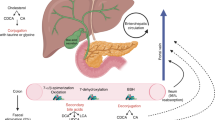
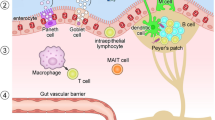
Abstract
Background
The gut microbiota, composed by several species of microorganisms, works to preserve the liver–gut homeostasis and plays an important role during digestion and absorption of nutrients, and in the immune response of the host. In this review, we analyzed the influence of microbiota in patients with cholangiocarcinoma (CCA) who were candidates for elective surgery.
Methods
A literature review was conducted to identify papers that provided empiric evidence to support that the altered microbiota composition (dysbiosis) is related also to CCA development.
Results
Bacteria such as Helicobacter pylori, Helicobacter hepaticus, and Opisthorchis viverrini increase the risk of CCA. The most abundant genera were Enterococcus, Streptococcus, Bacteroides, Klebsiella, and Pyramidobacter in CCA’s biliary microbiota. Additionally, levels of Bacteroides, Geobacillus, Meiothermus, and Anoxybacillus genera were significantly higher. An enrichment of Bifidobacteriaceae, Enterobacteriaceae, and Enterococcaceae families has also been observed in CCA tumor tissue. Microbiota is related to postoperative outcomes in abdominal surgery. The combination of caloric restriction diets in liver cancer or CCA increases the effect of the chemotherapy treatment.
Conclusion
The correct use of nutrition for microbiota modulation according to each patient’s needs could be a therapeutic tool in combination with elective surgery and chemotherapy to diminish side effects and improve prognosis. Further investigations are needed to fully understand the mechanisms by which they are related.

Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Code availability
The data for this project are confidential but may be obtained with Data Use Agreements with the Massachusetts Department of Elementary and Secondary Education (DESE). Researchers interested in access to the data may contact Victor Lopez-Lopez. It can take some months to negotiate data use agreements and gain access to the data. The author will assist with any reasonable replication attempts for 2 years following publication.
References
Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, Cardinale V et al (2020) Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol 17:557–588
Wu HJ, Chu PY (2019) Role of cancer stem cells in cholangiocarcinoma and therapeutic implications. Int J Mol Sci 20:4154
Asadi H, Hollingsworth R, Pennycooke K, Thanaratnam P, Given M, Keeling A, Lee M (2016) A review of percutaneous transhepatic biliary drainage at a tertiary referral centre. Clin Radiol 71:1312 e1317-1312 e1311
DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA et al (2007) Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 245:755–762
Thursby E, Juge N (2017) Introduction to the human gut microbiota. Biochem J 474:1823–1836
Underhill DM, Iliev ID (2014) The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol 14:405–416
Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, Knight R (2018) The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol 15:397–411
Arab JP, Martin-Mateos RM, Shah VH (2018) Gut-liver axis, cirrhosis and portal hypertension: the chicken and the egg. Hepatol Int 12:24–33
Sun J, Zhang J, Wang X, Ji F, Ronco C, Tian J, Yin Y (2020) Gut-liver crosstalk in sepsis-induced liver injury. Crit Care 24:614
Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ (2015) Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis 26:26191
Segura-López FK, Güitrón-Cantú A, Torres J (2015) Association between Helicobacter spp. infections and hepatobiliary malignancies: a review. World J Gastroenterol 21:1414–1423
Zhou D, Wang JD, Weng MZ, Zhang Y, Wang XF, Gong W, Quan ZW (2013) Infections of Helicobacter spp. in the biliary system are associated with biliary tract cancer: a meta-analysis. Eur J Gastroenterol Hepatol 25:447–454
Jala I, Almanfaluthi ML, Laha T, Kanthawong S, Tangkawattana S, Saichua P, Suttiprapa S et al (2021) Helicobacter pylori GroEL seropositivity is associated with an increased risk of Opisthorchis viverrini-associated hepatobiliary abnormalities and cholangiocarcinoma. Korean J Parasitol 59:363–368
Shimoyama T, Takahashi R, Abe D, Mizuki I, Endo T, Fukuda S (2010) Serological analysis of Helicobacter hepaticus infection in patients with biliary and pancreatic diseases. J Gastroenterol Hepatol 25(Suppl 1):S86-89
Thinkhamrop K, Khuntikeo N, Laohasiriwong W, Chupanit P, Kelly M, Suwannatrai AT (2021) Association of comorbidity between Opisthorchis viverrini infection and diabetes mellitus in the development of cholangiocarcinoma among a high-risk population, northeastern Thailand. PLoS Negl Trop Dis 15:e0009741
Avilés-Jiménez F, Guitron A, Segura-López F, Méndez-Tenorio A, Iwai S, Hernández-Guerrero A, Torres J (2016) Microbiota studies in the bile duct strongly suggest a role for Helicobacter pylori in extrahepatic cholangiocarcinoma. Clin Microbiol Infect 22:178.e111-178.e122
Rao B, Ren T, Wang X, Wang H, Zou Y, Sun Y, Liu S et al (2021) Dysbiosis in the human microbiome of Cholangiocarcinoma. Front Physiol 12:715536
Saab M, Mestivier D, Sohrabi M, Rodriguez C, Khonsari MR, Faraji A, Sobhani I (2021) Characterization of biliary microbiota dysbiosis in extrahepatic cholangiocarcinoma. PLoS One 16:e0247798
Chng KR, Chan SH, Ng AHQ, Li C, Jusakul A, Bertrand D, Wilm A et al (2016) Tissue microbiome profiling identifies an enrichment of specific enteric bacteria in Opisthorchis viverrini associated cholangiocarcinoma. EBioMedicine 8:195–202
Chen B, Fu SW, Lu L, Zhao H (2019) A Preliminary study of biliary microbiota in patients with bile duct stones or distal cholangiocarcinoma. Biomed Res Int 2019:1092563
Jia X, Lu S, Zeng Z, Liu Q, Dong Z, Chen Y, Zhu Z et al (2020) Characterization of gut microbiota, bile acid metabolism, and cytokines in intrahepatic cholangiocarcinoma. Hepatology 71:893–906
Lee H, Lee HK, Min SK, Lee WH (2020) 16S rDNA microbiome composition pattern analysis as a diagnostic biomarker for biliary tract cancer. World J Surg Oncol 18(1):19
Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, Lind GE et al (2016) Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 13:261–280
Kirstein MM, Vogel A (2016) Epidemiology and risk factors of cholangiocarcinoma. Visc Med 32:395–400
Kim TS, Pak JH, Kim JB, Bahk YY (2016) Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: a brief review. BMB Rep 49:590–597
Zheng S, Zhu Y, Zhao Z, Wu Z, Okanurak K, Lv Z (2017) Liver fluke infection and cholangiocarcinoma: a review. Parasitol Res 116:11–19
Ketpueak T, Thiennimitr P, Apaijai N, Chattipakorn SC, Chattipakorn N (2020) Association of chronic opisthorchis infestation and microbiota alteration on tumorigenesis in cholangiocarcinoma. Clin Transl Gastroenterol 12:e00292
Thinkhamrop K, Khuntikeo N, Phonjitt P, Chamadol N, Thinkhamrop B, Moore MA, Promthet S (2015) Association between diabetes mellitus and fatty liver based on ultrasonography screening in the world’s highest cholangiocarcinoma incidence region, Northeast Thailand. Asian Pac J Cancer Prev 16:3931–3936
Park JH, Hong JY, Kwon M, Lee J, Han K, Han IW, Kang W et al (2021) Association between non-alcoholic fatty liver disease and the risk of biliary tract cancers: a South Korean nationwide cohort study. Eur J Cancer 150:73–82
Zhang Q, Ma C, Duan Y, Heinrich B, Rosato U, Diggs LP, Ma L et al (2021) Gut microbiome directs hepatocytes to recruit MDS’s and promote cholangiocarcinoma. Cancer Discov 11:1248–1267
Labib PL, Goodchild G, Pereira SP (2019) Molecular pathogenesis of cholangiocarcinoma. BMC Cancer 19:185
Lin J, Shi J, Guo H, Yang X, Jiang Y, Long J, Bai Y et al (2019) Alterations in DNA damage repair genes in primary liver cancer. Clin Cancer Res 25:4701–4711
Mar WA, Shon AM, Lu Y, Yu JH, Berggruen SM, Guzman G, Ray CE Jr et al (2016) Imaging spectrum of cholangiocarcinoma: role in diagnosis, staging, and posttreatment evaluation. Abdom Radiol (NY) 41:553–567
Ruys AT, Busch OR, Rauws EA, Gouma DJ, van Gulik TM (2013) Prognostic impact of preoperative imaging parameters on resectability of hilar cholangiocarcinoma. HPB Surg 2013:657309
Choi YH, Lee JM, Lee JY, Han CJ, Choi JY, Han JK, Choi BI (2008) Biliary malignancy: value of arterial, pancreatic, and hepatic phase imaging with multidetector-row computed tomography. J Comput Assist Tomogr 32:362–368
Yeh BM, Liu PS, Soto JA, Corvera CA, Hussain HK (2009) MR imaging and CT of the biliary tract. Radiographics 29:1669–1688
Park HS, Lee JM, Choi JY, Lee MW, Kim HJ, Han JK, Choi BI (2008) Preoperative evaluation of bile duct cancer: MRI combined with MR cholangiopancreatography versus MDCT with direct cholangiography. AJR Am J Roentgenol 190:396–405
Yin L, Zhao S, Zhu H, Ji G, Zhang X (2021) Primary tumor resection improves survival in patients with multifocal intrahepatic cholangiocarcinoma based on a population study. Sci Rep 11:12166
Groot Koerkamp B, Wiggers JK, Gonen M, Doussot A, Allen PJ, Besselink MGH, Blumgart LH et al (2015) Survival after resection of perihilar cholangiocarcinoma-development and external validation of a prognostic nomogram. Ann Oncol 26:1930–1935
Strijker M, Belkouz A, van der Geest LG, van Gulik TM, van Hooft JE, de Meijer VE, Haj Mohammad N et al (2019) Treatment and survival of resected and unresected distal cholangiocarcinoma: a nationwide study. Acta Oncol 58:1048–1055
Lee SG, Song GW, Hwang S, Ha TY, Moon DB, Jung DH, Kim KH et al (2010) Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci 17:476–489
Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM (2014) Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg 149:565–574
Robles R, Figueras J, Turrión VS, Margarit C, Moya A, Varo E, Calleja J et al (2004) Spanish experience in liver transplantation for hilar and peripheral cholangiocarcinoma. Ann Surg 239:265–271
Panayotova GG, Paterno F, Guarrera JV, Lunsford KE (2020) Liver transplantation for cholangiocarcinoma: insights into the prognosis and the evolving indications. Curr Oncol Rep 22:49
Sapisochin G, Facciuto M, Rubbia-Brandt L, Marti J, Mehta N, Yao FY, Vibert E et al (2016) Liver transplantation for “very early” intrahepatic cholangiocarcinoma: international retrospective study supporting a prospective assessment. Hepatology 64:1178–1188
De Martin E, Rayar M, Golse N, Dupeux M, Gelli M, Gnemmi V, Allard MA et al (2020) Analysis of liver resection versus liver transplantation on outcome of small intrahepatic cholangiocarcinoma and combined hepatocellular-cholangiocarcinoma in the setting of cirrhosis. Liver Transpl 26:785–798
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S et al (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273–1281
Agnes A, Puccioni C, D’Ugo D, Gasbarrini A, Biondi A, Persiani R (2021) The gut microbiota and colorectal surgery outcomes: facts or hype? A narrative review. BMC Surg 21:83
Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Bieda J, Chaemsaithong P et al (2014) The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome 2:18
Shogan BD, Belogortseva N, Luong PM, Zaborin A, Lax S, Bethel C, Ward M et al (2015) Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci Transl Med 7:286ra268
Paun BC, Cassie S, MacLean AR, Dixon E, Buie WD (2010) Postoperative complications following surgery for rectal cancer. Ann Surg 251:807–818
Schardey HM, Joosten U, Finke U, Staubach KH, Schauer R, Heiss A, Kooistra A et al (1997) The prevention of anastomotic leakage after total gastrectomy with local decontamination. A prospective, randomized, double-blind, placebo-controlled multicenter trial. Ann Surg 225:172–180
Shogan BD, Chen J, Duchalais E, Collins D, Chang M, Krull K, Krezalek MA et al (2020) Alterations of the rectal microbiome are associated with the development of postoperative ileus in patients undergoing colorectal surgery. J Gastrointest Surg 24:1663–1672
Carpelan-Holmstrom M, Koskenvuo L, Haapamaki C, Renkonen-Sinisalo L, Lepisto A (2020) Clinical management of 52 consecutive retro-rectal tumours treated at a tertiary referral centre. Colorectal Dis 22:1279–1285
Shin SY, Hussain Z, Lee YJ, Park H (2021) An altered composition of fecal microbiota, organic acids, and the effect of probiotics in the guinea pig model of postoperative ileus. Neurogastroenterol Motil 33:e13966
Lederer AK, Chikhladze S, Kohnert E, Huber R, Müller A (2021) Current insights: the impact of gut microbiota on postoperative complications in visceral surgery-a barrative review. Diagnostics (Basel) 11:2099
Alverdy JC, Hyoju SK, Weigerinck M, Gilbert JA (2017) The gut microbiome and the mechanism of surgical infection. Br J Surg 104:e14–e23
Rodriguez-Palacios A, Harding A, Menghini P, Himmelman C, Retuerto M, Nickerson KP, Lam M et al (2018) The artificial sweetener splenda promotes gut proteobacteria, dysbiosis, and myeloperoxidase reactivity in Crohn’s disease-like ileitis. Inflamm Bowel Dis 24:1005–1020
Vernocchi P, Del Chierico F, Putignani L (2020) Gut microbiota metabolism and interaction with food components. Int J Mol Sci 21:3688
de Roos B, Brennan L (2017) Personalised interventions-a precision approach for the next generation of dietary intervention studies. Nutrients 9:847
Funding
This work was funded by the Institute of Health “Carlos III” (ISCIII), and co-funded by the Fondo Europeo de Desarrollo Regional-FEDER (grant number PI20/00505). B.R-M was supported by the “Miguel Servet Type I” program (CP19/00098, ISCIII, Spain; co-funded by the Fondo Europeo de Desarrollo Regional-FEDER). M.A.M-S was supported by a PFIS contract from the ISCIII (FI21/00003, ISCIII, Spain; co-funded by the Fondo Europeo de Desarrollo Regional-FEDER).
Author information
Authors and Affiliations
Contributions
Study conception and design: OLR, VLL, RRC, and BRM. Acquisition of data: OLR, VLL, ABR, MMS, DE, CLT, KM, ABM, and PR. Analysis and interpretation of data: OLR, VLL, ABR, MMS, DE, CLT, KM, ABM, PR, RRC, and BRM. Drafting of manuscript: OLR, VLL, RRC, and BRM. Critical revision of manuscript: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Oriana Lo Re and Victor López-López shared first authorship.
Ricardo Robles-Campos and Bruno Ramos-Molina shared last authorship.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Re, O.L., López-López, V., Balaguer-Román, A. et al. New challenges in cholangiocarcinoma candidates for elective surgery: harnessing the microbiome dysbiosis. Langenbecks Arch Surg 408, 134 (2023). https://doi.org/10.1007/s00423-023-02867-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00423-023-02867-8