory pressure 6 cmH2O and inspiratory time 0.4 s. FIO2 was set according to individual requirements. Actual expiratory times and respiratory rates varied according to patient triggering. Air leak around the endotracheal tube was less than 10% and trigger threshold sensitivity was set at maximum. Skin temperature was maintained at 36.5–37 °C, arterial blood O2 saturation at 92–97%, PaO2 at 50–80 mmHg and arterial PCO2 at 35–45 mmHg. The recordings were performed when the premature infants were quiet and in synchrony with the ventilator. Synchrony was defined as chest wall movement occurring in phase with ventilator inflation, and was confirmed by a visual inspection of the flow tracing [2].
Data acquisition
Signal recording
All the infants were monitored with a polygraphic monitor (Omnicare 24, Hewlett Packard, Boeblingen, Germany). Transthoracic impedance and electrocardiographic (ECG) signals were obtained from thoracic electrodes located under left and right clavicles and in the left subcostal area. Arterial BP was obtained via an indwelling umbilical arterial catheter. Digitised signals of DII ECG, transthoracic impedance and BP traces were recorded simultaneously, transferred and saved on-line as binary files on a computer for later analysis. Sampling frequencies were 125 Hz for transthoracic impedance and BP signals and 500 Hz for the ECG. The recordings were made under stable conditions for at least 2 min (steady respiratory rate, no crying, agitation or asynchronous respiration, no damping of the arterial signal, no artefacts).
Echocardiographic measurements
A cardiovascular ultrasound scan was performed at the end of the recording by the same investigator (A. Beuchée) as previously described [17, 18]. After ruling out structural anomalies of the heart, haemodynamic function was assessed by Doppler echocardiography using a ultrasonoscope (Kontron, Montigny, France). The flow pattern of the transductal shunting was recorded and classified according to Su et al. [22] as pulmonary hypertension, growing, pulsatile, closing or closed patterns. A good quality transductal flow recording was obtained in all babies but one. In this case diastolic disturbance of the flow pattern in the pulmonary artery confirmed the ductal patency. The peak left-to-right ductal flow velocity (V td,max) was determined to calculate the maximum transductal pressure gradient according to the Bernoulli equation [22]. The resistance index in the subdiaphragmatic aorta (AoRI) [(PSV−ESV)/PSV, where PSV and ESV are the peak systolic and end-diastolic flow velocities respectively], and the ratio of the areas under diastolic and systolic flow portions of the Doppler trace (−D/S), obtained from a transabdominal view, were measured to assess the systemic effects of transductal flow [18, 21]. The left ventricle diameters, the internal diameter of the ascending aorta and the left atrium diameter were obtained from the parasternal long-axis view (M-mode). The ratios of the left atrial and left ventricle end-diastolic diameters to that of the aorta (LA/Ao and LVEDD/Ao, respectively) were calculated as estimates of the preload. Cardiac output (CO, in millilitre/minute per kilogram) was calculated using the formula: CO=V ao×S ao×60/Wt, where S ao is the cross-sectional area of, and V ao the mean flow velocity in the aorta, obtained from a subcostal view and computed from the M-mode-measured diameter and Wt the body weight. Doppler and M-mode measurements were averaged from five consecutive beats at a steady state.
Data analysis
Extraction of the parameters
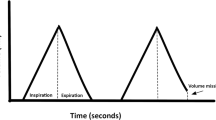
A sequence of 256 successive cardiac beats was isolated from the 2-min recordings obtained for each baby. Discrete series of parameters (systologram, diastologram and respirogram) were extracted from these sequences. These series are presented as functions of the heart beat index i [3]. A set of measurements was extracted from ECG, BP and respiration signals recorded simultaneously, at each ith cardiac cycle: R-R interval duration (R-R), diastolic and systolic BP (DBP and SBP) and respiratory amplitude (RespA) (Fig. 1). These data, sampled at each cardiac cycle, were then stored as ASCII files, which were used for data analysis.
Extraction of the parameters respiratory amplitude (RespA i ). R-R interval duration (R-R i ). diastolic and systolic blood pressure (DBP i ; SBP i ). Conversion of the initial signal sampled at 125 Hz [500 Hz for the electrocardiographic (ECG) signal] to a time series function of the heart beat index i (lower x-axis) or function of time in the respiratory cycle with the onset of expiratory phase taken as 0% (upper x-axis)
Time-domain analysis
Means were calculated for each discrete series of DBP, SBP and R-R interval duration. SD and coefficient of variation (CV) were used to assess global variability. The root-mean-square of successive differences (rMSSD) was used to measure short-term, beat-to-beat variability [23].
Frequency-domain analysis
Spectral analysis and cross-spectral analysis were performed on the same series of 256 successive beats. Before analysis, a least-squares linear procedure was used to remove any linear offset and trend from the data. Respiration amplitude series were normalised by dividing the centred-on-zero and detrended series of data by their SD. To prevent cardiac aliasing effects we checked that respiratory rate was less than half the heart rate. Spectral analysis of the data was performed using the Fourier fast transform (FFT) algorithm in the analysis software (Origin v. 4.1, Microcal Software, Northampton, Mass., USA) with a Hamming window. Frequency units with Hertz dimension were obtained multiplying frequencies measured in cycles/beat by 1/mean R-R and are referred to as "equivalent Hertz" (eq. Hz). This approximation is allowed by the low R-R interval variability in premature neonates with respiratory distress syndrome [3].
The total power spectral density (TP) was calculated for each discrete variable series by integrating the power spectra over the frequency range [0.02; ƒ eq. Hz], where ƒ is 0.5/mean R-R. Since the sampling frequency of the data is on a beat-to-beat basis, the maximum frequency for which a meaningful spectrum can be calculated is half the beat-to-beat sampling frequency. TP is an index of global variability and is expressed in (millimetre Hg)2. The spectral densities in the low-frequency (LF) range [0.02; 0.2 eq. Hz] and in the high-frequency (HF) range [0.2; ƒ eq. Hz] were also calculated. Very low frequency variations [0; 0.02 eq. Hz] were ignored so as to avoid the possibility of artefacts due to long-term slow oscillations.
Statistical analysis
Data were analysed using a statistics software package (Statistica, StatSoft, Tulsa, Okla., USA). Data are presented as median values and extremes. The Spearman rank test was used to study the correlation between continuous variables. The squared coherence function (k 2) was used to evaluate the reliability of cross-spectral analysis for assessing frequency. Linear regressions between continuous variables were estimated using least-squares procedures. Multiple linear regression analysis was used to assess the influence of the parameters studied on the relation between short-term BPV measurements (rMSSD and HF) and −D/S or AoRI. P<0.05 was considered significant.
The quality of respiratory-related BPV measurements was checked by comparing the results obtained in the time- and the frequency-domain analyses, according [23]. The strength of the positive correlation between rMSSD and HF was good (R 2=0.56 and 0.89 for DBP and SBP respectively, P<0.01) and was stronger between rMSSD and √HF (R 2=0.99 and0.98 for DBP and SBP respectively, P<0.001).
Results
Patients
Ten premature babies were included. Median gestational age was 29 weeks 2 days (27 weeks–31 weeks 5 days) and median birth weight was 1313 g (700–1,900 g). No baby was excluded. Univariate analysis did not show any significant relation between maternal, foetal, or neonatal conditions (hypertension during pregnancy, foetal heart rate abnormality, clinical chorioamnionitis, type of anaesthesia, APGAR scores, gestational age, birth weight, postnatal age, PaO2, FIO2, FIO2×P aw/PaO2) and rMSSD, HF, −D/S and AoRI. Clinical and echocardiographic data at the time of the recording are reported in the Table 1. The ductus arteriosus was patent in all babies.
Relation between the frequency of arterial BP and the respiratory cycle
The influence of respiratory cycles on DBP and SBP variations is obvious in the individual time series (Fig. 2A) as well as when the time series are normalised as a function of time in the respiratory cycle (Fig. 2B). These periodic fluctuations of BP with the respiratory cycle involve both DBP and SBP but the magnitude was higher for SBP than DBP in all recordings. The influence of respiratory cycles on DBP and SBP variation was confirmed by cross-spectral analysis. Cross-spectral amplitudes of the series of RespA for each series of DBP and SBP in the HF range were maximal at the peak respiration frequency in all recordings with a median k 2 of 0.91 (0.75–0.99) for DBP and 0.93 (0.64–0.99) for SBP. The direction of change of blood pressure with the respiratory cycle was not consistent.
Examples of interrelation between ventilation cycles and blood pressure from three premature infants. A Respirograms (RespA in arbitrary units), systolograms and diastolograms (SBP and DBP in mmHg) recorded on sequences of 256 beats. B The points represent the values of RespA measured by impedancemetry, SBP or DBP; data are normalized as a function of time in respiratory cycle with the onset of expiratory phase taken as 0%
Quantification of respiratory-related BPV
The measurements of BPV in time- and frequency-domain analyses are presented in Table 2. The short-term variability calculated in the time domain by rMSSD (Table 2), as well as the proportion of spectral density in the HF range (23–98%) varied widely between patients.
Haemodynamics, patent ductus arteriosus and respiratory-related BPV
AoRI and −D/S correlated strongly with each other (r=0.96; P<0.001) and both correlated with the rMSSD of BP. The coefficient of correlation between −D/S and rMSSD was r=0.86 (P=0.002) for DBP and r=0.80 (P=0.006) for SBP (Fig. 3). Between AoRI and rMSSD r was 0.89 (P=0.001) for DBP 0.85 (P=0.002) for SBP. The −D/S ratio also correlated significantly with SD (P<0.05) but not with other time-domain indicators of global BPV. The analysis in the frequency domain confirmed that the correlation between −D/S or AoRI and BPV involved the HF but not the LF component. The absence of influence of gestational age, birth weight, postnatal age, PaO2, FIO2, FIO2×P aw/PaO2 on the relation between short-term BPV measurements (rMSSD and HF) and −D/S or AoRI was verified by multiple linear regression.
The short-term BPV estimates (rMSSD, HF) did not correlate with any of the following variables: the absolute values of DBP or SBP, left ventricle output and maximal transductal velocity or gradient. It was also independent of the cardiac preload indices: left ventricular end-diastolic and left atrial diameter to aortic diameter ratio.
Heart rate variability
Mean R-R interval duration was 408 ms (341–467). Global heart rate variability indices were: SD 3.97 ms (2–10.53), CV 0.97% (0.59–2.25) and TP 454 10−6 ms2 (108–3,227). Time- and frequency-domain estimates of short-term variability were rMSSD 2.69 ms (1.77–3.54) and HF 177·10–6 ms2 (83–395). According to cross-spectral analysis of each series of DBP, SBP and RespA on the series of R-R interval duration there was no significant heart rate variability at the peak respiration frequency except for patients B, H and J.
Discussion
Principal findings
In ventilated premature infants with patent ductus arteriosus, short-term BPV involves both DBP and SBP. This BPV is correlated in frequency with the respiratory cycle and in magnitude with −D/S or AoRI, which are indexes of the extent of left-to-right transductal shunting. These results suggest for the first time that when the duct is open, respiratory-related BPV is more associated with the magnitude of transductal shunting than with cardiac loading conditions.
Strength and weakness of the study in relation to others
Different methods have been used to quantify BPV. Most previous studies in the premature infant have used the CV. This calculated parameter depends on the series number of data points and it is therefore not possible to make reliable comparisons between studies. Additionally, this global variability index does not distinguish the distribution of the BPV components, which varied a great deal between the newborns we studied. The values of short-term beat-to-beat BPV in our study were in accordance with the study of Amitay et al. performed in the premature infant with an umbilical arterial catheter [2]. We also measured BP via an umbilical artery catheter because this site is usually considered to be the most reliable in neonatal practice [10]. As the site of measurement is known to affect the morphology of the blood pressure wave we cannot rule out a possible attenuation of BPV magnitude in distal arteries but we think that it is likely that a high aortic BPV will result in a high distal BPV.
Pulmonary mechanics are known to influence the magnitude of respiration-related BPV. Asynchrony and raised intrathoracic pressures are known to increase this magnitude [2, 4, 16]. This did not influence our results since synchronised ventilation and identical ventilator pressure settings used for all the babies excluded interference of these parameters with the main judgement criteria, i.e. the degree of high-frequency BPV. The effect of tidal volume and compliance were not evaluated and we cannot rule out a possible effect of these parameters on BPV. Nevertheless, there was no significant influence of weight, blood gas PaO2 or FIO2 on BPV indicators.
Meaning of the study, possible mechanisms and implications
Fluctuation of arterial BP with respiration is a well-described phenomenon that has been reinvestigated recently in adult humans by spectral analysis [3, 4, 8, 20, 24]. De Boer et al. have introduced a model describing short-term fluctuations in arterial BP as a mechanical coupling of respiration to changes in left ventricle stroke volume and systolic arterial BP which, in turn, cause changes in the R-R interval via arterial baroreflexes [8]. Moreover, it has been shown that respiration affects arterial BP in proportion to changes in lung volume, while heart rate fluctuations are eliminated by pharmacological blockade [20]. In this latter situation, respiratory-induced fluctuations in stroke volume are not counteracted by inverse heart rate fluctuations and the direct influence of the stroke volume on mean arterial BP is consequently increased [4, 24].
As in other studies related to heart rate variability in premature infants [1], we found that overall R-R interval variability was low in all cases. We would therefore have expected BPV to depend mainly on haemodynamics, i.e. stroke volume and cardiovascular loading conditions [14]. Indeed, in the premature infant under positive-pressure ventilation, the respiration cycle is known to affect the ventricular ejection time [12], diastolic ventricular filling [19] and the subsequent left ventricle stroke volume.
When the duct is patent, total vascular resistance is determined by the systemic resistance and the resistance of a system which associates the duct and the pulmonary vascular resistance [5, 17]. In this situation it is likely that respiratory-related fluctuations in pulmonary vascular resistance [7] could induce respiratory-related BPV. In such a model (patent ductus arteriosus and low heart rate variability) we can assume respiratory-related BPV to be determined mainly by the respiratory-related fluctuations of both pulmonary vascular resistance and stroke volume.
In premature infants with respiratory distress and patent ductus arteriosus the respiratory-related BPV was independent of the indicators of left ventricle preload, i.e. the ratios of the left atrial and left ventricle end-diastolic diameters to that of the aorta. This result differs from experimental and clinical data [6, 13, 16] that have demonstrated an inverse correlation between left ventricular preload and respiratory-related BPV magnitude. In addition, the more significant the transductal shunting, the greater the BPV. This seems to be inconsistent since, with a patent ductus arteriosus, the left ventricle preload increases with the magnitude of left-to-right transductal shunting [5]. We would therefore have expected the increase in the transductal shunting to lead to a decrease in beat-to-beat variability of the stroke volume and subsequent BPV. This apparent paradox could be explained by our hypothesis of a direct influence of respiratory-related fluctuations of pulmonary vascular resistance on BPV when the duct is patent. Indeed, we believe that this phenomenon could have blunted the effects of left ventricular preload on the respiratory-related BPV.
Clinical implications
Patency of the ductus arteriosus should be ruled out by echocardiography before using respiratory-related fluctuation of BP as an indicator of hypovolaemia in premature infants with respiratory distress [6, 13, 16].
Respiratory-induced fluctuations of blood pressure were as large as 5 mmHg in some of our premature infants. These fluctuations could contribute to the explanation of some discrepancies reported between methods of measuring arterial BP.
We have shown respiratory-induced BPV to be correlated with transductal shunting in premature infants of less than 32 weeks of gestation with respiratory distress syndrome. In those neonates both high BPV [9, 15] and a patent ductus arteriosus [11, 18] have been associated with the subsequent development of severe intra/periventricular haemorrhage. The role of this interrelationship on the incidence of cerebral haemorrhage remains to be determined.
References
Äärimaa T, Oja R, Antila K (1988) Interaction of heart rate and respiration in newborn babies. Pediatr Res 24:745–750
Amitay M, Etches PC, Finer NN, Maidens JM (1993) Synchronous mechanical ventilation of the neonate with respiratory disease. Crit Care Med 21:118–124
Baselli G, Cerutti S, Civardi S, Liberati D, Lombardi F, Malliani A (1986) Spectral and cross-spectral analysis of heart rate and arterial blood pressure variability signals. Comput Biomed Res 19:520–534
Blaber AP, Hughson RL (1986) Cardiorespiratory interactions during fixed pace resistive breathing. J Appl Physiol 80:1618–1626
Clyman RI, Mauray F, Heymann MA, Roman C (1995) Cardiovascular effects of patent ductus arteriosus in preterm lambs with respiratory distress. J Pediatr 111:579–587
Coriat P, Vrillon M, Perel A, Baron JF, Le Bret F, Saada M (1994) A comparison of systolic blood pressure variations and echocardiographic estimates of end-diastolic left ventricular size in patients after aortic surgery. Anesth Analg 78:46–53
Dawson CA, Grimm DJ, Linehan JH (1979) Lung inflation and longitudinal distribution of pulmonary vascular resistances during hypoxia. J Appl Physiol 47:532–536
DeBoer RW, Karemaker JM, Strackee J (1987) Hemodynamic fluctuations and baroreflex sensitivity in humans: a beat-to-beat model. Am J Physiol 253:H680–H689
D'Souza SW, Janakova H, Minors D, Suri R, Waterhouse J, Appleton G (1995) Blood pressure, heart rate and skin temperature in preterm infants: associations with periventricular haemorrhage. Arch Dis Child Fetal Neonatal Ed 72:F162–F167
Evans DH, Lark JM, Archer LN, Levene MI (1986) The continuous measurement of intra-arterial pressure in the neonate: method and accuracy. Clin Phys Physiol Meas 7:179–84
Evans N, Kluckow M (1996) Early ductal shunting and intraventricular haemorrhage in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed 75:F183–F186
Maroto E, Foulon JC, Teyssier G, Bard H, van Doesburg NH, Cartwright D (1990) Effect of intermittent positive pressure ventilation on diastolic ventricular filling patterns in premature infants. J Am Coll Cardiol 16:171–174
Perel A, Pizov R, Cotev S (1987) The systolic blood pressure variation is a sensitive indicator of hypovolaemia in ventilated dogs subjected to graded haemorrhage. Anesthesiology 67:498–502
Perlman JM (1988) Respiratory origin of fluctuations in arterial blood pressure in premature infants with respiratory distress syndrome. Pediatrics 81:399–403
Perlman JM, Volpe JJ (1990) Fluctuating blood pressure and ventricular hemorrhage. Pediatrics 85:620–621
Pizov R, Cohen M, Weiss Y, Segal E, Shamay C, Perel A (1996) Positive end-expiratory pressure-induced hemodynamic changes are reflected in the arterial pressure waveform. Crit Care Med 24:1381–1387
Pladys P, Wodey E, Beuchée A, Branger B, Bétrémieux P (1999) Mean arterial blood pressure and cardiac output in preterm infants during the first day of life. Eur J Pediatr 158:817–824
Pladys P, Beuchée A, Wodey E, Tréguier C, Lassel L, Bétrémieux P (2001) Patent ductus arteriosus and cystic periventricular leucomalacia in preterm infants. Acta Paediatr 90:309–315
Reller M, Kotagal UR, Meyer RA, Kaplan S (1986) Duration of ventricular ejection during spontaneous breathing and positive pressure ventilation in newborn infants. Biol Neonate 50:130–135
Saul JP (1995) Respiration and blood pressure variability: mechanical and autonomic influences. Fundam Clin Pharmacol 112:17s–22s
Serwer GA, Armstrong BE, Anderson PAW (1982) Continuous wave Doppler ultrasonographic quantification of patent ductus arteriosus flow. J Pediatr 100:297–299
Su BH, Watanabe T, Shimizu M, Yanagisawa M (1997) Echocardiographic assessment of patent ductus arteriosus shunt flow pattern in premature infants. Arch Dis Child Fetal Neonatal Ed 77:F36–F40
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (1996) Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation 93:1043–1065
Toska K, Eriksen M (1993) Respiration-synchronous fluctuations in stroke volume, heart rate and arterial pressure in humans. J Physiol (Lond) 472:501–512
Välimäki I, Rantonen T (1999) Spectral analysis of heart rate and blood pressure variability. Clin Perinatol 26:967–980
Acknowledgements
This work was supported by the COREC, Rennes, France. The authors are grateful to Sylvain Jasson for valuable technical support and Nina Deschamps for the translation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beuchée, A., Pladys, P., Senhadji, L. et al. Beat-to-beat blood pressure variability and patent ductus arteriosus in ventilated, premature infants. Pflugers Arch - Eur J Physiol 446, 154–160 (2003). https://doi.org/10.1007/s00424-002-0961-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-002-0961-3