Abstract
The use of sodium bicarbonate (NaHCO3) supplementation to improve repeated high-intensity performance is recommended; however, most swimming performance studies examine time trial efforts rather than repeated swims with interspersed recovery that are more indicative of training sessions. The aim of this study, therefore, was to investigate the effects of 0.3 g.kg−1 BM NaHCO3 supplementation on sprint interval swimming (8 × 50 m) in regionally trained swimmers. Fourteen regionally competitive male swimmers (body mass (BM): 73 ± 8 kg) volunteered for this double-blind, randomised, crossover designed study. Each participant was asked to swim 8 × 50 m (front crawl) at a maximum intensity from a diving block, interspersed with 50 m active recovery swimming. After one familiarisation trial, this was repeated on two separate occasions whereby participants ingested either 0.3 g.kg−1 BM NaHCO3 or 0.05 g.kg−1 BM sodium chloride (placebo) in solution 60 min prior to exercise. Whilst there were no differences in time to complete between sprints 1–4 (p > 0.05), improvements were observed in sprint 5 (p = 0.011; ES = 0.26), 6 (p = 0.014; ES = 0.39), 7 (p = 0.005; ES = 0.60), and 8 (p = 0.004; ES = 0.79). Following NaHCO3 supplementation, pH was greater at 60 min (p < 0.001; ES = 3.09), whilst HCO3− was greater at 60 min (p < 0.001; ES = 3.23) and post-exercise (p = 0.016; ES = 0.53) compared to placebo. These findings suggest NaHCO3 supplementation can improve the latter stages of sprint interval swimming performance, which is likely due to the augmentation of pH and HCO3− prior to exercise and the subsequent increase in buffering capacity during exercise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
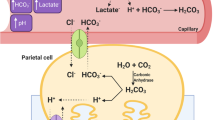
Sodium bicarbonate (NaHCO3) is a known legal ergogenic aid that was recommended in the most recent International Olympics Committee (IOC) consensus statement on dietary supplements and the high-performance athlete (Maughan et al. 2018). Most research in swimming opts for 0.3 g.kg− 1 BM as this typically achieves the balance between performance enhancement and the management of gastrointestinal (GI) discomfort (Gough et al. 2017a; Newbury et al. 2021; McNaughton et al. 2019). The mechanism to explain the performance effects following NaHCO3 supplementation is related to the control of ion fluxes (e.g. hydrogen ions [H+]) during high-intensity exercise (McNaughton et al. 2016, 2019). Whilst a topic of contention (Westerblad 2016), exponential H+ accumulation is suggested to contribute to fatigue by reducing calcium ion (Ca2+) sensitivity and cross-bridge binding, disrupting glycolytic enzyme activity, and the excitation–contraction coupling during high-intensity exercise (Fitts 2008; Spriet et al. 1987). The ingestion of NaHCO3 can mitigate the exponential rises in H+ during high-intensity exercise, by increasing the flux of H+ from active musculature to the blood to be buffered (Bishop et al. 2004). Such mechanisms can potentially be beneficial to swimming due to the high-intensity nature of training and competitive events (Pyne and Sharp 2014).
Ergogenic benefits of NaHCO3 supplementation on interval swimming have been mixed within adult cohorts. Gao et al. (1988) reported a significant improvement compared to a placebo in the 4th and 5th sprint of a 5 × 100 yd (91 m) repetitions with 2 min passive rest in-between efforts following ingestion of 2.9 mmol.l–1 NaHCO3 60 min prior to exercise. The distance employed (91 m) is not common in swimming, however, whereby a 25, 50 or 100 m distance would be more replicable of competitive swimming. In contrast, Campos et al. (2012) reported no significant impact of NaHCO3 ingestion during 6 × 100 m maximal swimming bouts (6 min passive rest in-between), whereby completion times were near identical compared to the placebo. In the latter study, there were no measures for acid base balance (e.g. pH, HCO3−) and, therefore, it is difficult to debate why no performance effects were observed. However, the authors did opt for capsule ingestion 60 min prior to exercise and it is therefore unlikely HCO3– had reached a suitable level to elicit ergogenic effects, as research suggests this peak is around ~ 120 min (Newbury et al. 2021; Jones 2016). Whilst these findings may suggest NaHCO3 supplementation is not ergogenic for interval swimming performance, if appropriate methodologies were employed a performance benefit may be seen (e.g., dose timing and exercise protocol). Due to the equivocal nature of the performance benefit to interval swimming following NaHCO3, further research is warranted.
One limitation of the work to date is passive rest periods between intervals have been employed. It is intuitive to suggest that active recovery periods could lead to greater acid base balance recovery, and clearance of H+ (Dodd et al. 1984), and with the addition of NaHCO3, an even greater recovery and potentially improved exercise performance (Siegler et al. 2008). Specifically, Siegler et al. (2008) reported using a 15 min post-exercise active recovery increased the recovery of HCO3– compared to a passive recovery following NaHCO3 supplementation. The authors reported that recovery of acid base balance (i.e. pH and HCO3−) recovered at a faster rate in the active vs. passive recovery, and led to a small, non-significant performance improvement compared to passive recovery during 3 × 30 s cycling sprints. Importantly, after the first sprint, HCO3– was approximately 3 mmol.l−1 greater following NaHCO3 ingestion at 180 s recovery following the first 30 s sprint, with similar effects seen in sprint 2 and 3. Based on these findings, it is plausible that if a longer interval set (e.g. 8 × 50 m) was used the accumulative recovery benefits of NaHCO3 supplementation and active recovery may lead to greater performance benefits. Equally, longer sets would be more reflective of a typical training set within a cohort of highly trained swimmers (Pollock et al. 2019). To date, however, this has not been explored in the context of interval swimming performance.
If NaHCO3 supplementation can improve interval swimming there is potential for enhanced adaptation to training, based on findings related to PGC-1α and Heat Shock Protein 72 (HSP72) (Peart et al. 2013; Percival et al. 2015). Indeed, Peart et al. (2013) reported 4 h post a 4 min ‘all-out’ cycling bout, NaHCO3 supplementation attenuated the stress response, as HSP72 expression was lower compared to the placebo at 30 min, 1 h, and 2 h post-exercise compared to the placebo (average increase from pre–post-exercise, placebo = 42% vs. experimental = 5%). These findings suggest that the stress response was attenuated using NaHCO3/alkalosis, and this might allow for a greater level of recovery between training bouts. However, this study was in cycling and featured a single ‘all-out’ exercise bout; therefore, it is unknown how this could be applied to swimming training. Moreover, Percival et al. (2015) reported PGC-1α mRNA expression was greater (~ 28%) 3 h post-exercise following NaHCO3 supplementation versus placebo, after completion of a HIIT protocol (10 × 60 s cycling sprints at 90% VO2max). As PGC-1α is a well-known transcriptional coactivator of several genes required for mitochondrial biogenesis, these increases following NaHCO3 supplementation might lead to greater adaptation.
It is unknown, however, if improvements can be seen during interval swimming in a highly trained cohort or if long-term supplementation can improve training adaptation. Evidence from studies using NaHCO3 as a training aid is mixed, although few exist in cycling. Specifically, Siegler et al. (2018) reported no effect of NaHCO3 on resistance training metrics (e.g. maximal voluntary torque; MVT, rate of torque development; RTD) over 10 weeks. Whilst Bishop et al. (Bishop et al. 2004) reported significant improvements in TTE following NaHCO3 compared to a placebo following five training sessions per week for 5 weeks, although this was in mice. It is plausible that if improvements in exercise performance are observed in this cohort NaHCO3 supplementation could be an important training aid to improve training performance of similar intensity and volume, which might then lead to greater adaptation. Based on the premise that NaHCO3 could improve interval training swimming performance, the purpose of this study was to investigate the effects of 0.3 g.kg−1 BM NaHCO3 supplementation in a highly trained cohort on 8 × 50 m interval swimming performance interspersed with an active recovery.
Methods
Participants
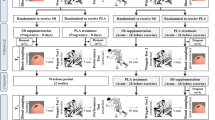
Fourteen trained male competitive swimmers (BM: 73 ± 8 kg) participated in this double-blind, randomised, crossover study. Randomisation was completed using a 4 × 4 block design (NaHCO3 first treatment for n = 6, placebo first treatment for n = 8) accounting for n = 16, however, n = 2 dropped out due to time commitments. Participants met the category of tier two (‘trained/developmental’) using the classifications provided by McKay et al. (2022). All participants were recruited through a local nationally ranked swimming club’s squads where ages varied from 17 to 22 years old (19 ± 2 years). Participants were trained swimmers that swam 7–10 times a week accumulating to 15–20 h a week. Land-based training was also undertaken 3 to 5 times a week primarily consisting of resistance training. Participants competed in up to 12 competitions a year, which included national/international standard competitions that required qualification through the British Swimming Association standards. Swimmers were mixed in terms of stroke preference and participated across middle (100–400 m) and long distance (> 400 m) primarily (n = 11), although some (n = 3) did also do sprint (50–100 m). All participants were informed of the procedures and possible side effects of the study through written informed consent; parental consent was given for participants under the age of 18. This study received institutional ethical approval (Newbury/7595/HELSFAEC).
Exercise protocol
All participants completed the exercise protocol during their normal training time and at the same time of day (± 1 h) (range: 5–8 pm). Following a familiarisation trial that entailed completing the interval sets, the experimental protocol was repeated on two occasions 1 week apart. Each participant was asked to swim 50 m (front crawl), from a competitive diving block, at their maximum effort and repeat this 8 times. After each 50 m sprint, participants undertook a 50 m active recovery swim requiring them to swim at their normal warm–up pace. Each exercise bout was run off a 5 min base, which left the participants with approximately 3 min passive recovery. Although similar exercise protocols have utilised passive recovery (Gao et al. 1988; Zajac et al. 2009), the current protocol replicated a typical training session for the squad. The exercise protocol was designed following consultation with both regional and national coaches in the United Kingdom who had oversight of high-performance swim programs.
On both testing sessions participants ingested either a solution of NaHCO3 (0.3 g.kg−1 BM) or sodium chloride (0.05 g.kg−1 BM) mixed with low calorie orange squash (0.2 ml.kg−1·bm) and tap water (0.3 ml.kg−1 BM). This dose was selected as it has been shown to produce ergogenic effects whilst balancing GI discomfort compared to lower or higher doses (McNaughton et al. 2016). Treatments were double blind and administered in a block randomised order. The sodium chloride dosage was used to replicate the taste of sodium bicarbonate with participants agreeing to similar tastes (Price et al. 2003), and no participants in the current study could identify which treatment they had received upon verbal questioning following each experimental trial. Each solution was consumed 60 min prior to testing to allow time for absorption into the blood and for an adequate warm-up period. The warm-up was approximately 2000 m as determined by the head coach and replicated a typical squad session. Participants maintained a low to moderate intensity pace that kept their heart rate between 130 and 160 bts·min−1. All warm-ups were consistent between participants.
Fingertip capillary blood samples were taken at three time points; baseline (pre-ingestion), 60 min post-NaHCO3 ingestion, and immediately post-exercise. A 5 µL sample was taken for blood lactate concentration ([BLa]) and was analysed using a hand-held device (Lactate Pro LT-1710). Blood pH and HCO3− concentrations were analysed using a reliable blood gas analyser (Radiometer, ABL5, Copenhagen, Denmark) after taking a 70 µL blood sample (Gough et al. 2017; Stadlbauer et al. 2011). The blood gas analyser was calibrated prior to use as per the manufacturer instructions and all quality control checks passed prior to use. Heart rate (HR; Polar beat heart rate monitor), ratings of perceived (RPE) (Borg scale; 6–20; Borg 1982) and sprint time were recorded after every 50 m sprint by experienced coaches. Participants were also asked to wear their usual training swimwear for each experimental trial, as well as only consuming either water or squash during each test. Assessments for GI were conducted every 10 min from ingestion to pre-exercise using visual analogue scales (VAS), as per previous research (Gough et al. 2017a). Symptoms for nausea, flatulence, stomach cramp, bowel urgency, diarrhoea, vomiting, stomach bloating, belching, stomachache, headache, and thirst were collected.
Statistical analysis
Data were initially checked for normality using Shapiro–Wilk and standard geographical methods (e.g. Q–Q plots and Histograms). For time to complete each 50 m set, a two-way (treatment: NaHCO3 or PLA x each 50 m set) repeated measures Analysis of Variance (ANOVA) was conducted, using a Bonferroni correction. Homogeneity of variance/sphericity were analysed using Mauchly tests and any violations were corrected if required (e.g. via Greenhouse–Geisser or Huynh–Feldt adjustments). This test was also used for blood variables (pH, HCO3− and [BLa]), HR, and RPE. For any ANOVA interaction or main effects, partial eta squared effect size is reported (ɳ2). A paired samples t test was used for aggregated GI discomfort, and aggregated swimming performance (sum of 8 × 50 m in seconds). To determine individual changes in time to complete, the Smallest Worthwhile Change (SWC) statistic was used (0.3* between-subject standard deviation; SD) for each interval sprint (Paton and Hopkins 2006). Hedge’s g effect size was calculated (due to n < 20) by dividing the mean difference between trials by the pooled SD (Lakens 2013), and interpretation was as follows: trivial (≤ 0.2), small (0.2–0.49), moderate (0.5–0.79) or large (≥ 0.8) (Cohen 1988). Data are presented as Mean ± SD (unless stated otherwise). Statistical significance was set at p < 0.05 and all statistical data were analysed using SPSS software version 28 (IBM, Chicago, IL, USA).
Results
Swimming performance
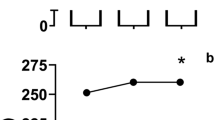
Performance was improved by NaHCO3 ingestion during the interval swimming protocol (p = 0.005, ɳ2 = 0.301). No improvements were observed between sprints 1–4 (p > 0.05), however, improvements were observed in sprint 5 (mean difference = – 0.5 s; p = 0.011; ES = 0.26), 6 (mean difference = – 0.8 s; p = 0.014; ES = 0.39), 7 (mean difference = – 1.0 s; p = 0.005; ES = 0.6), and 8 (mean difference = – 1.4 s; p = 0.004; ES = 0.79) (Table 1). Aggregated performance (sum of 8 intervals) was faster following NaHCO3 ingestion compared to placebo (NaHCO3 = 226.5 ± 14.0 s, PLA = 227.4 ± 14.3 s; p = 0.008, ES = 0.06; Fig. 1). Following NaHCO3, the drop off in performance from sets 1–4 was – 0.4 s and – 0.1 s between 5 and 8, whilst the placebo dropped off by – 0.7 s and – 0.9 s, respectively.
Blood variables
Following NaHCO3 supplementation pH was greater at 60 min (+ 0.07 a.u; p < 0.001; ES = 3.09), whilst HCO3− was greater at both 60 min (+ 5.7 mmol.l−1; p < 0.001; ES = 3.23) and post-exercise (+ 2 mmol.l−1; p = 0.016; ES = 0.53) (Fig. 2). The change in HCO3− during exercise was greater following NaHCO3 supplementation compared to placebo (+ 3.5 mmol.l−1; p < 0.001; ES = 0.88; Table 2). Blood lactate concentration was greater following NaHCO3 supplementation post-exercise compared to placebo (17.6 ± 4.9 vs. 14.7 ± 3.8 mmol.l−1; p < 0.001; ES = 0.64). There was a large variation in blood responses from baseline to 60 min following NaHCO3 ingestion (Table 2).
Heart rate, GI and RPE
There were no differences in HR at any time point between NaHCO3 and placebo (p = 0.883, Pƞ2 = 0.061). Aggregated GI discomfort was higher for NaHCO3 compared to placebo (21 ± 12 vs. 3 ± 1 a.u; p = 0.021; ES = 2.05). There were no differences in RPE between NaHCO3 and the placebo (p = 0.754, Pƞ2 = 0.024).
Discussion
This study aimed to investigate the effects of NaHCO3 ingestion on swimming interval performance in trained competitive swimmers. The results suggest NaHCO3 ingestion improves swimming interval performance in the latter stages of the set compared to a placebo, which was likely caused by the increase in buffering capacity. However, not all participants improved and GI discomfort was apparent in several swimmers. Based on these findings, NaHCO3 ingestion should be tested on an individual basis for suitability. These findings are still important for swimmers nonetheless that are aiming to increase their performance in training and is an effective ergogenic aid. The current study also reports, for the first time, that NaHCO3 is a suitable ergogenic aid when an appropriate dose and timing of ingestion is employed, along with an active recovery period between bouts. Lastly, due to the improvement in performance, there is scope for this supplement to improve overall training adaptation; however, further research is warranted to investigate NaHCO3 ingestion over a training block to confirm this inference.
The current study corroborates with previous research reporting improvements in interval swimming performance following NaHCO3 ingestion (Siegler and Gledall-Sidall 2010; Gao et al. 1988; Zajac et al. 2009). Of note, is the greater training status of participants employed in the current study compared to Siegler and Gledall-Sidall (2010), such that our findings can be applied to those that are at the competitive end of swimming performance. Furthermore, as the current study utilised an active recovery and an interval set of exercise, which is commonly employed by coaches in training (Pollock et al. 2019), our results can be applied directly to improve swimming interval performance. Building upon previous work by Zajac et al. (2009) in adolescent athletes, the current study reports that improvements can be seen in adults. Moreover, as performance improved and similar acid base balance responses were observed in the current study compared to Percival et al. (2015) and Peart et al. (2013), it is plausible that if this strategy was applied chronically greater adaptations to training might be observed through changes in PGC-1α and HSP72. Favourable adaptations in PGC-1α could improve mitochondrial biogenesis, which may increase mitochondrial content and oxidative enzyme activity (Gollnick et al. 1973), particularly following training exercises (i.e. high intensity) like that employed in the current study (MacInnis et al. 2017). As the study was not mechanistic in nature we cannot confirm if these changes were present, however, this opens an area for future research.
As a group, there was an improvement in swim performance during the latter half of the repeated sprints (sprints 5–8). However, the ergogenic effects of NaHCO3 were not apparent across all the participants at the same time points. Specifically, 10 of the 14 participants improved above the SWC in sprint interval eight, whereas only 4 improved by sprint interval three. These observations indicate that the ergogenic effects became apparent towards the latter part of the exercise, which was approximately three minutes into the exercise. It can be speculated that those participants not improving performance may have had sub-optimal ingestion strategies. Recently, a time to peak HCO3− approach, whereby participants ingest NaHCO3 at their respective time to peak HCO3− prior to exercise, has been shown to increase the chances of securing an ergogenic benefit (Boegman et al. 2020; Gough et al. 2018). Using this strategy might have allowed the other participants to obtain ergogenic effects, as Boegman et al. (2020) showed this approach was more effective at improving 2000 m TT rowing performance compared to the standardised ingestion strategy (as used in the current study). However, this procedure was not conducted in the current study due to logistical constraints with this highly trained cohort. Future research could, therefore, adopt the time to peak HCO3− strategy and compare with a standardised time frame to see if this allows more participants to benefit from NaHCO3 supplementation.
The group mean increase in HCO3− following NaHCO3 ingestion was ~ 6 mmol.l−1, demonstrating the study protocol was successful at achieving a level of alkalosis similar to other studies, and the increase purported to improve exercise performance (Carr et al. 2011; Lopes-Silva et al. 2022). It is important to note, however, that not all participants met this threshold and still managed to improve their performance, and vice versa, which subsequently questions whether this ergogenic threshold is required. Following on from previous findings (Gough et al. 2021), both participants six and seven in the current study increased their HCO3− from baseline to post-supplementation comfortably over this purported threshold, yet did not improve performance (7 and 12 mmol.l−1, respectively). Conversely, participant 12 and 13 increased by 4.3 and 4.8 mmol.l−1, respectively, yet improved their performance over the SWC of the test. This evidence suggests that a threshold of change in HCO3− is not as clear as first seems, and instead, it is more important to trial different doses to identify the physiologically optimal dose on an individual basis.
Whilst the current study was more applied in nature, it does offer insight into the mechanism of action for NaHCO3 ingestion. Indeed, significant pre-exercise increases in pH and HCO3− were observed compared to placebo and this infers an increased buffering capacity during exercise. This is also shown by the significantly increased blood lactate, although this could have been due to the increased exercise intensity in the NaHCO3 performance, and not a marker of increased buffering capacity. Nonetheless, in agreement with the findings of Bishop et al. (2004), the current study infers the ingestion of NaHCO3 increased the movement of H+ from intracellular to extracellular compartments, and this could have reduced the level of fatigue in participants. Of note, the change in HCO3− during exercise corroborates this notion as this was significantly greater following NaHCO3 ingestion from pre- to post-exercise compared to placebo, in line with previous studies (Gough et al. 2018, 2019). Moreover, the current study employed an active recovery between bouts, and this may have heightened the ergogenic effects compared to previous studies using a passive rest and reporting no effect. This is based on the accelerated recovery of acid base balance previously reported by Siegler et al. (2008) following NaHCO3 compared to placebo. Notwithstanding, these inferences are restricted to extracellular measurements, and as such, future research more mechanistic in nature could continue to explore this theory of fatigue to elucidate the full mechanisms following NaHCO3 ingestion. Equally, future research may include blood measurements during each interval if this is logistically possible.
Applied studies are always open to limitations, and this study presents limitations in the ingestion strategy adopted and the lack of mechanistic insight. Recently, a string of studies (Gough et al. 2017b, 2018, 2019; Boegman et al. 2020) have suggested/evidenced that using an individual time to peak HCO3− strategy (mapping ingestion timing to each individual time to peak HCO3−) improves performance compared to the standardised ingestion strategy in this study. However, it was logistically too difficult to determine individual time to peak HCO3−, and this approach also has some criticisms in that an ergogenic window might be present following NaHCO3 ingestion of up to 2 h; therefore, the individual peak may not be required (Farias de Oliveira et al. 2020). Moreover, it was also observed that a number of swimmers suffered GI discomfort which may limit the practical use of NaHCO3 in a real-world setting. Specifically, two participants suffered from diarrhoea that caused disruption to their usual preparation for training. On the other hand, the other participants in the study had general mild-moderate stomachache, belching and bloating. It is important to, therefore, trial NaHCO3 on an individual basis; however, for most, it will be tolerable.
Conclusion
To conclude, this study reports that the ingestion of NaHCO3 can improve swimming interval performance reflective of a training bout. Not all participants improved their performance, however, and therefore, this supplement should be trialled on an individual basis. Further research is warranted to explore how NaHCO3 could impact training adaptation.
Data availability
Data is available upon request to the corresponding author.
Abbreviations
- NaHCO3 :
-
Sodium bicarbonate
- HCO3 – :
-
Bicarbonate
- H+ :
-
Hydrogen ion
- pH:
-
Potential hydrogen
- Ca2+ :
-
Calcium
- GI:
-
Gastrointestinal discomfort
- VAS:
-
Visual analogue scale
- HSP72:
-
Heat shock protein 72
- ANOVA:
-
Analysis of variance
- ɳ 2 :
-
Partial eta squared
References
Bishop D, Edge J, Davis C, Goodman C (2004) Induced metabolic alkalosis affects muscle metabolism and repeated-sprint ability. Med Sci Sports Exerc 36(5):807–813
Boegman S, Stellingwerff T, Shaw G, Clarke N, Graham K, Cross R, Siegler JC (2020) The impact of individualizing sodium bicarbonate supplementation strategies on world-class rowing performance. Front Nutr 7:138
Borg G (1982) Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. Int J Sports Med 3(03):153–158
Campos EZ, Sangali EB, Neto JG, Gobbi RB, Freitas Junior IF, Papoti M (2012) Effects of sodium bicarbonate ingestion during an intermittent exercise on blood lactate, stroke parameters, and performance of swimmers. J Exercise Physiol Online 15(6):84–92
Carr AJ, Hopkins WG, Gore CJ (2011) Effects of acute alkalosis and acidosis on performance. Sports Med 41(10):801–814
Cohen J (1988) Statistical power analysis for the behavioral sciences. Routledge, Abingdon, pp 77–83
Dodd S, Powers SK, Callender T, Brooks E (1984) Blood lactate disappearance at various intensities of recovery exercise. J Appl Physiol 57(5):1462–1465
Egger F, Meyer T, Such U, Hecksteden A (2014) Effects of sodium bicarbonate on high-intensity endurance performance in cyclists: a double-blind, randomized cross-over trial. PLoS ONE 9(12):e114729
Farias de Oliveira L, Saunders B, Yamaguchi G, Swinton P, GianniniArtioli G (2020) Is individualization of sodium bicarbonate ingestion based on time to peak necessary? Med Sci Sports Exercise 52(8):1801–1808
Fitts RH (2008) The cross-bridge cycle and skeletal muscle fatigue. J Appl Physiol 104(2):551–558
Gao J, Costill DL, Horswill CA, Park SH (1988) Sodium bicarbonate ingestion improves performance in interval swimming. Eur J Appl Physiol 58(1):171–174
Gollnick PD, Armstrong RB, Saltin B, Saubert CW, Sembrowich WL, Shepherd RE (1973) Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol 34:107–111
Gough LA, Deb SK, Sparks AS, McNaughton LR (2017a) The reproducibility of blood acid base responses in male collegiate athletes following individualised doses of sodium bicarbonate: a randomised controlled crossover study. Sports Med 47(10):2117–2127
Gough LA, Deb SK, Sparks A, McNaughton LR (2017b) The reproducibility of 4-km time trial (TT) performance following individualised sodium bicarbonate supplementation: a randomised controlled trial in trained cyclists. Sports Medicine-Open 3(1):1–10
Gough LA, Deb SK, Sparks SA, McNaughton LR (2018) Sodium bicarbonate improves 4 km time trial cycling performance when individualised to time to peak blood bicarbonate in trained male cyclists. J Sports Sci 36(15):1705–1712
Gough LA, Deb SK, Brown D, Sparks SA, McNaughton LR (2019) The effects of sodium bicarbonate ingestion on cycling performance and acid base balance recovery in acute normobaric hypoxia. J Sports Sci 37(13):1464–1471
Gough LA, Williams JJ, Newbury JW, Gurton WH (2021) The effects of sodium bicarbonate supplementation at individual time-to-peak blood bicarbonate on 4-km cycling time trial performance in the heat. Eur J Sport Sci 22(12):1856–1864
Hebestreit H, Meyer F, Htay H, Heigenhauser G, Bar-Or O (1996) Plasma metabolites, volume and electrolytes following 30s high intensity exercise in boys and men. Eur J Appl Physiol Occup Physiol 72:563–569
Inbar O, Bar-Or O (1986) Anaerobic characteristics in male children and adolescents. Med Sci Sports Exerc 18:264–269
Jones RL, Stellingwerff T, Artioli GG, Saunders B, Cooper S, Sale C (2016) Dose-response of sodium bicarbonate ingestion highlights individuality in time course of blood analyte responses. Int J Sport Nutr Exerc Metab 26(5):445–453
Kahle LE, Kelly PV, Eliot KA, Weiss EP (2013) Acute sodium bicarbonate loading has negligible effects on resting and exercise blood pressure but causes gastrointestinal distress. Nutr Res 33(6):479–486
Lakens D (2013) Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 4:863
Lindh AM, Peyrebrune MC, Ingham SA, Bailey DM, Folland JP (2008) Sodium bicarbonate improves swimming performance. Int J Sports Med 29(06):519–523
Lopes-Silva JP, Correia-Oliveira CR (2022) Acute effects of sodium bicarbonate ingestion on cycling time-trial performance: a systematic review and meta-analysis of randomized controlled trials. Eur J Sport Sci. https://doi.org/10.1080/17461391.2022.2071171
MacInnis MJ, Zacharewicz E, Martin BJ, Haikalis ME, Skelly LE, Tarnopolsky MA, Gibala MJ (2017) Superior mitochondrial adaptations in human skeletal muscle after interval compared to continuous single-leg cycling matched for total work: aerobic exercise intensity mediates skeletal muscle adaptations. J Physiol 595:2955–2968
Maughan RJ, Burke LM, Dvorak J, Larson-Meyer DE, Peeling P, Phillips SM, Engebretsen L (2018) IOC consensus statement: dietary supplements and the high-performance athlete. Int J Sport Nutr Exercise Metab 28(2):104–125
McKay AK, Stellingwerff T, Smith ES, Martin DT, Mujika I, Goosey-Tolfrey VL, Burke LM (2022) Defining training and performance caliber: a participant classification framework. Int J Sports Physiol Perform 17(2):317–331
McNaughton LR, Gough L, Deb S, Bentley D, Sparks SA (2016) Recent developments in the use of sodium bicarbonate as an ergogenic aid. Curr Sports Med Rep 15(4):233–244
McNaughton LR, Deb S, Gough LA, Higgins M, Price M, Sale C, Sparks A (2019) The BASES expert statement on extracellular buffering agents. Bases Expert Statements. Available at: https://www.bases.org.uk/spage-resources-bases_expert_statements.html
Newbury JW, Cole M, Kelly AL, Chessor RJ, Sparks SA, McNaughton LR, Gough LA (2021) The time to peak blood bicarbonate (HCO3–), pH, and the strong ion difference (SID) following sodium bicarbonate (NaHCO3) ingestion in highly trained adolescent swimmers. PLoS ONE 16(7):e0248456
Paton CD, Hopkins WG (2006) Variation in performance of elite cyclists from race to race. Eur J Sport Sci 6(01):25–31
Peart DJ, Kirk RJ, Hillman AR, Madden LA, Siegler JC, Vince RV (2013) The physiological stress response to high-intensity sprint exercise following the ingestion of sodium bicarbonate. Eur J Appl Physiol 113(1):127–134
Percival ME, Martin BJ, Gillen JB, Skelly LE, MacInnis MJ, Green AE, Gibala MJ (2015) Sodium bicarbonate ingestion augments the increase in PGC-1α mRNA expression during recovery from intense interval exercise in human skeletal muscle. J Appl Physiol 119(11):1303–1312
Pollock S, Gaoua N, Johnston MJ, Cooke K, Girard O, Mileva KN (2019) Training regimes and recovery monitoring practices of elite British swimmers. J Sports Sci Med 18(3):577
Price M, Moss P, Rance S (2003) Effects of sodium bicarbonate ingestion on prolonged intermittent exercise. Med Sci Sports Exerc 35(8):1303–1308
Pyne DB, Sharp RL (2014) Physical and energy requirements of competitive swimming events. Int J Sport Nutr Exerc Metab 24(4):351–359
Saunders B, Sale C, Harris RC, Sunderland C (2014) Sodium bicarbonate and high-intensity-cycling capacity: variability in responses. Int J Sports Physiol Perform 9(4):627–632
Siegler JC, Gleadall-Siddall DO (2010) Sodium bicarbonate ingestion and repeated swim sprint performance. J Strength Cond Res 24(11):3105–3111
Siegler JC, Keatley SIMON, Midgley AW, Nevill AM, McNaughton LR (2008) Pre-exercise alkalosis and acid-base recovery. Int J Sports Med 29(07):545–551
Siegler JC, Marshall PW, Finn H, Cross R, Mudie K (2018) Acute attenuation of fatigue after sodium bicarbonate supplementation does not manifest into greater training adaptations after 10-weeks of resistance training exercise. PLoS ONE 13(5):e0196677
Spriet L, Soderlund K, Bergstrom M, Hultman E (1987) Anaerobic energy release in skeletal muscle during electrical stimulation in men. J Appl Physiol 62(2):611–615
Stadlbauer V, Wallner S, Stojakovic T, Smolle KH (2011) Comparison of 3 different multianalyte point-of-care devices during clinical routine on a medical intensive care unit. J Crit Care 26(4):433-e1
Westerblad H (2016) Acidosis is not a significant cause of skeletal muscle fatigue. Med Sci Sports Exerc 48(11):2339–2342
Zajac A, Cholewa J, Poprzecki S, Waskiewicz Z, Langfort J (2009) Effects of sodium bicarbonate ingestion on swim performance in youth athletes. J Sports Sci Med 8(1):45
Acknowledgements
We would like to thank the participants for taking part in the research.
Funding
The study received no funding.
Author information
Authors and Affiliations
Contributions
LAG and MP designed this work. LAG, MP, and JWN collected data. LAG drafted the work with contributions from MP and JWN. MP and JWN reviewed and approved the manuscript. All the authors are accountable for the accuracy and integrity of the work.
Corresponding author
Ethics declarations
Conflict of interest
All the authors have no competing interests to declare.
Additional information
Communicated by Michael I Lindinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gough, L.A., Newbury, J.W. & Price, M. The effects of sodium bicarbonate ingestion on swimming interval performance in trained competitive swimmers. Eur J Appl Physiol 123, 1763–1771 (2023). https://doi.org/10.1007/s00421-023-05192-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05192-6