Abstract
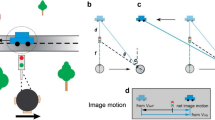
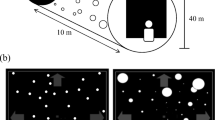
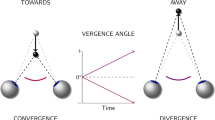
The object of this study is to mathematically specify important characteristics of visual flow during translation of the eye for the perception of depth and self-motion. We address various strategies by which the central nervous system may estimate self-motion and depth from motion parallax, using equations for the visual velocity field generated by translation of the eye through space. Our results focus on information provided by the movement and deformation of three-dimensional objects and on local flow behavior around a fixated point. All of these issues are addressed mathematically in terms of definite equations for the optic flow. This formal characterization of the visual information presented to the observer is then considered in parallel with other sensory cues to self-motion in order to see how these contribute to the effective use of visual motion parallax, and how parallactic flow can, conversely, contribute to the sense of self-motion. This article will focus on a central case, for understanding of motion parallax in spacious real-world environments, of monocular visual cues observable during pure horizontal translation of the eye through a stationary environment. We suggest that the global optokinetic stimulus associated with visual motion parallax must converge in significant fashion with vestibular and proprioceptive pathways that carry signals related to self-motion. Suggestions of experiments to test some of the predictions of this study are made.
Similar content being viewed by others
References
Angelaki DE, Hess BJM (2005) Self-motion-induced eye movements: effects on visual acuity and navigation. Nature Rev Neurosci 6(12): 966–976
Andersen RA (1997) Multimodal integration for the representation of space in the posterior parietal cortex. Phli as Trans R Soc Lond B 352: 1421–1428
Banks MS, Ehrlich SM, Backus BT, Crowell JA (1996) Estimating heading during real and simulated eye movements. Vision Res 36(3): 431–443
Becker W, Raab S, Jürgens R (2002) Circular vection during voluntary suppression of optokinetic reflex. Exp Brain Res 144(4): 554–557
Beintema J, Berg AV (1998) Heading detection using motion templates and eye velocity gain fields. Vision Res 38: 2155–2179
Berthoz A, Israël I, Georges-Francois P, Grasso R, Tsuzuku T (1995) Spatial memory of body linear displacement: what is being stored. Science 269: 95–98
Bertin RJV, Berthoz A (2004) Visuo-vestibular interaction in the reconstruction of travelled trajectories. Exp Brain Res 154: 11–21
Bertin RJV, Israël I (2005) Optic-flow-based perception of two-dimensional trajectories and the effects of a single landmark. Perception 34(4): 453–475
Bertin RJV, Israël I, Lappe M (2000) Perception of two-dimensional, simulated ego-motion trajectories from optic flow. Vision Research 40: 2951–2971
Beusmans JMH (1998) Perceived object shape affects the perceived direction of self-movement. Perception 27(9): 1079–1085
Beverly KI, Regan D (1979) Separable aftereffects of changing size and motion in depth: Different neural mechanisms?. Vision Res 19: 727–733
Bremmer F, Duhamel JR, Ben Hamed S, Graf W (2002) Heading encoding in the macaque ventral intraparietal area (VIP). Eur J Neurosci 16(8): 1554–1568
Bremmer F, Kubischik M, Pekel M, Lappe M, Hoffmann KP (1999) Linear vestibular self-motion signals in monkey medial superior temporal area. Ann NY Acad Sci 871: 272–281
Bremmer F, Lappe M (1999) The use of optical velocities for distance discrimination and reproduction during visually simulated self motion. Exp Brain Res 127(1): 33–42
Bronstein AM, Buckwell D (1997) Automatic control of postural sway by visual motion parallax. Exp Brain Res 113: 243–248
Chen-Huang C, McCrea RA (1999a) Effects of viewing distance on the responses of horizontal canal-related secondary vestibular neurons during angular head rotation. J Neurophysiol 81: 2517–2537
Chen-Huang C, McCrea RA (1999b) Effects of viewing distance on the responses of vestibular neurons to combined angular and linear vestibular stimulation. J Neurophysiol 81: 2538–2557
Cooper HM, Magnin M (1986) A common mammalian plan of accessory optic system organization revealed in all primates. Nature 324(6096): 457–459
Crowell JA, Banks MS, Shenoy KV, Andersen RA (1998) Visual self-motion perception during head turns. Nature Neurosci 1(8): 732–737
Cutting JE (1996) Wayfinding from multiple sources of local information in retinal flow. J Exp Psychol: Human Percept Perform 22: 1299–1313
Cutting JE, Vishton PM, Flückiger M, Baumberger B, Gerndt JD (1997) Heading and path information from retinal flow in naturalistic environments. Percept Psychophys 59(3): 426–441
Droulez J, Conilleau-Péres (1990) Visual perception of surface curvature. The spin variation and its physiological implications. Biol Cybern 62: 211–224
Duffy CJ (1998) MST neurons respond to optic flow and translational movement. J Neurophysiol 80: 1816–1827
Faubert J (2001) Motion parallax, stereoscopy, and the perception of depth: Practical and theoretical issues. In: Javidi B, Okano F (eds) Three-Dimensional Video and Display: Devices and Systems. Proceedings of SPIE, vol. CR76, pp 168–191
Fite KV (1985) Pretectal and accessory-optic visual nuclei of fish, amphibia and reptiles: theme and variations. Brain Behav Evol 16: 192–202
Franz MO, Chahl JS, Krapp HG (2004) Insect-inspired estimation of egomotion. Neural Comput 16(11): 2245–2260
Freeman TCA, Fowler TA (2000) Unequal retinal and extra-retinal motion signals produce different perceived slants of moving surfaces. Vision Res 40: 1857–1868
Frenz H, Lappe M (2005) Absolute travel distance from optic flow. Vision Res 45: 1679–1692
Frost BJ (1993) Subcortical analysis of visual motion: Relative motion, figure-ground discrimination and self-induced optic flow. In: Miles FA, Wallman J(eds) Visual Motion and its Role in the Stabilization of the Gaze. Elsevier, Amsterdam, pp 159–175
Frost BJ, Wylie DR, Wang Y-C (1990) The processing of object and self-motion in the tectofugal and accessory optic pathways of birds. Vision Res 30(11): 1677–1688
Gibson JJ (1950) The Perception of the Visual World. Houghton Mifflin, Boston
Gibson JJ, Olum P, Rosenblatt F (1955) Parallax and perspective during aircraft landings. Am J Psychol 68: 372–385
Goldberg JM, Fernández C (1984) The vestibular system. In: Brookhart JM, Mountcastle VB, Darian-Smith I(eds) Handbook of Physiology – The Nervous System III. American Physiological Society, Baltimore, pp 977–1022
Gordon DA (1965) Static and dynamic fields in human space perception. J Opt Soc Am 55: 1296–1303
Graziano MSA, Andersen RA, Snowden RJ (1994) Tuning of MST neurons to spiral motions. J Neurosci 14(1): 54–67
Grigo A, Lappe M (1998) An analysis of heading towards a wall. In: Harris LR, Jenkin M(eds) Vision and action. Cambridge University Press, Cambridge, pp 215–229
Grimm RJ, Hemenway WG, Lebray PR, Black FO (1989) The perilymph fistula syndrome defined in mild head trauma. Acta Otolaryngol Suppl 464: 1–40
Hanes DA, McCollum G (2006) Variables contributing to the coordination of eye/head gaze shifts. Biol Cybern 94(4): 300–324
Harris LR (1994) Visual motion caused by movements of the eye, head, and body. In: Smith AT, Snowden R(eds) Visual detection of motion. Academic Press, London, pp 397–436
Harris LR, Jenkin M, Zikowitz DC (2000) Visual and non-visual cues in the perception of linear self motion. Exp Brain Res 135: 12–21
Heeger DJ, Jepson AD (1992) Subspace methods for recovering rigid motion I: algorithm and implementation. Int J Comput. Vision 7(2): 95–117
Israël I, Grasso R, Georges-Francois P, Tsuzuku T, Berthoz A (1997) Spatial memory and path integration studied by self-driven linear displacement. I. Basic properties. J Neurophysiol 77: 3180–3192
Jacob RG, Furman JM, Durrant JD, Turner SM (1996) Panic, Agoraphobia, and Vestibular Dysfunction. Am J Psychiatry 153: 503–512
Keshner EA, Kenyon RV (2000) The influence of an immersive virtual environment on the segmental organization of postural stabilizing responses. J Vestib Res 10: 207–219
Koenderink JJ, Doorn AJ (1987) Facts on optic flow. Biol Cybern 56(4): 247–254
Koenderink JJ, Doorn AJ (1976) Local structure of movement parallax of the plane. J Opt Soc Am 66: 717–723
Lackner JR, DiZio P (1988) Visual stimulation affects the perception of voluntary leg movements during walking. Perception 17: 71–80
Land MF, Lee DN (1994) Where we look when we steer. Nature 369(6483): 742–4
Lappe M (1998) A model of the combination of optic flow and extraretinal eye movement signals in primate extrastriate visual cortex. Neural Netw 11: 397–414
Lappe M, Bremmer F, van den Berg AV (1999) Perception of self-motion from visual flow. Trends Cogn Sci 3(9): 329–336
Lappe M, Rauschecker JP (1993) A neural network for the processing of optic flow from ego-motion in man and higher mammals. Neural Comput 5: 374–391
Longuet-Higgins HC, Prazdny K (1980) The interpretation of a moving retinal image. Proc R Soc Lond B 208: 385–397
Mergner T, Wertheim A, Rumberger A (2000) Which retinal and extra-retinal information is crucial for circular vection?. Arch Ital Biol 138(2): 123–38
Miles FA (1993) The sensing of rotational and translational optic flow by the primate optokinetic system. In: Miles FA, Wallman J(eds) Visual motion and its role in the stabilization of gaze. Elsevier, Amsterdam, pp 393–403
Miles FA (1998) The neural processing of 3-D visual information: evidence from eye movements. Eur J Neurosci 10: 811–822
Nakamura S (2006) Effects of depth, eccentricity and size of additional static stimulus on visually induced self-motion perception. Vision Res 46(15): 2344–53
Nakayama K, Loomis JM (1974) Optical velocity patterns, velocity-sensitive neurons, and space perception: a hypothesis. Perception 3(1): 63–80
Nawrot M (2003a) Eye movements provide the extra-retinal signal required for the perception of depth from motion parallax. Vision Res 43: 1553–1562
Nawrot M (2003b) Depth from motion parallax scales with eye movement gain. J Vision 3: 841–851
Nawrot M (2000) Viewing distance, eye movements, and the perception of relative depth from motion parallax. Investigative Ophthalmol Visual Sci 39(4): S462
Ono ME, Rivest J, Ono H (1986) Depth perception as a function of motion parallax and absolute-distance information. J Exp Psychol: Human Percept Perform 12: 331–337
Ono H, Sato T, Shioiri S, Ujike H (1998) An aftereffect of William Dember in motion: probing the signal for motion parallax. In: Hoffman RR, Sherrick MF, Warm JS(eds) Viewing psychology as a whole. APA, Washington, DC
Ono H, Ujike H (1994) Apparent depth with motion aftereffect and head movement. Perception 23: 1241–1248
Perrone JA, Stone LS (1994) A model of self-motion estimation within primate extrastriate visual cortex. Vision Res 34: 2917–2938
Regan D (1986) Visual processing of four kinds of relative motion. Vision Res 26(1): 127–145
Regan D, Beverly KI (1982) How do we avoid cofounding the direction we are looking with the direction we are moving?. Science 215: 194–196
Regan D, Gray R, Portfors CV, Hamstra SJ, Vincent A, Hong XH, Kohly R, Beverly K (1998) Catching, hitting, and collision avoidance. In: Harris LR, Jenkin M(eds) Vision and action. Cambridge University press, Cambridge
Rogers BJ (1993) Motion parallax and other dynamic cues for depth in humans. Rev Oculomot Res 5: 119–37
Rogers B, Graham M (1979) Motion parallax as an independent cue for depth perception. Perception 8: 125–134
Royden CS (1994) Analysis of misperceived observer motion during simulated eye rotations. Vision Res 34: 3215–3222
Thompson AM, Nawrot M (1999) Abnormal depth perception from motion parallax in amblyopic observers. Vision Res 39: 1407–1413
Ujike H, Ono H (2001) Depth thresholds of motion parallax as a function of head movement velocity. Vision Res 41: 2835–2843
Wang RF, Cutting JE (1999) Where we go with a little good information. Psychol Sci 10(1): 71–75
Warren WH, Hannon DJ (1990) Eye movements and optical flow. J Opt Soc Am A 7(1): 160–169
Wei M, DeAngelis GC, Angelaki DE (2003) Do visual cues contribute to the neural estimate of viewing distance used by the oculomotor system?. J Neurosci 23(23): 8340–8350
Yong NA, Pasige GD, Seidman SH (2007) Multiple sensory cues underlying the perception of translation and path. J Neurophysiol 97(2): 1100–1113
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hanes, D.A., Keller, J. & McCollum, G. Motion parallax contribution to perception of self-motion and depth. Biol Cybern 98, 273–293 (2008). https://doi.org/10.1007/s00422-008-0224-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-008-0224-2