Abstract
Background
TSH receptor autoantibodies (TRAbs) are pathognomonic for Graves’ disease and are thought to also underly the pathogenesis of Graves’ ophthalmopathy (GO). A decline in TRAb levels has been documented post-total thyroidectomy (TTx) in GO, however with conflicting correlations with disease outcomes. The aim of the study was to compare the effectiveness of TTx to other treatment modalities of Graves’ disease and examine whether the lowering of TRAbs is associated with GO improvements.
Method
We searched electronic databases including Medline, Embase, Scopus, and Web of Science until 31 September 2022 using a broad range of keywords. Patients with GO undergoing TTx with measurements of both TRAbs and progression of the disease using a validated GO scoring system were included. Fourteen studies encompassing data from 1047 patients with GO met our eligibility criteria. The PRISMA guidelines were followed, and five studies had comparable data that were suitable for a meta-analysis.
Results
The Cochrane Risk of Bias tool for RCTs showed low risk of bias across most domains. The pooled odds ratio showed that more patients significantly had normalized TRAb levels post-TTx as compared to other interventions (OR: 1.36, 95% CI: 1.02–1.81, p = 0.035). But, there was no significant difference in GO improvement post-TTx as compared with other intervention groups.
Conclusions
This meta-analysis shows that TRAb levels may decline largely post-TTx, but may not predict added improvements to the progression of GO. Thus, future studies with uniform designs are required to assess the minimal significant GO improvements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Graves’ ophthalmopathy (GO) is an autoimmune disorder of the eye that is characterized by orbital soft tissue swelling, exophthalmos, and resulting visual symptoms. It is the most common extrathyroidal manifestation of Graves’ disease, which may be clinically relevant in 25–50% of patients [1]. While the usual course is benign, it may progress to compress the optic nerve to cause vision loss in 3–5% of patients with Graves’ disease [2]. The underlying pathology is thought to be linked to the shared TSH receptor antigen found in the orbital fibroblasts [3]. It is postulated that in Graves’ disease patients, TSH receptor autoantibodies (TRAbs) produced by intrathyroidal B-cells are central to the disease. These autoantibodies lead to the overstimulation of the TSH receptor on retro-ocular fibroblasts and adipocytes, resulting in orbital fat expansion and increased tissue volume [3]. Total thyroidectomy (TTx) is a well-established treatment option for the condition that aims to achieve complete removal of the thyroid gland [4]. The American Thyroid Association recommends TTx as one of the acceptable first-line treatment options for both Graves’ disease and Graves’ ophthalmopathy [5]. Moreover, it is listed as a preferred option over radioactive iodine (RAI) in moderate-to-severe or sight-threatening GO [5]. It is proposed that TTx works by removing the target tissue for autoantibodies and cause decline in TRAb post-treatment. This observation has been documented by several studies [6,7,8,9]. Previous meta-analysis that compared subtotal thyroidectomy (STx) to TTx found no difference in regression of GO with either surgical technique, suggesting that the total removal of thyroid antigens may be less relevant than previously suggested [10]. However, this review was limited by the number of studies with measurements of TRAb level to comment on the difference in decline in TRAb level post-TTx. Thus, little is known whether the decline in autoantibody post-TTx is significant and if it has an impact on GO outcomes. Therefore, in this study, we aimed to determine if the decline in TRAbs is associated with improvements in GO.
Material and methods
Literature search strategy
This study was conducted following the PRISMA guidelines [11]. We searched for articles across four publicly available electronic databases, including Medline, Embase, Scopus, and Web of Science. The search was limited to the English language or English translations with no limit on publication dates up to 30th September 2022. We utilized common keywords and MESH terms on Medline and adopted our search strategy for other databases (Supplementary Fig. S1). The comparator and outcome terms were omitted to avoid missing relevant studies. The search terms on Medline were as follows: “Graves’ Disease” OR “Thyroid Associated Orbitopathy” OR “Thyroid Eye Disease” OR “Graves’ Ophthalmopathy” OR “Graves Orbitopathy” AND “Thyroidectomy.” The data extraction was done in duplicate by two authors, AA and FA. We also searched manually the reference list from the eligible studies and systemic reviews to identify any additional articles.
Inclusion criteria
We selected studies that included patients with GO undergoing TTx with measurements of both TRAb levels and progression of the disease using a validated scoring system. This included randomized controlled trials, cohort, case-control, and qualitative studies. A validated scoring system included CAS (Clinical Activity Score), NOSPECS (no physical signs or symptoms, only signs, soft tissue involvement, proptosis, extraocular muscle involvement, corneal involvement and sight loss), EUGOGO (European Group of Graves’ Orbitopathy), VISA (vision, inflammation, strabismus, appearance), and their respected variants. The diagnostic criteria for GO were defined as: (i) characteristic ocular abnormalities on clinical examination, (ii) biochemically confirmed current or past Graves’ hyperthyroidism (low TSH and high T3/T4 levels), and (iii) presence of TRAbs.
Data extraction and quality assessment
Microsoft Excel table was used to summarize key information from the eligible studies. The information extracted from the studies included in the meta-analysis were as follows: primary author’s name, year of publication, interventions included in the study, concurrent additional treatments, country of origin of the study, age of participants, follow-up duration, GO scoring system used, the number of patients with normalized or unnormalized TRAb levels after the last follow-up post-TTx and intervention groups, and the number of patients with improved, unchanged, or worsened GO outcomes after the last follow-up post-TTx and intervention groups. Normalized TRAb levels in our study refer to a return to the baseline normal range as defined by the biochemical assay used. As a corollary, unnormalized TRAb levels refer to elevated TRAb levels. This reporting system for TRAb levels and GO outcomes was used as it allowed cross-comparison and captured the largest dataset. Similar information was extracted from studies included in the qualitative analysis. Variations in the reporting system used for TRAb levels and GO outcomes were documented where applicable.
The risk of bias assessment for all randomized controlled trials (RCTs) was performed using the Cochrane risk of bias tool for randomized trials (ROB 2) according to the Cochrane Handbook for Systematic Reviews of Interventions [12]. The risk of bias assessment for all cohort studies was performed using the Newcastle-Ottawa Scale (NOS) (Supplementary Fig. S3) [13].
Statistical analysis
A random-effects model was used to calculate the pooled odds ratio (OR) and 95% confidence interval to compare between TTx and other interventions concerning the number of patients with normalized TRAb levels, unnormalized TRAb levels, improved GO scores, unchanged GO, and worsened GO. The heterogeneity among the studies was assessed using the I2 statistic where I2 values of 25%, 50%, and 75% were considered to indicate low, moderate, and high heterogeneity, respectively. To examine the publication bias, we used Egger’s regression model where a p-value of <0.05 was considered to indicate publication bias.
Results
Literature search
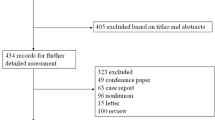
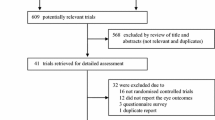
The search strategy results are summarized using the PRISMA flowchart in Fig. 1. After the exclusion of duplicates, a total of 817 articles were identified. Of these, 790 were excluded based on their title and abstract due to their ineligibility with the inclusion criteria. A total of 27 articles underwent full-text examination, of which 13 articles were excluded. The reasons for exclusion were as follows: no TTx performed (n = 2), no GO-specific data (n = 5), no intervention-specific data (n = 4), and no TRAb levels measured (n = 2). Therefore, 14 articles (six RCTs, eight cohort studies) encompassing data from 1047 patients with GO were included in this systematic review [7, 9, 14,15,16,17,18,19,20,21,22,23,24]. Furthermore, five of these articles (four RCTs, one cohort study) had comparable data that were suitable for a meta-analysis [14,15,16,17,18].
Studies included
The characteristics of all 14 studies included in the review are summarized in Table 1. Most studies were conducted in European population groups while Catz et al. examined North American patients, Erdogan et al. and Nart et al. examined Turkish patients [7, 23]. Most studies included predominately adult patients and sample sizes varied from 32 to 200 patients. The follow-up duration post-surgery ranged from as early as 21 days for some patients in the study by Nart et al. to 9 years in the study by Catz et al. [7, 20]. One study compared TTx to RAI, four included STx comparison groups, four included total thyroid ablation groups (near or total thyroidectomy followed by RAI), three included ATD (antithyroid drugs), and three studies had no comparison groups.
Five studies were included in the meta-analyses and comprised a total of 530 patients with GO. The meta-analysis included one study with the RAI group, two studies with STx groups, and two studies with total thyroid ablation. These studies were conducted in European population groups and included predominately adult patients. The follow-up duration ranged from as early as 18 months in the study conducted by Witte et al. to 5 years post-surgery in the study by Barcyznski et al. [15, 16].
Risk of bias
The risk of bias assessment of the RCTs and cohort studies are presented in Supplementary Fig. 2A and Supplementary Table S1, respectively. The risk of bias in the RCTs included in our study indicated that while there was a low risk of bias across most of the domains of the Cochrane risk of bias tool, there was overall some level of concern (Supplementary Fig. 2B). This mostly stemmed from the lack of blinding in most of the RCTs included in the review. The RCTs by Witte et al., Moleti et al., Leo et al., and Jarhult et al. were single-blinded as either the investigators or patients were aware of the procedure. The RCT of 200 patients comparing TTx to STx by Barcyznski et al. was the only RCT that was double-blinded as both investigators and ophthalmologists were masked to the group assignment [15]. The RCT of 42 patients by Erdogan et al. did not account for blinding in their analysis [23]. However, this study was not included in our meta-analysis since it did not have comparable GO data. The risk of bias in the cohort studies included in our review was assessed using the Newcastle-Ottawa Scale. All studies received a score of above 5, and the only cohort study included in our meta-analysis, Kautbally et al., received a score of 8, indicating a low risk of bias (Supplementary Table S1) [14].
The decline in TRAbs levels
All studies included in the review showed a decline in TRAb levels post-TTx, and a statistically significant decline was documented by seven studies [7, 9, 18, 19, 21,22,23]. Five studies (four RCTs and one cohort study) that had a comparison group and also made qualitative assessments in regard to the normalization of TRAbs were included in our meta-analysis [14,15,16,17,18]. As shown in Fig. 2, while individually no study demonstrated a significant effect, the effective pooled data suggest that TRAb levels were significantly normalized after TTx as compared to other intervention groups (OR: 1.36, 95% CI: 1.02–1.81, p = 0.035). Similarly, significantly fewer patients had unnormalized TRAb levels post-TTx as compared to other intervention groups (OR: 0.60, 95% CI: 0.37–0.99, p = 0.046, Supplementary Fig. 6A). However, we found discrepancies when compared to the results of the studies that were included in this systematic review. The retrospective cohort study of 61 patients by Konturek et al. and an RCT of 42 patients by Erdogan et al. did find a significant reduction in TRAbs levels post-TTx as compared to STx and ATD, respectively [14, 15]. But De Bellis et al., Myer Zu Horste et al., and Jarhult et al. respectively found no difference when compared to thyroid ablation, ATDs, and STx, respectively [9, 21, 24].
Improvement in Graves’ ophthalmopathy score
All studies included in the review showed improvement in GO outcomes post-TTx, but significant improvement was documented by five studies [7, 9, 21,22,23]. These five studies (four RCTs and one cohort study) had a comparison group and also made qualitative assessments in regard to GO progression, hence, were included in our meta-analysis [14,15,16,17,18]. There was also no significant differences found in improvement, worsening, or unchanging outcomes of GO in post-TTx as compared with other intervention groups (according to Figures 3, 4 and Supplementary Fig. 6B). This finding was consistent with the results of all of the individual studies included in the meta-analysis. Moreover, no significant difference in GO outcomes was also noted between TTx and other interventions in all but two studies included in this review [17, 21]. In the single-blinded RCT of 40 patients, Moleti et al. found that GO outcomes improved significantly following total thyroid ablation as compared to the TTx alone [17]. Similarly, the retrospective cohort study of 92 patients by Myer Zu Horste et al. showed that TTx improved the outcome of GO significantly as compared to ATD alone [21].
Discussion
TRAb levels post-TTx
Our results suggest that TRAb levels were significantly normalized after TTx as compared to other intervention groups (OR: 1.36, 95% CI: 1.02–1.81, p = 0.035, Fig. 2). This finding was consistent with Kautbally et al. and Barcyznski et al. which showed a significant reduction in TRAb levels post-TTx as compared to RAI and STx, respectively [14, 15]. But we also found that there was no significant difference in the outcomes of GO post-TTx as compared to other intervention groups (Figs. 3, 4, Supplementary Fig. 6B). These results indicate that while TRAb levels may undergo decline more post-TTx, but their is no evidence it offered any added improvements to the progression of GO. The decline in TRAb levels post-TTx has been documented by previous studies, however with conflicting correlations to improvements in the GO outcomes [6,7,8,9].
To the best of our knowledge, we understand that this is the first meta-analysis to demonstrate a significant decline in the TRAb level post-TTx and with no clinical correlation with GO outcomes. There may be several explanations for this finding, which include variability of GO scoring systems and TRAb assays used, independent variables affecting GO outcomes, alternative disease mechanisms, and limitations of our current study.
GO scoring systems and TRAb assays
There were technical challenges in this analysis due to certain nuances in the GO scoring systems and TRAb assays used in the studies. The instruments for GO scoring systems differ in the amount of objective and subjective data that are collected from the patients. The CAS and EUGOGO classifications are mainly based on objective data and are considered to be good predictors of disease, as compared to GO quality of life questionnaires, which have been found to show only a moderate correlation with disease severity [25,26,27]. Moreover, the NOSPECS grading system only measures disease severity but not activity unlike the newer classifications systems such as VISA and EUGOGO [7]. While most of the studies included in our analysis used a 2nd-generation TRAb immunoassay, they do not differentiate between the stimulating (TSI) and blocking antibodies (TBII) subtypes of TRAbs. This is important to note as TSIs are known to provide a stronger positive correlation to GO severity [28,29,30].
Independent variables affecting GO outcomes
TTx is preferred over ATD and RAI for more severe GO cases and those with thyrotoxicosis and a large goiter size [5]. Hence, differences in GO severity could impact the results seen when comparing TTx to other treatment options. More severe GO and higher thyrotoxicosis are also associated with higher TRAb levels and therefore are more likely to cause persistence after treatment [31]. The smoking status of the patient is also another independent factor associated with higher TRAb levels and worse clinical outcomes [32]. Moreover, there may be a timepoint variability to the results seen when comparing surgery to RAI ablation. It has been demonstrated by studies examining the course of TRAb levels that RAI ablation results in a temporary surge of antibodies after treatment followed by a gradual decline [14, 30, 33,34,35]. While this may be related to the dose of RAI delivered, Kautbally et al. observed a marked rise in TSI levels over the first 6 months followed by a gradual decrease, and eventual normalization of TSI levels at 18 months [13].
Alternative disease mechanisms
The TRAb overstimulation of the orbital fibroblast TSH receptor model is currently the most well-accepted disease mechanism underlying GO progression [3]; however, new insights into the pathophysiology of GO have implicated the role of insulin-like growth factor 1 (IGF1) [36]. Along with the TSH receptor, IGF1 receptor expression in orbital fibroblasts is also increased in GO [37, 38]. Evidence from in vitro studies suggests that GO results from the stimulation of the IGF1 receptor on orbital fibroblasts with a possible synergistic interaction between TRAbs and IGR1 in increasing orbital fat expansion [36, 39]. Further evidence of this crosstalk between TRAbs and IGF was demonstrated by Krieger et al. The study showed that M22, a stimulating TRAb, which did not bind the IGF1 receptor, was also inhibited by the IGF1 receptor antagonists [40]. Further elucidation of this signalling pathway has led researchers to trial various immunosuppressive and biological agents with varying levels of success [39]. Future developments in this area could lead to new non-surgical treatment options in GO.
Limitations of our study
Our analysis was limited by the small sample sizes of each study and a few robust RCTs with qualitative analysis of TRAb levels and GO outcomes in patients after TTx. There were nine studies not included in our meta-analysis which all showed conflicting correlations between TRAb levels and GO outcomes [7, 9, 19,20,21,22,23,24, 41].
While we do demonstrate low statistical heterogeneity in our analysis, there may still be clinical heterogeneity in our study when comparing TTx to other intervention groups which include a combination of surgical and non-surgical treatments. This was particularly evident in studies that included TTx in both control and treatment arms such as those which compared TTx to thyroid ablation. A subgroup analysis was not possible in this review due to the limited studies measuring TRAb levels and the differences in the reporting of outcomes. Future studies in this area could perform a subgroup analysis to compare the decline in TRAb levels after TTx to other surgical or non-surgical interventions. Going forward, being able to quantify the amount of TRAb and how its decline correlates with the outcome of Graves’s opthalmopathy would aid in its diagnostic applicability and management. This is an important implication to consider as TTx carries a risk of adverse effects such as permanent hypoparathyroidism or post-operative complications such as damage to the recurrent laryngeal nerve [10, 42].
Conclusion
We found that significantly more patients had normalized TRAb levels post-TTx as compared to other interventions. However, there was no significant difference in the outcome and progression of GO post-TTx as compared with other intervention groups. These results suggest that while TRAb levels decline more post-TTx, they may not predict added improvements to GO progression.
Data availability
Original data generated and analyzed during this study are included in this published article or in the data repositories listed in the References.
References
Hiromatsu Y, Eguchi H, Tani J, Kasaoka M, Teshima Y (2014) Graves’ ophthalmopathy: epidemiology and natural history. Internal Medicine 53(5):353–360. https://doi.org/10.2169/internalmedicine.53.1518
Wiersinga WM, Bartalena L (2002) Epidemiology and prevention of Graves’ ophthalmopathy. Thyroid. 12(10):855–860. https://doi.org/10.1089/105072502761016476
Bahn RS (2015) Current insights into the pathogenesis of Graves’ ophthalmopathy. Hormone and Metabolic Research 47(10):773–778. https://doi.org/10.1055/s-0035-1555762
Kotwal A, Stan M (2018) Current and future treatments for Graves’ disease and Graves’ ophthalmopathy. Hormone and Metabolic Research 50(12):871–886. https://doi.org/10.1055/a-0739-8134
Ross DS, Burch HB, Cooper DS et al (2016) 2016 American Thyroid Association Guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 26(10):1343–1421. https://doi.org/10.1089/thy.2016.0229
Takamura Y, Nakano K, Uruno T et al (2003) Changes in serum TSH receptor antibody (TRAb) values in patients with Graves’ disease after total or subtotal thyroidectomy. Endocrine Journal 50(5):595–601. https://doi.org/10.1507/endocrj.50.595
Nart A, Uslu A, Aykas A et al (2008) Total thyroidectomy for the treatment of recurrent Graves’ disease with ophthalmopathy. Asian Journal of Surgery 31:115
Lowery AJ, Kerin MJ (2009) Graves’ ophthalmopathy: the case for thyroid surgery. Surgeon. 7(5):290–296. https://doi.org/10.1016/S1479-666X(09)80007-3
De Bellis A, Conzo G, Cennamo G et al (2012) Time course of Graves’ ophthalmopathy after total thyroidectomy alone or followed by radioiodine therapy: a 2-year longitudinal study. Endocrine. 41(2):320–326. https://doi.org/10.1007/s12020-011-9559-x
Liu ZW, Masterson L, Fish B, Jani P, Chatterjee K (2015) Thyroid surgery for Graves’ disease and Graves’ ophthalmopathy. Cochrane Database of Systematic Reviews 2015(11). https://doi.org/10.1002/14651858.CD010576.pub2
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 372:n71. https://doi.org/10.1136/bmj.n71
Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA Assessing risk of bias in a randomized trial. Cochrane Handbook for Systematic Reviews of Interventions. https://doi.org/10.1002/9781119536604.ch8
Wells GA, Shea B, O’Connell D, Peterson J, Welch V LM et al. Newcastle-Ottawa Scale for assessing the quality of nonrandomized studies in meta-analysis. Published 2019. https://www.researchgate.net/publication/261773681_The_Newcastle-Ottawa_Scale_NOS_for_Assessing_the_Quality_of_Non-Randomized_Studies_in_Meta-Analysis. Accessed August 16, 2020
Kautbally S, Alexopoulou O, Daumerie C, Jamar F, Mourad M, Maiter D (2012) Greater efficacy of total thyroidectomy versus radioiodine therapy on hyperthyroidism and thyroid-stimulating immunoglobulin levels in patients with Graves’ disease previously treated with antithyroid drugs. Eur Thyroid J 1(2):122–128. https://doi.org/10.1159/000339473
Barczyński M, Konturek A, Hubalewska-Dydejczyk A, Gołkowski F, Nowak W (2012) Randomized clinical trial of bilateral subtotal thyroidectomy versus total thyroidectomy for Graves’ disease with a 5-year follow-up. The British Journal of Surgery 99(4):515–522. https://doi.org/10.1002/bjs.8660
Witte J, Goretzki PE, Dotzenrath C et al (2000) Surgery for Graves’ disease: total versus subtotal thyroidectomy — results of a prospective randomized trial. World Journal of Surgery 24:1303–1311. https://doi.org/10.1007/s002680010216
Moleti M, Violi MA, Montanini D et al (2014) Radioiodine ablation of postsurgical thyroid remnants after treatment with recombinant human TSH (rhTSH) in patients with moderate-to-severe Graves’ orbitopathy (GO): a prospective, randomized, single-blind clinical trial. The Journal of Clinical Endocrinology and Metabolism 99(5):1783–1789. https://doi.org/10.1210/jc.2013-3093
Leo M, Marcocci C, Pinchera A et al (2012) Outcome of Graves’ orbitopathy after total thyroid ablation and glucocorticoid treatment: follow-up of a randomized clinical trial. The Journal of Clinical Endocrinology and Metabolism 97(1). https://doi.org/10.1210/jc.2011-2077
Domosławski P, Łukieńczuk T, Forkasiewicz Z et al Influence of total thyroidectomy on orbital opthalmopathy and levels of antithyroid antibodies in patients with Graves’ disease. Polish Journal of Surgery. https://doi.org/10.2478/v10035-007-0026-6
Catz B, Perzik SL (1969) Total thyroidectomy in the management of thyrotoxic and euthyroid Graves’ disease. American Journal of Surgery 118(3):434–439. https://doi.org/10.1016/0002-9610(69)90151-2
Meyer Zu Horste M, Pateronis K, Walz MK et al (2016) The effect of early thyroidectomy on the course of active Graves’ orbitopathy (GO): a retrospective case study. Hormone and Metabolic Research 48(7):433–439. https://doi.org/10.1055/s-0042-108855
Konturek A, Barczyński M, Cichoń S et al Total thyroidectomy in treatment of Graves’ ophthalmopathy. Polish Journal of Surgery. https://doi.org/10.2478/v10035-008-0066-6
Erdoğan MF, Demir Ö, Ersoy RÜ et al (2016) Comparison of early total thyroidectomy with antithyroid treatment in patients with moderate-severe Graves’ orbitopathy: a randomized prospective trial. Eur Thyroid J 5(2):106–111. https://doi.org/10.1159/000444796
Järhult J, Rudberg C, Larsson E et al (2005) Graves’ disease with moderate-severe endocrine ophthalmopathy-long term results of a prospective, randomized study of total or subtotal thyroid resection. Thyroid. 15(10):1157–1164. https://doi.org/10.1089/thy.2005.15.1157
Barrio-Barrio J, Sabater AL, Bonet-Farriol E, Velázquez-Villoria Á, Galofré JC (2015) Graves’ ophthalmopathy: VISA versus EUGOGO classification, assessment, and management. Journal of Ophthalmology 2015. https://doi.org/10.1155/2015/249125
Yang M, Wiersinga WM, Soeters MR, Mourits MP (2014) What is the aim of immunosuppressive treatment in patients with Graves’ orbitopathy? Ophthalmic Plastic & Reconstructive Surgery 30(2):157–161. https://doi.org/10.1097/IOP.0000000000000036
Fayers T, Dolman PJ (2011) Validity and reliability of the TED-QOL: a new three-item questionnaire to assess quality of life in thyroid eye disease. The British Journal of Ophthalmology 95(12):1670–1674. https://doi.org/10.1136/BJOPHTHALMOL-2011-300487
Kampmann E, Diana T, Kanitz M, Hoppe D, Kahaly GJ (2015) Thyroid stimulating but not blocking autoantibodies are highly prevalent in severe and active thyroid-associated orbitopathy: a prospective study. International Journal of Endocrinology 2015. https://doi.org/10.1155/2015/678194
Ponto KA, Kanitz M, Olivo PD, Pitz S, Pfeiffer N, Kahaly GJ (2011) Clinical relevance of thyroid-stimulating immunoglobulins in Graves’ ophthalmopathy. Ophthalmology. 118(11):2279–2285. https://doi.org/10.1016/j.ophtha.2011.03.030
Teng CS, Yeung RTT, Khoo RKK, Alagaratnam TT (1980) A prospective study of the changes in thyrotropin binding inhibitory immunoglobulins in Graves’ disease treated by subtotal thyroidectomy or radioactive iodine*. The Journal of Clinical Endocrinology and Metabolism 50(6):1005–1010. https://doi.org/10.1210/jcem-50-6-1005
Stan MN, Bahn RS (2010) Risk factors for development or deterioration of Graves’ ophthalmopathy. Thyroid. 20(7):777. https://doi.org/10.1089/THY.2010.1634
Eckstein A, Quadbeck B, Mueller G et al (2003) Impact of smoking on the response to treatment of thyroid associated ophthalmopathy. The British Journal of Ophthalmology 87(6):773–776. https://doi.org/10.1136/BJO.87.6.773
Atkinson S, McGregor AM, Kendall-Taylor P, Peterson MM, Smith BR (1982) Effect of radioiodine on stimulatory activity of Graves’ immunoglobulins. Clinical Endocrinology 16(6):537–543. https://doi.org/10.1111/j.1365-2265.1982.tb03170.x
Laurberg P, Wallin G, Tallstedt L, Abraham-Nordling M, Lundell G, Törring O (2008) TSH-receptor autoimmunity in Graves’ disease after therapy with anti-thyroid drugs, surgery, or radioiodine: a 5-year prospective randomized study. European Journal of Endocrinology 158(1):69–75. https://doi.org/10.1530/EJE-07-0450
Dong Q, Liu X, Wang F et al (2017) Dynamic changes of TRAb and TPOAb after radioiodine therapy in Graves’ disease. Acta Endocrinologica 13(1):72–76. https://doi.org/10.4183/aeb.2017.72
Smith TJ, Janssen JAMJL (2016) Building the case for insulin-like growth factor receptor-I involvement in thyroid-associated ophthalmopathy. Front Endocrinol (Lausanne) 7:1. https://doi.org/10.3389/FENDO.2016.00167
Brenner-Gati L, Berg KA, Gershengorn MC (1988) Thyroid-stimulating hormone and insulin-like growth factor-1 synergize to elevate 1,2-diacylglycerol in rat thyroid cells. Stimulation of DNA synthesis via interaction between lipid and adenylyl cyclase signal transduction systems. Journal of Clinical Investigation 82(3):1144. https://doi.org/10.1172/JCI113672
Starkey KJ, Janezic A, Jones G, Jordan N, Baker G, Ludgate M (2003) Adipose thyrotrophin receptor expression is elevated in Graves’ and thyroid eye diseases ex vivo and indicates adipogenesis in progress in vivo. Journal of Molecular Endocrinology 30(3):369–380. https://doi.org/10.1677/JME.0.0300369
Taylor PN, Zhang L, Lee RWJ et al (2020) New insights into the pathogenesis and nonsurgical management of Graves orbitopathy. Nature Reviews. Endocrinology 16(2):104–116. https://doi.org/10.1038/S41574-019-0305-4
Krieger CC, Neumann S, Marcus-Samuels B, Gershengorn MC (2017) TSHR/IGF-1R cross-talk, not IGF-1R stimulating antibodies, mediates Graves’ ophthalmopathy pathogenesis. Thyroid. 27(5):746. https://doi.org/10.1089/THY.2017.0105
Lanzolla G, Menconi F, Nicolì F et al (2021) Beneficial effect of low-dose radioiodine ablation for Graves’ orbitopathy: results of a retrospective study. Journal of Endocrinological Investigation 44(12):2575–2579. https://doi.org/10.1007/s40618-021-01544-1
Bhattacharyya N, Marvin PF (2002) Assessment of the morbidity and complications of total thyroidectomy. Archives of Otolaryngology. Head & Neck Surgery 128(4):389–392. https://doi.org/10.1001/archotol.128.4.389
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
AA: writing—original draft; writing—review and editing (equal). FA: writing—review and editing (equal). GE: formal analysis; writing—review and editing (equal). SE: supervision; conceptualization; writing—review and editing (equal).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1:
Figures S1-S6 and Table S1 (DOCX 1192 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anees, A., Ayeni, F.E., Eslick, G.D. et al. TSH receptor autoantibody levels post-total thyroidectomy in Graves’ ophthalmopathy: a meta-analysis. Langenbecks Arch Surg 408, 415 (2023). https://doi.org/10.1007/s00423-023-03153-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-023-03153-3