Abstract
Purpose
Professional boxing is a sport that requires a high aerobic capacity to prevent fatigue and allow athletes to perform over 4–12 rounds. Typically, athletes will go into a heavy training period in a pre-bout camp lasting 6 to 9 weeks. This study investigates the impact of 3 weeks of repeated Wingate sprint interval training, performed on standard gym ergometer bikes, on skeletal muscle endurance and mitochondrial function.
Methods
Ten male professional boxers (age: 26 ± 4 years, height: 175 ± 5 cm, weight: 70 ± 5 kg) participated in the study. Baseline testing involved a NIRS monitor attached to the rectus femoris muscle prior to an incremental time to exhaustion test on a treadmill. After the treadmill test participants underwent a series of arterial occlusions to determine mitochondrial function post-volitional exhaustion. Participants then continued their own training for 3 weeks and then repeated baseline testing. After the second testing session, participants undertook three weekly sprint sessions consisting of 3 × 30 s maximal sprints with 60 s recovery. Testing was repeated 3 weeks later.
Results
The time to exhaustion increased by > 6% after 3 weeks of sprint interval training as compared to baseline and control (p < 0.05). Skeletal muscle oxygen saturation (SmO2) at exhaustion was increased by 5.5% after 3 weeks of sprint interval training as compared to baseline and control (p = 0.008). Skeletal muscle mitochondrial rate post exhaustion was increased by 160% after 3 weeks of sprint interval training as compared to baseline and control (p < 0.001).
Conclusion
The study demonstrated that SIT led to increased incremental time to exhaustion, higher SmO2 levels at volitional exhaustion and increased mitochondrial rates in professional boxers. These findings suggest that SIT should be an integral part of a boxe’s conditioning regimen to improve performance and safety within the ring.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amateur boxing is typically seen as a highly intermittent sport, a classification supported by studies like Nassib et al. (2017), which also notes the unique feature of one-minute recovery periods between rounds. The debate around the energetic costs of boxing, whether it leans more towards anaerobic (Ghosh AK, Goswami A 1995) or aerobic energy use (Davis et al. 2014), highlights the role of anaerobic energy production in the sport. However, professional boxing demonstrates a more intricate pattern of oxygen use and recovery, particularly in the rectus femoris muscle. Recent research (Usher and Babraj 2023) suggests a three-component model for skeletal muscle oxygenation and recovery, and consistent muscle activity as opposed to intermittency. This consistent activity suggests a continuous metabolic engagement of the mitochondria within the rectus femoris, maintaining a stable equilibrium between oxygen delivery and use throughout the rounds (Usher and Babraj 2023). The recovery from exercise has been shown to be an aerobic process (Zoll et al. 2006; Porter et al. 2015) and improved mitochondrial function will enhance recovery between rounds. Therefore, there is a need for appropriate training methods that enhance mitochondrial rate and function in professional boxing.
Sprint interval training (SIT) has been defined as short duration, typically 30 s or less, repeated supramaximal efforts (Gibala et al. 2012). Most studies utilising this approach use 7.5% of body mass to control intensity (Burgomaster et al. 2008; MacInnis and Gibala 2017) and has been shown to increase mitochondrial content and function within skeletal muscle after just 6 sessions, along with increases in glycolytic enzyme activity, buffering capacity and glycogen stores (Burgomaster et al. 2008; Little et al. 2010; Jacobs and Lundby 2013). To date the impact of SIT on mitochondrial function has been limited to biopsy pre- and post training and then measuring mitochondrial enzyme content and activity (Perry et al. 2010; MacInnis and Gibala 2017) and as such has not been explored in professional athletes. As well as changes in mitochondrial capacity, SIT has been shown to improve oxygen extraction into the skeletal muscle (Bailey et al. 2009) and increase mitochondrial oxygen affinity (Larsen et al. 2020) which will lead to improved energy production and utilisation (Booth and Thomason 1991; Mathuram et al. 2022) and can improve an athlete’s ability to sustain high-intensity efforts (Juel et al. 2004; Nielsen et al. 2017) and delay the onset of fatigue (Yamagishi and Babraj 2017).
The use of near infrared spectroscopy (NIRS) with rapid arterial occlusion (AO) have been used to noninvasively assess mitochondrial function post exercise (Ryan et al. 2013, 2014; Sumner et al. 2020; Hanna et al. 2021). Mitochondrial measurement with NIRS involves using a blood pressure cuff to create an AO, creating ischemic conditions in muscle tissue which separates the process of oxygen delivery and consumption (Maliszewski et al. 2024). Lagerwaard et al. (2019) reported a 40% faster mVO2 recovery in the gastrocnemius muscle of participants with a high endurance capacity as compared to low endurance capacity (Lagerwaard et al. 2019). Brizendine et al. (2013) showed an approximate twice the amount of mitochondrial capacity in the vastus lateralis muscle in endurance trained athletes as compared to inactive individuals (Brizendine et al. 2013). However, to date this approach has only been looked at during submaximal exercise efforts and has not been used in elite sport.
Given the short duration of training camps in professional boxing and the need to enhance muscle oxidative capacity then the use for a SIT based intervention may be beneficial. The efficiency and brief duration of these exercises make them easy to integrate into tight training schedules and would allow more time to technical components of the sport. Therefore, this study sought to determine the impact of a 3 week SIT intervention on mitochondrial capacity in trained professional boxers. We believe this study is the first to examine mitochondrial values with professional boxers as well as the first study to examine it following volitional exhaustion. We hypothesised that SIT would improve skeletal muscle fatiguability and improve post-exercise mitochondrial function in professional boxers.
Methods
Subjects
9 male professional boxers (age: 26 ± 4 years, height: 175 ± 5 cm, weight: 70 ± 5 kg body fat: 11 ± 3%, rectus femoris fat thickness: 4.6 ± 2.0 mm) were recruited for this study. Body composition remained unchanged throughout the study (p > 0.05). Each athlete held a current professional licence with the British Boxing Board of Control (bouts: 15 ± 5). Participants were excluded if there was a loss of training days over the last 3 months due to musculoskeletal injury or sanction from British Boxing Board of Control preventing the athlete from taking part in training or sparring. Participants were informed verbally and in writing on the risks and benefits of the study prior to signing informed consent form and was approved by Abertay University Research Ethics Committee (EMS5604). The study was carried out in line with the Declaration of Helsinki, except for the registration in a database.
Testing
Participants were instructed to adhere to their regular training routines and dietary habits throughout the study duration. They were also advised to avoid intense physical activities and alcohol consumption for 24 h prior to each laboratory visit. Additionally, participants were required to be in a fasted state, having abstained from food or drink intake for at least 4 h prior to their arrival at the Human Performance Laboratory.
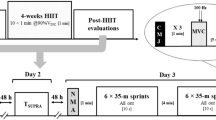
The research protocol comprised three distinct laboratory visits: an initial familiarisation session, a control period assessment, and a final evaluation post a 3 week sprint training intervention (Fig. 1). During each visit, participants had near infrared spectroscopy (NIRS; Moxy Monitor, Fortiori Design LLC, USA) taped to the rectus femoris muscle of the left and right leg. The Moxy is a lightweight (48 g) and small (62 × 52 × 15 mm) device that uses continuous four wavelengths (680 nm, 720 nm, 760 nm, and 800 nm) reporting values of SmO2 in the form of a 0–100 percentage scale (Feldmann et al. 2019).
The moxy monitor was set to high speed, 0.5 s update with no smoothing. The Moxy monitor has been shown to be valid and reliable during exercise with a greater dynamic scale than the Portamon monitor (McManus et al. 2018; Feldmann et al. 2019); and has been used to explore muscle oxygenation during a wide range of sporting environment and training domains (Paquette et al. 2018; Perrey and Ferrari 2018). Participants then attached a heart rate monitor (Polar H10, Polar Electro, UK). All data was then collected via Bluetooth transmission to the VO2 master app (VO2 Master Health Sensors Inc, Canada).
Body composition
All participants had height determined by stadiometer (Seca 264, Seca, UK) and body composition was determined by bioimpedance (Tanita MC-780, Tanita, Japan). The fat thickness on the rectus femoris muscle was determined by ultrasound (EagleView, Wellue, Diamond Bar, CA, USA) as a fat thickness of greater than 14 mm will impede NIRS signal (Feldmann et al. 2019).
Testing protocol
The occlusion protocol involved the application of a blood pressure cuff to the upper thigh, connected to a rapid inflation system, and the leg and was rapidly inflated to > 300 mmHg. The protocol (Fig. 1) included three rapid occlusions at rest in the supine position, each lasting 15 s with 30 s between each occlusion (see S1 for SmO2 during occlusion). Cuff inflation reaching 300 mmHg in under 2 s. The time from cuff inflation to decline in rectus femoris muscle oxygenation was 0.319 ± 0.114 s. Participants then completed an incremental treadmill test to volitional exhaustion, starting at a speed of 10 km.h−1 with a 0% incline, increasing by 1 km.h−1 every minute until reaching 18 km.h−1, after which incline increased by 1% every minute. Two minutes post-treadmill run, participants underwent a series of 10 rapid occlusions (15 s each with 30 s between each occlusion) followed by a single 5 min occlusion whilst in a supine position.
Control period
Participants were then instructed to continue with their own training for the next 3 weeks after the first testing session before returning to the lab at the same time of day and repeating the testing procedure.
Intervention
Following the second visit to the lab participants were given a familiarisation of 30 s effort against 7.5% body mass on the ergometer (LODE). Briefly, participants were instructed to bring the speed up to 85 rpm and then given a 3, 2, 1 countdown and instructed to cycle as fast as possible when hearing 1. Heart rate was recorded throughout, and athletes instructed that this is where the heart rate needs to be during the first sprint in subsequent training sessions. The intervention entailed participants performing 3 × 30 s maximal efforts with 60 s recovery on 3 days of the week for 3 weeks. Participants were instructed that ideally there should be 48 h between sprint sessions. Effort levels during these remote bike sprints were monitored using the Polar H10 heart rate data to ensure consistent exertion, with a self-reported adherence of 100% (Fig. 1).
Data analysis
Heart rate and SmO2 were exported from VO2 master as a 1 s average and processed in Python Jupyter Lab (version 3.3.2). A median average 5 s filter was applied to the data to smooth any movement artefacts (Buchheit and Laursen, 2011). SmO2 was used for analysis as it gives a better indication of skeletal muscle oxygenation when blood flow is not steady (Buchheit and Ufland 2011). Correcting for blood volume changes during occlusions to accurately assess changes due to oxygen consumption is required as blood volume changes can obscure the true NIRS signals reflecting muscular oxygen dynamics (Ryan et al. 2012). To effectively correct these signals, the blood volume change must be proportioned between oxygenated and deoxygenated blood components. This differentiation is important because it allows for a more precise interpretation of the NIRS data, isolating the oxygen consumption component from the confounding effects of blood volume fluctuations (Ryan et al. 2012).
Post-exercise occlusion a linear regression model was used for partial curve fitting to calculate the rate of muscle oxygen consumption (mVO2) during arterial occlusion. Curve fitting was expressed via a monoexponential curve fit to determine mitochondrial rate function (Ryan et al 2012). SmO2 during the treadmill test was analysed using linear curve fitting from the start of the final desaturation period to plateau before exhaustion (Fig. 1) with the slope representing rate of fast desaturation and rate of fast resaturation taken as the slope from the linear part of post-exercise recovery (Fig. 1). Area under the SmO2 post-treadmill recovery curve was calculated using the trapezoidal method and reflects oxygen availability during the recovery phase.
Statistical analysis
All data is presented as means ± standard deviation. Main and interaction effects are detailed, with post hoc pairwise analyses available in subsequent tables. Statistical analyses were conducted using Jamovi software (version 2.3.13). Normality checks were performed using the Shapiro–Wilks test, and all data were found to be normally distributed. Grubbs test for outliers was applied to the time to exhaustion and mitochondrial rate datasets, with an alpha value of 0.05. The G statistic for time to exhaustion was 1.959 and the critical value was 2.215, the G statistic for mitochondrial rate was 1.639 and the critical value was 2.651. Since the G statistic is lower than the critical value for both time to exhaustion and mitochondrial rate then there were no outliers within the dataset. All data was analysed using a repeated measures ANOVA for occlusions. Where there was a significant main effect then a least squares difference post hoc test was applied to determine where differences occurred. Significance was accepted at p < 0.05.
Results
There was a significant main effect for time in incremental time to exhaustion (p = 0.004, Fig. 2). Following the control period there was no significant difference in time to exhaustion between session 1 and 2 (session 1: 662 ± 100 s, session 2: 651 ± 107 s, p = 0.490, Fig. 2). There was a significant difference in time to exhaustion following the 3 week sprint interval training protocol between session 1 and 3 (session 1: 662 ± 100 s, session 3: 702 ± 106 s, p = 0.023, Fig. 2) and session 2 and 3 (session 2: 651 ± 107 s, session 3: 702 ± 106 s, p = 0.001, Fig. 2).
There was no significant difference in pre-exercise SmO2 (p = 0.153, Table 1), the change in SmO2 across the test (p = 0.804, Table 1) or the rate of fast desaturation at the end of the test (p = 0.268, Table 1, Fig. 1). There was a significant main effect for time (p = 0.008, Table 1) in the SmO2 value at exhaustion. There was no significant difference in the value of SmO2 at exhaustion between session 1 and session 2 (p = 0.773, Table 1). Following 3 weeks of sprint interval training there was a significant difference in SmO2 at exhaustion as compared to session 1 (p = 0.017, Table 1) or session 2 (p = 0.006, Table 1). There was a significant main effect for time (p = 0.037, Table 1) in the SmO2 post-treadmill recovery area under the curve. There was no significant difference in the value of AUC between session 1 and session 2 (p = 0.350, Table 1). Following 3 weeks of sprint interval training there was a significant difference in AUC as compared to session 1 (p = 0.020, Table 1) or session 2 (p = 0.050, Table 1).
Heart rate across the occlusion period was 104 ± 15 bpm in session 1, 109 ± 14 bpm in session 2, 108 ± 16 bpm in session 3, with no significant difference between mean values (p = 0.061) or standard deviation in heart rate across the occlusions (p = 0.161). Saturation in the exponential curve fit was only seen in session 3 post-sprint training but not in session 1 or session 2 (Fig. 3A–C). There was a significant main effect for time for mitochondrial rate function post-treadmill run to volitional exhaustion (p < 0.001, Fig. 3D). Following the control period there was no significant difference in mitochondrial rate function between session 1 and 2 (session 1: 0.203 ± 0.086 min−1, session 2: 0.222 ± 0.97 min−1, p = 0.422, Fig. 3D). There was a significant difference in mitochondrial rate function following the 3 week sprint interval training protocol between session 1 and 3 (session 1: 0.203 ± 0.086 min−1, session 3: 0.578 ± 0.187 min−1, p < 0.001, Fig. 3D) and session 2 and 3 (session 2: 0.222 ± 0.097 min−1, session 3: 0.578 ± 0.187 min−1, p < 0.001, Fig. 3D).
There was a significant occlusion effect for SmO2 fast resaturation rate (p = 0.029; Table 2) but no session effect (p = 0.139; Table 2) or session by occlusion effect (p = 0.992). In session 1, occlusion 3 was significantly different from occlusion 4, 5 and 10. In session 2, occlusion 10 was significantly different from occlusion 3, 4 and 5 and occlusion 3 was significantly different from occlusion 5.
Discussion
The aim of this study was to examine mitochondrial changes and improvements in incremental time to exhaustion over a 3 week period using standard gym ergometers, as opposed to the more costly lab-based alternatives. This is the first paper to demonstrate the effectiveness of repeated sprint training within professional boxing, as well as its effect on rectus femoris oxygenation and mitochondrial function. The rectus femoris plays a crucial role in key boxing activities like movement and generating punch force during a boxing match (Dunn et al. 2020; Lenetsky et al. 2020; Usher and Babraj 2023). Enhancing this muscle’s oxidative capacity is therefore critical, as it enables boxers to maintain consistent performance across multiple rounds, effectively reducing the risk of peripheral fatigue that could lead to a decline in performance. This study presents a promising training intervention due to its flexibility and short duration, allowing it to be periodised easily into a training camp.
Time to exhaustion
There was a significant increase in incremental treadmill time to exhaustion following 3 weeks of sprint interval training as compared to testing session 1 and 2 but no change across the control period (Fig. 2). Time to exhaustion is an important aspect of incremental exercise testing but the exact reason for failure is not known. In trained runners, incremental time to exhaustion has been shown to increase by 6.4% following 2 weeks of SIT with a 1:3 work to rest ratio (Kavaliauskas, Rodrigo R Aspe and Babraj 2015), which is similar to the size of change seen in session 3 as compared to session 1 or 2 in the current study (Fig. 2). Following SIT, incremental time to exhaustion can be increased with no change in VO2 max (Kavaliauskas et al. 2015). When low intensity training is done then incremental TTE is only increased with blood flow restriction (Corvino et al. 2014). Taken together this shows that the point of failure during an incremental test is largely derived from peripheral rather than central adaptation. At the point of failure, the same muscular metabolic disturbance is seen after sprint interval training, although with a much greater workload and with a reduction in central fatigue developed due to a resistance of central drive to the type III/IV afferent signal (O’Leary et al. 2017). At the point of failure, we see a greater smO2 in the skeletal muscle as compared to presprint interval training in professional boxers (Table 1). This may reflect a greater type III/IV afferent activity following training which would promote a greater ventilatory reflex response leading to an increased muscle perfusion during higher workloads allowing for a greater sustained effort (Amann 2011). Further, following 7 sessions of sprint interval training consisting of 4–7 30 s sprints, Laursen et al. (2020) report a greater mitochondrial affinity for oxygen suggesting improved efficiency of oxygen use across increasing workloads. This may explain the greater smO2 seen that we report at the point of failure (Table 1). More research is needed to determine the underpinning physiological changes that prevent fatigue following sprint interval training, but this adaptation is crucial to allow sustained performance during competition for a professional boxer.
Mitochondrial rate of change
After 3 weeks of sprint interval training there was a significant increase in mitochondrial rate compared to session 1 or 2, as measured post-volitional exhaustion (Fig. 3). Across the occlusion period heart rate was relatively consistent at all 3 testing sessions, suggesting a similar systemic delivery of blood to the recovering skeletal muscle. This is the first study to utilise recovery mitochondrial rate following volitional exhaustion, all other papers have carried our exercise either at a low percentage of maximum force or following electrical stimulation (Ryan, Brizendine and McCully 2013; Maliszewski et al. 2024). In session 1 and 2 there is no saturation in the monoexponential curve across the occlusions in well trained professional boxers (Fig. 3A, B, Table 2). This is similar to the response seen following electrical stimulation in people with spinal cord injury which is suggested to involve mitochondrial disturbance (Ghatas et al. 2020). Therefore, no saturation in the monoexponential fit may reflect the nature of disturbance in the skeletal muscle at the point of volitional exhaustion with changes in mitochondrial structure, function and energy production suggested as important factors in fatigue development (Filler et al. 2014). Further, to the best of our knowledge this is the first intervention study looking at change in NIRS derived mitochondrial rate following a training intervention, although others have shown a relationship to aerobic fitness (Lagerwaard et al. 2019). Sprint interval training has been shown to change activity levels of mitochondrial enzymes (Macdougall et al. 1998) and increase the rate of mitochondrial protein synthesis post exercise leading to remodelling of mitochondrial function within the skeletal muscle (Scalzo et al. 2014). Further the increased mitochondrial rate function post training may also be related to better oxygen extraction at maximal exercise and during recovery and a lower mitochondrial sensitivity to ADP due to a reduction in p50mito (Larsen et al. 2020) leading to greater mitochondrial affinity for oxygen and faster muscular utilisation of oxygen during recovery. Improvement in oxygen affinity in the mitochondria has been suggested to be controlled by elevated cytochrome c oxidase activity (Larsen et al. 2020) which has been shown to be increased following SIT (Macdougall et al. 1998). In the current study we see an increase in the area under the post-exhaustion SmO2 curve (Table 1) which suggests a faster refill of oxygen availability of the skeletal muscle post sprints that would reflect greater efficiency of oxygen use during recovery. As such sprint interval training has been shown to improve PCr recovery kinetics post exercise (Forbes et al. 2008).
Recovery
Recovery from fatiguing exercise is a crucial component with sporting performance (Mika et al. 2016; Kellmann et al. 2018). In this study the lack of variation in muscle reoxygenation rates suggests a potential disparity between oxygen desaturation and resaturation at the point of voluntary exhaustion. This observation may align with findings that reoxygenation rates can diverge from desaturation and oxygen kinetics after initial sprints (Buchheit and Ufland 2011). Muscle reoxygenation post exercise is influenced by factors like blood flow, the metaboreflex response, and capillary dynamics. Enhanced blood flow and muscle oxidative enzyme activity are linked to better muscle reoxygenation post exercise (Kime et al. 2003; Puente-Maestu et al. 2003). The metaboreflex, particularly in ischemic conditions, boosts arterial haemoglobin, thus improving oxygen delivery (O’Leary et al. 2017). However, when lactate is elevated in muscles or blood, it has been suggested it can limit or restrict post-exercise reoxygenation (McCully et al. 1994). Despite similar levels of metabolic disturbance at the point of failure before and after training, lactate concentrations remain heightened post exhaustion and may be further elevated following interval training (Juel et al. 2004). Consequently, the increased oxygen levels at exhaustion after SIT could obscure observable changes in muscle reoxygenation due to a potentially stronger metaboreflex response. There’s a need for more research to understand SIT’s effects on muscle reoxygenation dynamics.
Adaptations in well trained athletes
Professional boxers typically engage in a well-established training regimen, which includes technical pad work, heavy bag exercises, strength and conditioning, and sparring. This training process, while standardized, often lacks a structured approach specifically aimed at physiological adaptation (Usher and Babraj 2023). Despite this, boxers are considered well-trained athletes, proficient in their specialised requirements. Similar studies to this one have shown that even well-trained athletes can experience further physiological adaptations from SIT training. For example, in well trained cyclists sprint training increased endurance capacity and time trial performance over 4 weeks. However, intensity is the key determinant of adaptation. In the current study, athletes controlled the intensity based on expected heart rate response to ensure they had the correct intensity on the different cycles used. This may represent a simpler way for athletes to control effort than using VO2 max (Laursen and Jenkins 2002) or anaerobic power reserve (Wang and Zhao 2023).
Limitations
This is a pre and post study of the effects of a three-week repeated sprint intervention with well trainer professional boxers. As such no attempt was made to control the athletes training load before taking part in the study. Repeated sprints were conducted on available gym-based bike ergometers, with all three sprint times decided by the athlete as per the study design. This may have affected the magnitude of adaptation, although heart rate data was utilised to gauge and guide the repeated sprint intensity levels. No attempt was made to monitor the athlete diets, any changes in diet over the course of the study may have the potential to alter mitochondrial function (Putti et al. 2015). However, athletes were told to maintain normal diet and it has been shown that people rarely change their diet (Pai and Sabharwal 2022) but future research should look to record dietary habits.
Perspectives
This research is the first to investigate the effects of SIT in professional boxers and on noninvasive of mitochondrial rate changes post volition. There is significant performance and physiological adaptations after SIT, demonstrating it is an effective training protocol for professional boxing. The study demonstrates increased incremental time to exhaustion and higher SmO2 at volitional fatigue, indicating improved oxygen affinity and use within the muscle. We also report increased mitochondrial rates post-training like those shown from biopsy (Holloszy 1982; Brizendine et al. 2013; Sumner et al. 2020). Together they highlight the importance of SIT for boxing, allowing greater endurance to allow the athlete to perform consistently across rounds and fatigue resistance will improve cognitive readiness (Aidman 2020) allowing the athlete to be safer in the ring. Further, SIT can be performed on any standard gym bike if the athlete knows the intensity that must be utilised. In the current study heart rate was used as a method to ensure intensity is met with athletes reaching 160 bpm in the first sprint. This is easier for an athlete or coach to implement than other approaches. Taken together we show the effectiveness of a short-term intervention for professional boxers, but more research is needed to determine optimal protocols and maximise adaptation.
Data availability
Data will be available upon reasonable request.
References
Aidman E (2020) Cognitive fitness framework: towards assessing, training and augmenting individual-difference factors underpinning high-performance cognition. Front Hum Neurosci. https://doi.org/10.3389/fnhum.2019.00466
Amann M (2011) Central and peripheral fatigue: interaction during cycling exercise in humans. Med Sci Sports Exerc. https://doi.org/10.1249/MSS.0b013e31821f59ab
Bailey SJ et al (2009) Influence of repeated sprint training on pulmonary O2 uptake and muscle deoxygenation kinetics in humans. J Appl Phys. https://doi.org/10.1152/japplphysiol.00144.2009
Booth FW, Thomason DB (1991) Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Phys Rev. https://doi.org/10.1152/physrev.1991.71.2.541
Brizendine JT et al (2013) Skeletal muscle metabolism in endurance athletes with near-infrared spectroscopy. Med Sci Sports Exerc. https://doi.org/10.1249/MSS.0b013e31827e0eb6
Buchheit M, Ufland P (2011) Effect of endurance training on performance and muscle reoxygenation rate during repeated-sprint running. Eur J Appl Physiol. https://doi.org/10.1007/s00421-010-1654-9
Buchheit M, Laursen PB (2013) High-intensity interval training, solutions to the programming puzzle: part I: cardiopulmonary emphasis. Sports Med. https://doi.org/10.1007/s40279-013-0029-x
Burgomaster KA et al (2008) Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol. https://doi.org/10.1113/jphysiol.2007.142109
Corvino RB et al (2014) Quatro semanas de treinamento com restrição de fluxo sanguineo aumenta o tempo de exaustão em exercício severo no ciclismo. Rev Bras Cineantropom Desempenho Hum. https://doi.org/10.5007/1980-0037.2014v16n5p570
Davis P, Leithäuser RM, Beneke R (2014) The energetics of semicontact 3 × 2-min amateur boxing. Int J Sports Phys Perform. https://doi.org/10.1123/IJSPP.2013-0006
Dunn EC et al (2020) Relationships between punch impact force and upper- and lower-body muscular strength and power in highly trained amateur boxers. J Strength Cond Res. https://doi.org/10.1519/jsc.0000000000003585
Feldmann A, Schmitz R, Erlacher D (2019) Near-infrared spectroscopy-derived muscle oxygen saturation on a 0% to 100% scale: reliability and validity of the Moxy Monitor. J Biomed Optics. https://doi.org/10.1117/1.jbo.24.11.115001
Filler K et al (2014) Association of mitochondrial dysfunction and fatigue: a review of the literature. BBA Clin. https://doi.org/10.1016/j.bbacli.2014.04.001
Forbes SC, Slade JM, Meyer RA (2008) Short-term high-intensity interval training improves phosphocreatine recovery kinetics following moderate-intensity exercise in humans. Appl Phys Nutr Metab. https://doi.org/10.1139/H08-099
Ghatas MP, Holman ME, Gorgey AS (2020) Methodological considerations for near-infrared spectroscopy to assess mitochondrial capacity after spinal cord injury. J Spinal Cord Med. https://doi.org/10.1080/10790268.2019.1631585
Ghosh AK, Goswami AAA (1995) Heart rate and blood lactate response in amateur competitive boxing. Indian J Med Res. https://doi.org/10.24985/ijass.2010.22.1.1
Gibala MJ et al (2012) Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Phys. https://doi.org/10.1113/jphysiol.2011.224725
Hanna R et al (2021) Bilateral NIRS measurements of muscle mitochondrial capacity Feasibility and repeatability. Phys Rep. https://doi.org/10.14814/phy2.14826
Holloszy JO (1982) Muscle metabolism during exercise. Arch Phys Med Rehab 63(5):231–234
Jacobs RA, Lundby C (2013) Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. J Appl Physiol. https://doi.org/10.1152/japplphysiol.01081.2012
Juel C et al (2004) Effect of high-intensity intermittent training on lactate and H + release from human skeletal muscle. Am J Phys Endocrinol Metab. https://doi.org/10.1152/ajpendo.00303.2003
Kavaliauskas M, Aspe RR, Babraj J (2015) High-Intensity Cycling Training: The Effect of Work-to-Rest Intervals on Running Performance Measures. The Journal of Strength and Conditioning Research. Available at: https://journals.lww.com/nsca-jscr/fulltext/2015/08000/high_intensity_cycling_training__the_effect_of.19.aspx
Kavaliauskas M, Aspe RR, Babraj J (2015b) High-intensity cycling training. J Strength Cond Res. https://doi.org/10.1519/jsc.0000000000000868
Kellmann M et al (2018) Recovery and performance in sport: consensus statement. Int J Sports Phys Perform. https://doi.org/10.1123/ijspp.2017-0759
Kime R et al (2003) Discrepancy between cardiorespiratory system and skeletal muscle in elite cyclists after hypoxic training. Dyn Med. https://doi.org/10.1186/1476-5918-2-4
Lagerwaard B et al (2019) In vivo assessment of muscle mitochondrial function in healthy, young males in relation to parameters of aerobic fitness. Eur J Appl Physiol. https://doi.org/10.1007/s00421-019-04169-8
Larsen FJ et al (2020) Mitochondrial oxygen affinity increases after sprint interval training and is related to the improvement in peak oxygen uptake. Acta Phys 229(3):1–9. https://doi.org/10.1111/apha.13463
Laursen PB, Jenkins DG (2002) The scientific basis for high-intensity interval training. Sports Med 32(1):53–73. https://doi.org/10.2165/00007256-200232010-00003
Lenetsky S et al (2020) Defining the phases of boxing punches: a mixed-method approach. J Strength Cond Res 34(4):1040–1051. https://doi.org/10.1519/JSC.0000000000002895
Little JP et al (2010) A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol 588(6):1011–1022. https://doi.org/10.1113/jphysiol.2009.181743
Macdougall JD et al (1998) Muscle performance and enzymatic adaptations to sprint interval training. J Appl Physiol 84(6):2138–2142. https://doi.org/10.1152/jappl.1998.84.6.2138
MacInnis MJ, Gibala MJ (2017) Physiological adaptations to interval training and the role of exercise intensity. J Physiol. https://doi.org/10.1113/JP273196
Maliszewski K et al (2024) A systematic review of the relationship between muscle oxygen dynamics and energy rich phosphates. Can NIRS help? BMC Sports Sci Med Rehabilit 16(1):1–23. https://doi.org/10.1186/s13102-024-00809-5
Mathuram TL et al (2022) A synthetic small RNA homologous to the D-loop transcript of mtDNA enhances mitochondrial bioenergetics. Front Physiol 13:1–16. https://doi.org/10.3389/fphys.2022.772313
McCully KK et al (1994) Simultaneous in vivo measurements of HbO2 saturation and PCr kinetics after exercise in normal humans. J Appl Physiol 77(1):5–10. https://doi.org/10.1152/jappl.1994.77.1.5
McManus CJ, Collison J, Cooper CE (2018) Performance comparison of the MOXY and PortaMon near-infrared spectroscopy muscle oximeters at rest and during exercise. J Biomed Optics 23(01):1. https://doi.org/10.1117/1.jbo.23.1.015007
Mika A et al (2016) Comparison of two different modes of active recovery on muscles performance after fatiguing exercise in mountain canoeist and football players. PLoS ONE 11(10):1–14. https://doi.org/10.1371/journal.pone.0164216
Nassib S et al (2017) Energetics demands and physiological responses to boxing match and subsequent recovery. J Sports Med Phys Fit 57(1–2):8–17
Nielsen J et al (2017) Plasticity in mitochondrial cristae density allows metabolic capacity modulation in human skeletal muscle. J Physiol 595(9):2839–2847. https://doi.org/10.1113/JP273040
O’Leary TJ et al (2017) Endurance capacity and neuromuscular fatigue following high- vs moderate-intensity endurance training: a randomized trial. Scand J Med Sci Sports 27(12):1648–1661. https://doi.org/10.1111/sms.12854
Pai A, Sabharwal A (2022) Food habits: insights from food diaries via computational recurrence measures. Sensors. https://doi.org/10.3390/s22072753
Paquette M, Bieuzen F, Billaut F (2018) Muscle oxygenation rather than VO2max as a strong predictor of performance in sprint canoe–Kayak. Int J Sports Physiol Perform 13(10):1299–1307. https://doi.org/10.1123/ijspp.2018-0077
Perrey S, Ferrari M (2018) Muscle oximetry in sports science: a systematic review. Sports Med. https://doi.org/10.1007/s40279-017-0820-1
Perry CGR et al (2010) Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol 588(23):4795–4810. https://doi.org/10.1113/jphysiol.2010.199448
Porter C et al (2015) Resistance exercise training alters mitochondrial function in human skeletal muscle. Med Sci Sports Exerc 47(9):1922–1931. https://doi.org/10.1249/MSS.0000000000000605
Puente-Maestu L et al (2003) Training improves muscle oxidative capacity and oxygenation recovery kinetics in patients with chronic obstructive pulmonary disease. Eur J Appl Physiol 88(6):580–587. https://doi.org/10.1007/s00421-002-0743-9
Putti R et al (2015) Diet impact on mitochondrial bioenergetics and dynamics. Front Physiol 6:1–7. https://doi.org/10.3389/fphys.2015.00109
Ryan TE et al (2012) Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes. J Appl Physiol 113(2):175–183. https://doi.org/10.1152/japplphysiol.00319.2012
Ryan TE et al (2013) A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. J Appl Physiol 115(12):1757–1766. https://doi.org/10.1152/japplphysiol.00835.2013
Ryan TE, Brizendine JT, McCully KK (2013) A comparison of exercise type and intensity on the noninvasive assessment of skeletal muscle mitochondrial function using near-infrared spectroscopy. J Appl Physiol 114(2):230–237. https://doi.org/10.1152/japplphysiol.01043.2012
Ryan TE et al (2014) Assessment of in vivo skeletal muscle mitochondrial respiratory capacity in humans by near-infrared spectroscopy: a comparison with in situ measurements. J Physiol 592(15):3231–3241. https://doi.org/10.1113/jphysiol.2014.274456
Scalzo RL et al (2014) Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. FASEB J 28(6):2705–2714. https://doi.org/10.1096/fj.13-246595
Sumner MD et al (2020) Near infrared spectroscopy measurements of mitochondrial capacity using partial recovery curves. Front Physiol 11:1–9. https://doi.org/10.3389/fphys.2020.00111
Usher A, Babraj J (2023) Use of NIRS to explore skeletal muscle oxygenation during different training sessions in professional boxing. Eur J Appl Physiol. https://doi.org/10.1007/s00421-023-05305-1
Wang X, Zhao L (2023) Adaptive responses of cardiorespiratory system and hormonal parameters to individualized high-intensity interval training using anaerobic power reserve in well-trained rowers. Front Physiol 14:1–11. https://doi.org/10.3389/fphys.2023.1177108
Yamagishi T, Babraj J (2017) Effects of reduced-volume of sprint interval training and the time course of physiological and performance adaptations. Scand J Med Sci Sports 27(12):1662–1672. https://doi.org/10.1111/sms.12831
Zoll J et al (2006) Exercise training in normobaric hypoxia in endurance runners. III. Muscular adjustments of selected gene transcripts. J Appl Physiol 100(4):1258–1266. https://doi.org/10.1152/japplphysiol.00359.2005
Author information
Authors and Affiliations
Contributions
Andrew Usher and John Babraj designed the study. Andrew Usher collected all data, Andrew Usher and John Babraj were involved in data analysis, manuscript drafting and reviewing of all drafts.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Ethical approval
Approval was obtained from the research ethics committee of Abertay University. The procedures used in this study adhere to the tenets of the Declaration of Helsinki with participants informed verbally and in writing about the study before giving their informed consent.
Additional information
Communicated by I. Mark Olfert.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Usher, A., Babraj, J. Impact of sprint interval training on post-fatigue mitochondrial rate in professional boxers. Eur J Appl Physiol (2024). https://doi.org/10.1007/s00421-024-05594-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00421-024-05594-0