Abstract
Extracellular vesicles (EVs) are secreted by various tissues and cells under normal physiological or pathological conditions. Exercise-induced EVs may be involved in the adaptation of exercise-induced fatigue. The 1500-m freestyle is the longest pool-based swimming event in the Olympic Games, and there is a paucity of information regarding changes in the miRNA profiles of circulating EVs after a single session of fatiguing swimming. In this study, 13 male freestyle swimmers conducted a fatiguing 1500-m freestyle swimming session at the speed of their best previously recorded swimming performance. Fasting venous blood was collected before and after the swimming session for analysis. 70 miRNAs from the circulating EVs were found to be differentially expressed after the fatiguing 1500-m freestyle swimming session, among which 45 and 25 miRNAs were up-regulated and down-regulated, respectively. As for the target genes of five miRNAs (miR-144-3p, miR-145-3p, miR-509-5p, miR-891b, and miR-890) with the largest expression-fold variation, their functional enrichment analysis demonstrated that the target genes were involved in the regulation of long-term potentiation (LTP), vascular endothelial growth factor (VEGF), glutathione metabolism pathway, dopaminergic synapse, signal transmission, and other biological processes. In summary, these findings reveal that a single session of fatiguing swimming modifies the miRNAs profiles of the circulating EVs, especially miR-144-3p, miR-145-3p, miR-509-5p, miR-891b, and miR-890, which clarifies new mechanisms for the adaptation to a single session of fatiguing exercise from the perspective of EV-miRNAs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extracellular vesicles (EVs), including in particular exosomes and microvesicles, are widely present in various humoral samples, such as blood, urine, saliva, etc. Various tissues and cells in the body have the ability to secrete EVs under normal physiological or pathological conditions. Studies have found that EVs are rich in various communication substances, such as nucleic acids, proteins, mRNA, and microRNAs (miRNAs). Among them, miRNAs play a key role in the exercise-regulated skeletal muscle energy metabolism (Zhang et al. 2018; Whitham et al. 2018; Hou et al. 2019). In recent years, studies have confirmed that acute exercise increases circulating EVs, and exercise intensity influences the response of EVs to endurance exercise (Amosse et al. 2018; Wilhelm et al. 2017). EVs can promote the repair and regeneration of skeletal muscle, enhance the growth of nerve cells, and inhibit the differentiation of neurons and the expression of pro-inflammatory factors (Qin and Dallas 2019).
The 1500-m freestyle is the longest pool-based swimming event in the Olympic Games. Some of the key aspects in the 1500-m freestyle performance include how to effectively improve energy utilization during competition, and how to delay the occurrence of exercise-induced fatigue. Exercise-induced fatigue is a common physiological phenomenon in which the body cannot continue to maintain a certain level or a predetermined intensity during exercise, resulting in a decline in exercise capacity (Russell et al. 2020; Kieran et al. 2018). The occurrence of exercise fatigue is often viewed as a common and complex phenomenon caused by abnormal neuromuscular functions, hormone disorders, protein imbalance, increased inflammation and oxidative stress, and overtraining (Chen et al. 2019; Arthur et al. 2017; Wu and Liu 2018). The essence of these abnormalities results from the decrease in synaptic excitability of the central nervous system (CNS) and the energy metabolism of skeletal muscles, which leads to disturbances in homeostasis and susceptibility to injury (Jian et al. 2012).However, studies on the expression of circulating EV-miRNAs after fatiguing exercise and the changes in plasma EV-miRNAs after 1500 m all-out freestyle swimming are still scarce (Lipinska et al. 2015).
EV-miRNAs may be involved in the adaptation to exercise-induced fatigue. A recent study on rats suggested that the expression of EVs carried by miR-1 increased after exercise, while the expressions of miR-133a, miR-133b, miR-206, miR-208a, and miR-499 increased immediately after, but returned to the baseline level after 48 h. The rats’ exosomes level remained unchanged at 24 h after 4 weeks of swimming training, but a significant increase was observed immediately after exercise. However, the expression of exosomes miR-1, miR-486, miR-208a, miR-3571, miR-122, miR-196b, miR-3591, miR-184, and miR-760 recovered after 24 h (Brisamar et al. 2020).These results provide evidence for physiological adaptations to physical activity in EV-miRNAs (Lovett et al. 2018; D’Souza et al. 2018). There is a relative paucity of studies specifically relating to the changes of EVs in plasma and the biological characteristics of miRNAs in EVs under an exercise-induced fatigue state. Therefore, the aim of the study was to reveal the characteristics of the EV-miRNAs in the plasma of swimmers in a state of exercise-induced fatigue, and provide a theoretical basis for finding new biomarkers of exercise fatigue. We hypothesized that the amount of EV-miRNAs in plasma would increase after a full 1500-m freestyle swimming session, and the differential profiles of circulating EV-miRNAs would be modified by the fatiguing freestyle swimming.
Materials and methods
Subjects
Thirteen male freestyle swimmers were recruited from Guangzhou Sport University. Inclusion criteria included: (1) nondrinker and nonsmoker; (2) non-fatigue in the past 2 days. Each subject was informed of the research procedures and objectives verbally and in writing, and a written informed consent form was signed by each participant. The study was approved by Guangzhou Sports University Ethics Committee (Approval No. 2020LCLL-006). The physical characteristics of the participants are summarized in Table 1.
Experimental program
One week before the swimming session, a baseline Wingate anaerobic power test was conducted on a MONARK-894E power bicycle (Sweden), consisting of 30 s of fastest possible pedaling at a power load of the athlete’s weight (kg) × 0.075. The maximum power (PP) and the relative maximum power (PP/kg) were recorded. Power decline (PD), relative power decline (PD/kg), power decline rate (FIpp), and fatigue percentage (PD%) were used to evaluate the anaerobic capacity of subjects.
In the morning of the swimming session, fasting venous blood samples were collected. After reporting a rating of perceived exertion (RPE) value and obtaining heart rate data with a heart rate monitor (POLAR RC3, Finland), all subjects commenced a 1500-m freestyle swimming session at the speed of their best previously recorded swimming performance (Matthews et al. 2016). After the swimming session, the RPE scale was recorded to monitor the degree of physical fatigue (Borg 1982); venous blood samples were collected again, then the second Wingate anaerobic power test was conducted.
Sample collection and pretreatment
Within half an hour after the collection of venous blood samples, serum components were separated by centrifugation at 1000g for 15 min, and stored in a freezer at −80 °C before further analysis.
Biochemical index assay
Blood lactic acid (Bla) was measured with EFK semi-automatic lactic acid analyzer (EFK, German). Creatine kinase (CK) in serum was detected using a fully automatic biochemical analyzer (Chemray-420, Rayto, China).
ELISA
Serum samples were taken out from the −80 °C freezer, thawed on ice and centrifuged at 4 °C with 2000g for 5 min. The level of Serotonin (5-HT) in serum was assayed by ELISA (ABN-KA1894 Serotonin ELISA Kit) using a Multiskan Spectrum (Thermo Scientific, USA). Samples were analyzed in duplicates.
EVs' isolation
Umibio Extracellular vesicles extraction kits (Umibio, China) were used to isolate the EVs from plasma. 3 mL of pre-chilled PBS and 1 mL of Blood Pure Exo Solution were added to the stored supernatant. The mixture was vortexed for 1 min, incubated at 4 °C for 2 h, and centrifuged at 4 °C for 60 min at 10,000g. The supernatant was discarded, while the pellet rich in EV particles was resuspended with 0.5 mL of PBS. After it dissolved, the resuspension was transferred to a new centrifuge tube.
Identification of EVs with microscope
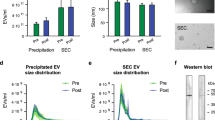
A transmission electron microscope (TEM) was used to directly observe the characteristics and morphology of the EVs for identification. After resuspending the extracted EVs with 50–100 μL of 2% paraformaldehyde, 50 μL of the EVs suspension were placed on copper mesh and allowed to stand still at room temperature for 20 min. 1% glutaraldehyde was fixed for 5 min; 4% uranyl acetate was used to negatively stain for 5 min, then the EVs pictures were photographed. The EVs were tracked using Nanosight nanoparticle tracking analysis technology (NTA) and distinguished from other particles, and finally the concentration and particle size distribution of EVs were detected.
Western blot
The isolated EVs were lysed with RIPA lysis buffer (Umibio, China), and the protein concentration was determined through BCA method. SDS-PAGE was performed on a 10% polyacrylamide gel and transferred to a PVDF membrane. This was sealed with 5% skimmed milk powder at room temperature for 1 h, incubated overnight at 4 °C with a primary antibody (CD63, ALIX) solution, and followed by a secondary antibody to block for 1 h at room temperature. Then, the protein bands were visualized using an enhanced chemi-luminescence (ECL) reagent.
Total RNA extraction and concentration assay
200 μL of EVs sample were placed into a RNase-Free centrifuge tube, mixed with an equal volume of 2 × denaturing solution, and placed on ice for 5 min. 200 μL of phenol:chloroform solution were added into the tube, and then vortexed for 30 s, centrifuged at 10,000g for 5 min at 4 °C. The supernatant was transferred to a new centrifuge tube, mixed with 1.25-fold volume of absolute ethanol, then 700 μL of the solution was transferred to the spin column and centrifuged at 10,000g for 30 s at 4 °C until it passed through the column. 700 μL of miRNA washing solution 1 was added, and centrifuged at 10,000g for 30 s at 4 °C until it passed through the spin column. 500 μL of washing solution 2 was added, and centrifuged at 10,000g for 30 s at 4 °C until it passed through the spin column. The supernatant was discarded and the spin column was put back into the collection tube. The resulting solution was centrifuged at 10,000g for 1 min at 4 °C until it all passed through the spin column. The adsorption column was put into a new collection tube and 50 μL of preheated washing solution were added at 95 °C. The precipitate (total RNA) was collected by centrifugation for 30 s, and the concentration of the total RNA extracted from EVs was detected by means of an Agilent 2100 Bioanalyzer System.
miRNA quality detection and library construction
The 3′ and 5′ adapters were connected. 2 μL of QIAseq miRNA NGS RT Initiator were added for RNA reverse transcription, and thoroughly mixed with QIAseq beads and QIAseq miRNA NGS Bead Binding Buffer for magnetic beads (QMN Beads) preparation. cDNA synthesis and purification were performed on ice, followed by library amplification. PCR product fragments were screened, and the concentration of the library was detected using Qubit dsDNA HS assay. The Agilent 2100 Bioanalyzer High Sensitivity DNA Assay was used to detect the fragment distribution range of the library. The main peak of the library was ~ 170–180 bp. Finally, the high-throughput Illumina 2 × 150 bp platform was used to detect the total miRNA extracted from EVs (Dillies et al. 2013).
Differential expressions of miRNAs
Using the Expdiff method, the known miRNAs in EVs was counted to determine whether there were significant differences in the expression levels between EVs, and the levels of miRNAs co-expressed between samples were compared using log2(fold-change) and scatter plots.
MiRNA pathway enrichment and datum analysis
The R language package DEGseq was used to identify differentially expressed genes. the R package based on differential expression analysis of negative binomial distribution, and the analysis of differentially expressed genes with biological repetition. Three database tools: targetscan, miRanda, and PITA were used to analyze the genetic sequence information of the subjects with known miRNAs and newly predicted, differentially expressed miRNAs (Wang et al. 2021; Zhang 2021). Finally, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis were performed on the set of miRNA target genes (Ashburner et al. 2000; Minoru et al. 2004). We used E-data software to select the RT and ACC data generated by Stroop task stimulus and imported it into WPS Excel 2019 to record.
Statistical analysis
All experimental data were recorded as mean ± standard deviation (Mean ± SD), and SPSS 23.0 software was used for statistical analysis. Each index before and after the exercise was analyzed by paired sample T-test, and the statistical significance was expressed at the p < 0.05 or p < 0.01 level. Graphpad Prism 7.0 software was employed for image drawing.
Results
HR, RPE results
The mean HR level of the subjects after a full 1500-m freestyle exercise was > 185 bpm. The RPE value was higher than 19, an extremely high level and a significant increase compared to the baseline (RPE 9).
Bla, CK, 5-HT test results
The subjects’ Bla significantly increased immediately after the full 1500-m freestyle exercise (p < 0.01) (Fig. 1A), reaching 11.2 mmol/L. The CK of the subjects increased significantly after the 1500-m exercise (p < 0.01) (Fig. 1B), reaching a maximum of 340 U/L.
As a central neurotransmitter in the brain, the concentration of 5-HT, one of the criteria for central nervous system fatigue, significantly increased after exercise (p < 0.05) (Fig. 1C).
Anaerobic test results
After the full 1500-m freestyle exercise, no significant changes were observed in the subjects' PP (w) and PP (w/kg) (Fig. 2A, B). In sharp contrast, a series of anaerobic exercise metrics significantly increased at the p < 0.05 level, including subjects' PD (w), PD (w/kg), FIpp, and PD (%) (Fig. 2C–F).
Changes in the subjects’ anaerobic capacity indicators as compared with baseline. PP (W), maximum power; PP (W/kg), relative maximum power; PD (W), power decline; PD (W/kg), relative power decline; FIpp, power decline rate; PD (%), fatigue percentage. A The subjects' PP (w) did not change significantly. B The subjects' PP (w/kg) did not change significantly. C The subjects' PD (w) significantly increased at p < 0.05. D The subjects' PD (w/kg) significantly increased at p < 0.05. E The subjects' FIpp significantly increased at p < 0.05. F The subjects' PD (%) significantly increased at p < 0.05
Changes of plasma EV-miRNAs in 1500-m freestyle swimmers under exercise fatigue
Identification results of EVs
To identify the EVs extracted from plasma samples, the morphology, particle size and distribution, and protein markers of plasma extracts were detected. Through TEM, the morphology of the extracted EVs in plasma was observed to be elliptical, and the diameter of the particles was ~ 100–200 nm (Fig. 3A). Through NTA analysis, we observed that the diameter of EV particles ranged from 40 to 200 nm, among which the particles with a diameter of 145 nm accounted for the highest proportion (Fig. 3B). The expression of EV marker proteins CD63 and ALIX were detected by WB (Fig. 3C).
Measurement of total RNA concentration
The concentration, total amount, and volume of total RNA were > 0.3 ng/μL, >13 ng, and 38 μL, respectively, as detected through Agilent 2100. As listed in Table 2, the quality of total miRNA extracted from the subjects' EVs in plasma met the test requirements, in that the concentration, volume, and purity of miRNAs could be satisfactorily used for database construction and subsequent trials.
Quality control of miRNAs’ detection
The miRNAs’ data obtained by preliminary filtering of the original miRNAs were further filtered. The number of bases with a quality value < 20 in the filtered data exceeded 1 read, and high-quality reads were obtained. After filtering out reads containing polyA and greater than 70% of the base reads, the small RNA clean tags sequence that could be used for subsequent analysis was finally acquired (Fig. 4).
The differential expressions of miRNAs in EVs
By integrating high-throughput sequencing with the three miRNAs’ target gene prediction databases (PITA, Targetscan, and miRand), subjects’ plasma EV-miRNAs expressions were analyzed in detail before and after exercise. In plasma, EV-miRNAs with a value of p < 0.05 and │log2(fold-change)│> 1 were considered to be differentially expressed. In total, 70 EV-miRNAs were found with significantly differential expression, among which 45 and 25 miRNAs were up-regulated and down-regulated, respectively (see Table 3). Three miRNAs were screened out due to their up-regulated expression after exercise and fold change being > 11, which included miR-144-3p, miR-145-3p, and miR-509-5p. Two miRNAs (miR-891b and miR-890) were filtered out due to their down-regulated expression and their fold change being > 9. Subsequently, the target genes regulated by the aforementioned five miRNAs were predicted prior to functional enrichment analysis.
Functional enrichment analyses of target genes regulated by EV-miRNAs
The five target genes of miR-144-3p, miR-145-3p, miR-509-5p, miR-891b and miR-890 were predicted to be differentially expressed through three databases of PITA, Targetscan, and miRand. Using GO and KEGG databases the functional annotation was analyzed to find the intersection relationship.
Figure 5 shows the statistical results of the comparison and classification of the target genes of differentially expressed EV-miRNAs in plasma through the GO database. In GO enrichment, three aspects are involved: biological process, cell composition, and molecular function, and each aspect is composed of eight items. Target genes are involved in cell metabolism, biological regulation, and signal transmission. They are mainly distributed in cell parts, membrane-enclosed cavities, and extracellular areas, and become enriched in molecular functions such as signal transmission, protein binding, and structural molecular activity.
The abscissa is the GO annotation, and the ordinate represents the number of genes. Green represents the biological process, red the cell composition, and blue the molecular function.
By screening the significantly enriched KEGG signaling pathways, the target genes regulated by the EV-miRNAs become mainly enriched in metabolic, calcium signaling, GnRH signaling and VEGF signaling pathways; long-term enhancement mechanism (long-term potentiation, or LTP); dopaminergic and cholinergic synapse; Alzheimer’s disease (AD); and glutathione, glycerophospholipid, and arachidonic acid metabolism, among others (Fig. 6). Consequently, it can be posited that EV-miRNAs are related to the enrichment of multiple signaling pathways, including those related to energy metabolism, skeletal muscle, central nervous system, immunity, and tumors. The database test shows that the metabolic pathways are the most significant and are closely related to EV-miRNAs target genes.
The color depth (Q value) indicates the enrichment degree of differentially expressed EV-miRNAs target genes in the signal pathway. The size of the circle (Gene number) denotes the number of genes with the differentially expressed EV-miRNAs target gene located under the signal pathway.
Discussion
Exercise fatigue is the main factor affecting athlete's training and competition status. In this study, we used the evaluation indicators of physiology and biochemistry to comprehensively observe the fatigue of freestyle athletes after a 1500-m freestyle swimming session. The profiles of plasma EV-miRNAs under fatigue condition was obtained by high-throughput detection technology, and the differentially expressed EV-miRNAs were screened out. It was found that 70 miRNAs changed differentially after exercise, 45 miRNAs were up-regulated, and 25 miRNAs were down-regulated. By screening the five target genes of miR-144-3p, miR-145-3p, miR-509-5p, miR-891b, and miR-890 with the largest fold change, functional enrichment analysis showed that the target genes are involved in the regulation of LTP, VEGF, glutathione metabolism pathway, dopaminergic synapse, signal transmission, biological regulation, and other biological processes. Our data suggested that the miRNA profiles of circulating extracellular vesicles could be modified by a single session of fatiguing swimming, and that EV-miRNAs might be involved in the mechanisms related to adaptation to fatiguing exercise.
During the full 1500-m freestyle swimming, the serum CK value reached 340 U/L, while the blood lactate reached 11.2 mmol/L. These results show that the subjects had gone all out for the full 1500-m freestyle swimming. This study also confirmed that going all out for the full 1500-m freestyle swimming can cause exercise-induced fatigue. The RPE scale reached above 19, sports ability decreased, physical function declined, and other fatigue indicators significantly changed. Skeletal muscle is the main place for lactate production, and accumulation of lactate may be related to the calcium signal pathway regulated by EV-miRNAs, which in turn might be involved in the blockade of neuromuscular conduction (Brooks 2020).
It is interesting to note that the characteristics of EV-miRNAs were modified after a single session of fatiguing swimming. A large number of EVs were released, and the CD63 and ALIX-labeled proteins were significantly expressed in the subjects’ plasma. Similar results have been reported in the literature: the expression of EVs markers Tsg101, HSP70, CD63, and Flot-1 increased immediately following EV release after exercise on power bicycles and treadmills, and returned to baseline after 90 min of recovery (Frühbeis et al. 2015). The same phenomenon was also observed in a study of exhaustive treadmill tests (Helmig et al. 2015; Karine et al. 2018); importantly, the study also observed the expression of various miRNAs in EVs after exercise. Another study found that miR-128-3p, miR-103-3p, miR-330-5p, miR-148a-3p, miR-191a-5p, miR-10b-5p, miR-93-5p and miR-25-3p in EVs have significant differential expression after acute exercise (Oliveira Getúlio et al. 2018). In this study, it was found that five EV-miRNAs were significantly differentially expressed after fatiguing swimming; three miRNAs of miR-144-3p, miR-145-3p, miR-509-5p had been up-regulated, and two miRNAs of miR-891b and miR-890 down-regulated. Studies have shown that the changes of miRNA and proteins carried by circulating exosomes help the body cope with the stress of acute fatigue exercise (Nair et al. 2020). Differentially expressed EV-miRNAs might inhibit the post-transcriptional translation process by inhibiting mRNAs and have biological effects in the process of fatigue (Assmann et al. 2019; Görgens et al. 2015).
Of the five EV-miRNAs differentially expressed after the fatiguing swimming, MiR-144-3p is related to a variety of biological processes, including nerve function, angiogenesis, adipogenesis, bone metabolism, and tumorigenesis (Lan et al. 2015; Liu et al. 2016; Sun et al. 2017); MiR-145-3p is involved in the regulation of VEGF related to angiogenesis and LTP related to nerve function; miR-509-5p is related the GnRH signaling pathway, which is associated with the potential signal pathways of fatigue. The down-regulation of miR-891b expression can inhibit the expression of GADD45β and Lnc-IRAK3-3, which can improve the body's antiviral immunity (Liao et al. 2019). Many studies have shown that the miRNAs carried by exercise-released EVs mediate cell-to-cell communication, participate in the regulation of energy metabolism during exercise, which have been identified as novel players in promoting systemic beneficial effects (Brisamar et al. 2020; Rong et al. 2020).
Through bioinformatics analysis, it was found that the main enriched biological processes of target genes that differentially express EV-miRNAs include regulation of metabolic processes, neuromuscular signal transmission, central nervous system regulation, and biological regulation involving EVs. Through KEGG functional enrichment analysis, it was found that the target genes that differentially express EV-miRNAs are mainly involved in metabolic pathways, VEGF, LTP, dopaminergic and cholinergic synapse, calcium signaling pathway, glutathione, glycerophospholipid, and arachidonic acid metabolism, and many other pathways. The LTP signaling pathway, dopaminergic synaptic pathway, and cholinergic synapse involved in the regulation of the target genes can improve brain synaptic plasticity, improve brain cognitive and executive functions, and promote the recovery of neurological function. It can thus be suggested that the differential EV-miRNAs involve the adaptation to a single fatiguing swimming session (D’Souza et al. 2017). A recent study showed that after acute aerobic exercise, the expression level of exosomal miRNAs (miR206, miR133b, and miR-181a-5p) increased (Oliveira Getúlio et al. 2018). Bioinformatics pathway analysis shows that exercise-induced exosomes are predicted to target genes involved in the MAPK pathway to promote muscle cell growth and differentiation. It can be seen that the up-regulation of selective muscle-specific miRNAs in exosomes may be related to the degree of muscle damage, thereby promoting the process of muscle repair and regeneration (Muroya et al. 2015). Therefore, after fatiguing swimming, the characteristics of EV-miRNAs had changed to adapt to exercise-induced physiological changes (Safdar and Tarnopolsky 2018). Differentially expressed miRNAs might cross-regulate multiple biological information processes and signaling pathways, indicating that exercise-induced EV-miRNAs changes might play an important role in skeletal muscle regulation and central nervous system regulation, which participate in the process of fatigue.
Conclusions
The miRNA profile of EVs in plasma changed significantly under the fatigue state after 1500-m freestyle swimming, especially for miR-144-3p, miR-145-3p, miR-509-5p, miR-891b, and miR-890. The changed EV-miRNAs might be involved in the mechanisms related to adaptation to fatiguing exercise, and can provide theoretical support for targeted prevention of exercise-induced fatigue.
Limitations
A number of limitations of this study should be considered. The study design lacked appropriate rest and non-fatiguing exercise controls, so the present findings cannot be solely ascribed to the impact of the fatiguing 1500 m freestyle swimming. In addition, only male athletes were included in this study.
Data availability
The datasets for this study are available as [raw data20220606.zip] at https://figshare.com/articles/dataset/raw_data20220606_zip/20029349.
References
Amosse J, Durcin M, Malloci M et al (2018) Phenotyping of circulating extracellular vesicles (EVs) in obesity identifies large EVs as functional conveyors of macrophage migration inhibitory factor. Mol Metab 2018(18):134–142
Arthur JC, Sarah JW, Christoph Z et al (2017) Post-exercise recovery of contractile function and endurance in humans and mice is accelerated by heating and slowed by cooling skeletal muscle. J Physiol 595(24):10–1113
Ashburner M, Ball CA, Blake JA et al (2000) Gene ontology: tool for the unification of biology. Gene 25(1):25–29
Assmann T, Milagro F, Martnez J (2019) Crosstalk between microRNAs, the putative target genes and the lncRNA network in metabolic diseases. Mol Med Rep 20(4):3543–3554
Borg G (1982) Psychophysical basis of perceived exertion. Med Sci Sports Exerc 14(5):377–381
Brisamar E, David J, Chun-Jung H et al (2020) Effects of exercise on exosomes release and cargo in vivo and ex vivo models: a systematic review. J Cell Physiol 236:3336–3353
Brooks GA (2020) Lactate as a fulcrum of metabolism. Redox Biol 35:101454
Chen HC, Huang CC, Lin TJ et al (2019) Ubiquinol supplementation alters exercise induced fatigue by increasing lipid utilization in mice. Nutrients 11(11):2550
D’Souza RF, Markworth JF, Aasen KMM et al (2017) Acute resistance exercise modulates microRNA expression profiles: combined tissue and circulatory targeted analyses. PLoS ONE 12(7):e0181594
D’Souza RF, Woodhead JST, Zeng N, Blenkiron C, Merry TL, Cameron-Smith D, Mitchell CJ (2018) Circulatory exosomal miRNA following intense exercise is unrelated to muscle and plasma miRNA abundances. Am J Physiol Endocrinol Metab 315(4):E723–E733
Dillies MA, Rau A, Aubert J et al (2013) A comprehensive evaluation of normalization methods for Illumina high-throughput RNA sequencing data analysis. Brief Bioinform 14(6):671–683
Frühbeis C, Helmig S, Tug S et al (2015) Physical exercise induces rapid release of small extracellular vesicles into the circulation. J Extracellular Ves 4(7):28239
Görgens SW, Eckardt K, Jensen J et al (2015) Exercise and regulation of adipokine and myokine production. Prog Mol Biol Trans Sci 135:313
Helmig S, Frühbeis C, Krämer-Albers E-M, Simon P, Tug S (2015) Release of bulk cell-free DNA during physical exercise occurs independently of extracellular vesicles. Eur J Appl Physiol 115(11):2271–2280
Hou ZX, Qin XH, Hu YY et al (2019) Long term exercise derived exosomal miR-342-5p: a novel exerkine for cardio protection. Circ Res 124(9):1386–1400
Jian QF, Gui ZM, Liang Y (2012) Effect of transcutaneous electrical acupoint stimulation on rats with chronic exercise-induced fatigue. J Acupunct Tuina Sci 10(5):265–270
Karine B, Laura RC, Bruna S et al (2018) Circulating extracellular vesicles in the aging process: impact of aerobic exercise. Mol Cell Biochem 440(1–2):10–1007
Kieran O, Peter BO, Tim JG (2018) Pain and fatigue in sport: are they so different? Br J Sports Med 52(9):10–1136
Lan F, Yu H, Hu M et al (2015) miR-144-3p exerts anti-tumor effects in glioblastoma by targeting c-Met. J Neurochem 135(2):274–286
Liao Y, Ho B, Chen M et al (2019) Host relieves Lnc-IRAK3-3-sequestered miR-891b to attenuate apoptosis in Enterovirus 71 infection. Cell Microbiol 21(9):e13043
Lipinska P, Allen SV, Hopkins WG (2015) Relationships between pacing parameters and performance of elite male 1500-m swimmers. Int J Sports Physiol Perform 11(2):159–163
Liu F, Chen N, Xiao R et al (2016) miR-144–3p serves as a tumor suppressor for renal cell carcinoma and inhibits its invasion and metastasis by targeting MAP3K8. Biochem Biophys Res Commun 480:87–93
Lovett JAC, Durcan PJ, Myburgh KH (2018) Investigation of circulating extracellular vesicle MicroRNA following two consecutive bouts of muscle-damaging exercise. Front Physiol 20(9):1149
Matthews MJ, Green D, Matthews H et al (2016) The effects of swimming fatigue on shoulder strength, range of motion, joint control, and performance in swimmers. Phys Ther Sport 23:118
Minoru K, Susumu G, Shuichi K et al (2004) The KEGG resource for deciphering the genome. Nucleic Acids Res 32:277–280
Muroya S, Ogasawara H, Hojito M (2015) Grazing affects exosomal circulating microRNAs in cattle. PLoS ONE 10:e0136475
Nair VD, Ge Y, Li S et al (2020) Sedentary and trained older men have distinct circulating exosomal microRNA profiles at baseline and in response to acute exercise. Front Physiol 11:605
Oliveira Getúlio P, Porto WF, Palu CC et al (2018) Effects of acute aerobic exercise on rats serum extracellular vesicles diameter, concentration and small RNAs content. Front Physiol 9:532
Qin W, Dallas SL (2019) Exosomes and extracellular RNA in muscle and bone aging and crosstalk. Curr Osteoporos Rep 17(3):548–559
Rong S, Wang L, Peng Z et al (2020) The mechanisms and treatments for sarcopenia: could exosomes be a perspective research strategy in the future? J Cachexia Sarcopenia Muscle 11(2):348–365
Russell S, Jenkins D, Halson S et al (2020) Changes in subjective mental and physical fatigue during netball games in elite development athletes. J Sci Med Sport 23(6):615–620
Safdar A, Tarnopolsky MA (2018) Exosomal as mediators of the systemic adaptations to endurance exercise. Cold Spring Harb Perspect Med 8(3):a029827
Sun L, Zhao M, Zhang J et al (2017) MiR-144 promotes β-amyloid accumulation-induced cognitive impairments by targeting ADAM10 following traumatic brain injury. Oncotarget 8(35):59181–59203
Wang H, Qi C, Wan D (2021) MicroRNA-377-3p targeting MMP-16 inhibits ovarian cancer cell growth, invasion, and interstitial transition. Ann Transl Med 9(2):124–124
Whitham M, Parker BL, Friedrichsen M et al (2018) Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab 27(1):237–251
Wilhelm EN, González-Alonso J, Chiesa ST, Trangmar SJ, Kalsi KK, Rakobowchuk M (2017) Whole-body heat stress and exercise stimulate the appearance of platelet microvesicles in plasma with limited influence of vascular shear stress. Physiol Rep 5(21):e13496
Wu CX, Liu ZF (2018) Proteomic profiling of sweat exosome suggests its involvement in skin immunity. J Invest Dermatol 138(1):10–1016
Zhang S (2021) An optimal piecewise cubic nonconforming finite element scheme for the planar biharmonic equation on general triangulations. Sci China (math) 64(11):2579–2602
Zhang A, Li M, Wang B et al (2018) miRNA-23a/27a attenuates muscle atrophy and renal fibrosis through muscle-kidney crosstalk. J Cachexia Sarcopenia Muscle 9(4):755–770
Acknowledgements
The authors wish to thank all participants, teachers from the Key Laboratory of Sports Biochemical Chemistry, Guangzhou Sport University, Prof. Xiquan Weng for revising the grammar and style of the manuscript, Weiwei Huang for their participation in the study.
Funding
This research was funded by the Characteristic Innovation Projects of Ordinary Colleges and Universities of Guangdong, China, grant number No. 2020KTSCX065 and the National Innovation and Entrepreneurship Projects of College Students, China, Grant number No. 5200080449. The APC was funded by Characteristic innovation projects of ordinary colleges and universities of Guangdong, China.
Author information
Authors and Affiliations
Contributions
Conceptualization, ZJL, WTL, and GQX; methodology, ZJL, and GQX; software, ZJL and GQX; validation, GQX, and WTL; formal analysis, ZJL, WTL, and GQX; investigation, ZJL and GQX; resources, WTL, and GQX; data curation, ZJL and GQX; writing-original draft preparation, ZJL, and XBC; writing-review and editing, WTL, XY, XBC and GQX; visualization, WTL, XY and GQX; supervision, WTL, and GQX; project administration, GQX. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
The study was approved by the Guangzhou Sports University Ethics Committee (Approval No. 2020LCLL-006).
Informed consent
Informed consent was obtained from all subjects involved in the study. Written informed consent to participate in this study was provided by the participants.
Additional information
Communicated by Michael I Lindinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lai, Z., Lin, W., Yan, X. et al. Fatiguing freestyle swimming modifies miRNA profiles of circulating extracellular vesicles in athletes. Eur J Appl Physiol 123, 2041–2051 (2023). https://doi.org/10.1007/s00421-023-05167-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-023-05167-7