Abstract
Given their importance in predicting clinical outcomes, cardiorespiratory fitness (CRF) and muscle status can be considered new vital signs. However, they are not routinely evaluated in healthcare settings. Here, we present a comprehensive review of the epidemiological, mechanistic, and practical bases of the evaluation of CRF and muscle status in adults in primary healthcare settings. We highlight the importance of CRF and muscle status as predictors of morbidity and mortality, focusing on their association with cardiovascular and metabolic outcomes. Notably, adults in the best quartile of CRF and muscle status have as low as one-fourth the risk of developing some of the most common chronic metabolic and cardiovascular diseases than those in the poorest quartile. The physiological mechanisms that underlie these epidemiological associations are addressed. These mechanisms include the fact that both CRF and muscle status reflect an integrative response to the body function. Indeed, muscle plays an active role in the development of many diseases by regulating the body’s metabolic rate and releasing myokines, which modulate metabolic and cardiovascular functions. We also go over the most relevant techniques for assessing peak oxygen uptake as a surrogate of CRF and muscle strength, mass, and quality as surrogates of muscle status in adults. Finally, a clinical case of a middle-aged adult is discussed to integrate and summarize the practical aspects of the information presented throughout. Their clinical importance, the ease with which we can assess CRF and muscle status using affordable techniques, and the availability of reference values, justify their routine evaluation in adults across primary healthcare settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poor cardiorespiratory fitness (CRF) and muscle status are associated with an increased risk of chronic disease and mortality from the fourth decade of life onward in both sexes (Kodama et al. 2009; Silventoinen et al. 2009; Leong et al. 2015; Schmid and Leitzmann 2015; Spahillari et al. 2016; de Santana et al. 2021; Qiu et al. 2021; Han et al. 2022). The CRF reflects the integrative capacity of the organism to transport oxygen from the atmosphere to the mitochondria during physical activity. For this reason, it is considered a robust indicator of the health of an individual (Kaminsky et al. 2013; Ross et al. 2016). Similarly, muscle strength, mass, and quality and performance in functional tests (Cooper et al. 2010; Cooper et al. 2014; Cruz-Jentoft et al. 2019) reflect the integrative capacity of the organism to perform physical activities where the skeletal muscle fulfills an essential function. A poor muscle status, characterized by low muscle strength, mass, and quality, and performance in functional tests, is associated with a greater risk of many chronic diseases, and mortality from all causes (Silventoinen et al. 2009; Cooper et al. 2010; Cooper et al. 2014; Lopez-Jaramillo et al. 2014; Leong et al. 2015; Cruz-Jentoft et al. 2019; Srikanthan and Karlamangla 2011; Londono et al. 2012; Chin et al. 2013; Park and Yoon 2013; Argiles et al. 2016), long before the formal criteria for the diagnosis of the disease known as sarcopenia are met. For this reason, muscle assessment should be done from early adulthood (i.e., since age 30). Skeletal muscle tissue is the main component of lean tissue reported in many studies, especially if the analyses are carried out in the extremities (i.e., appendicular mass), so in this text, and for simplicity, we will consider lean and muscular as synonyms (Wigodski et al. 2019).
Given the enormous potential of CRF and muscle status measurements as robust indicators of health status and predictors of important clinical outcomes whose evaluation and follow-up are beneficial for patients, they have become new vital signs (Bohannon 2008; Ahima and Park 2015; Ross et al. 2016). Even so, CRF and muscle status are not routinely assessed in adults in health practice (Bohannon 2008; Kaminsky et al. 2013; Ross et al. 2016; Ibrahim et al. 2018). This is likely because knowledge in the area has not reached health training programs. In the academic curricula, as well as in health practice and public health measures, there is an emphasis on the control of obesity and pharmacological interventions as strategies for health promotion and treatment of different chronic diseases (Pedersen and Saltin 2006; Kujala 2009; Gallo et al. 2010; Fletcher et al. 2018). In addition, until a few years ago, there were no reference values for multiple populations around the world against which to compare the results of simple tests of CRF and muscle status in adults. Finally, the emphasis on diagnosing and taking care of the older, certainly of paramount importance, have obscured the fact that the condition of the older is reached by walking a path which starts in the early adulthood and can be acknowledged since then (Sui et al. 2007; Cruz-Jentoft et al. 2019).
This review aims to show that the above limitations and paradigms can be overcome and challenged by recent knowledge, which now justifies the routine evaluation of the CRF and muscle status in primary healthcare settings starting the fourth decade of life, as a public health measure. The following aspects are addressed: (i) epidemiological evidence of the association of CRF and muscle status with morbidity and mortality, focused on cardiovascular and metabolic (i.e., cardiometabolic) outcomes; (ii) physiological mechanisms by which a better CRF and muscle status protect against adverse outcomes; (iii) methods to quantify CRF and muscle status; and (iv) application of concepts and translational recommendations based on a clinical case.
Association between CRF and morbidity and mortality
Inverse association between CRF and morbidity
In the last three decades, there has accumulated overwhelming evidence on the association between CRF and the risk of mortality and other adverse health outcomes (Blair et al. 1989; Myers et al. 2002; Kodama et al. 2009; Qiu et al. 2021; Han et al. 2022). A poor CRF is associated with an increased risk of cardiovascular disease and mortality from all causes after adjusting for age and other potential confounding variables (Kodama et al. 2009; Schmid and Leitzmann 2015; Qiu et al. 2021; Han et al. 2022). These findings have been reported in adults, asymptomatic men and women, people of different ethnic origins, and those with obesity, hypertension, type 2 diabetes mellitus, cardiovascular disease, and cancer (Blair et al. 1989; Myers et al. 2002; Church et al. 2004; Kodama et al. 2009; Kokkinos et al. 2013; Faselis et al. 2014; Ezzatvar et al. 2021a, 2021b). Additionally, a poor CRF has been associated with a greater risk of other adverse health outcomes, such as (i) cardiovascular and noncardiovascular outcomes after surgical procedures (Smith et al. 2009); (ii) time to heart transplant and hospitalization in patients with heart failure (Arena et al. 2014); (iii) incidence of stroke in older adults (Jefferis et al. 2014); (iv) dementia and Alzheimer's disease (Lee 2021); (v) metabolic syndrome and type 2 diabetes mellitus (Lee et al. 2009); and (vi) disability (Rabiee et al. 2015).
Inverse association between CRF and mortality
Different meta-analyses reported an inverse dose–response association between CRF expressed in terms of metabolic equivalents (MET, 3.5 mL O2/kg/min) and the risk of death (Kodama et al. 2009; Qiu et al. 2021; Han et al. 2022). An increase of 1 MET decreases mortality from all causes (12%), cardiovascular disease (13%), and cancer (7%) (Han et al. 2022). These findings have been described in apparently healthy people (Kodama et al. 2009; Han et al. 2022), in those with established cardiovascular disease (Ezzatvar et al. 2021a), and in cancer patients (Ezzatvar et al. 2021b). Likewise, a CRF > 10 METs in adults is associated with greater survival, while a CRF < 5 METs is associated with higher mortality (Kodama et al. 2009; Han et al. 2022). The protective effect of a better CRF on mortality is independent of age, ethnicity, adiposity, smoking, alcohol consumption, and the presence of comorbidities (Kodama et al. 2009; Han et al. 2022; Harber et al. 2017; Kaminsky et al. 2019a).
Usefulness of CRF in the reclassification of cardiovascular risk
CRF has a higher prognostic value than established classical risk factors (Myers et al. 2002; Church et al. 2004; Kodama et al. 2009; Kokkinos et al. 2013; Faselis et al. 2014) and some variables that are measured during the electrocardiographic stress test (ST-segment depression, symptoms, and hemodynamic response) (Kligfield and Lauer 2006) for the development of cardiovascular disease and mortality. Including the CRF as one of the risk factors improves the performance of prediction models of morbidity or mortality due to cardiovascular disease (Mora et al. 2005; Israel et al. 2016; Kondamudi et al. 2021). Interestingly, given that CRF is a variable that responds to treatments, its repeated measurement over time could contribute to risk stratification (Blair et al. 1995). People who increase their CRF between two measurements have a lower risk of adverse health outcomes than those whose physical fitness is steady or decreased over time (Blair et al. 1995).
Efforts have been made to incorporate CRF into predictive models of cardiovascular risk assessment (Framingham and European risk score) (Mora et al. 2005; Israel et al. 2016; Kondamudi et al. 2021) due to its potential to reclassify the risk of adverse outcomes (improvement in the net reclassification rate from one risk category to another of 10–40%) (Ross et al. 2016) and to monitor changes over time. Unfortunately, international public health organizations have lagged to include it in prevention strategies, delaying its widespread teaching and use.
Association between muscle status and morbidity and mortality
Inverse association between muscle status and morbidity
Studies conducted in adults have shown a negative association of handgrip strength and global or appendicular muscle mass with insulin resistance (IR), glycemic control, and the risk of developing metabolic syndrome and diabetes (Atlantis et al. 2009; Srikanthan and Karlamangla 2011; Londono et al. 2012; Park and Yoon 2013; Argiles et al. 2016; Kim and So 2016; Haines et al. 2022). For example, grip strength or global lean mass in the lowest quartile of the population is associated with up to 4 times a higher risk of developing metabolic syndrome (Atlantis et al. 2009; Yi et al. 2018). The spectrum of complications associated with lower grip strength or lower muscle mass includes an increased risk of falls, fractures, disability, infections, respiratory disease, and hospitalizations (Bohannon 2008; Leong et al. 2015; Argiles et al. 2016).
Qualitatively similar relationships have been reported between grip strength and knee extension strength and cardiovascular outcomes: the lower the strength, the greater the risk of myocardial infarction, cerebrovascular events, and heart failure (Silventoinen et al. 2009; Lopez-Jaramillo et al. 2014; Leong et al. 2015). Lower grip strength was a risk factor for falling in diabetic adults (Wen et al. 2021). The negative association of grip strength and muscle mass with blood pressure and cardiometabolic risk factors, such as pulse wave velocity and glycemic or lipidic alterations, has been observed since early adulthood (Cohen et al. 2014; Furushima et al. 2017). People with greater grip strength have better values of parameters of cardiometabolic importance, such as body mass index, waist circumference, blood pressure, and plasma glucose or lipids (Yi et al. 2018).
Low muscle quality, reported in terms of high myosteatosis, is also associated with worse cardiometabolic outcomes (Kim and Kim 2021). All the above associations are maintained even after adjusting for age, sex, adiposity or body size variables, and physical activity.
Inverse association between muscle status and mortality
In longitudinal studies, greater grip strength or total or appendicular muscle mass has been associated with a significant reduction in mortality from cardiovascular causes and from all causes in adults and elderly adults, both healthy and with glycemic alterations, in both men and women (Bohannon 2008; Cooper et al. 2010; Cooper et al. 2014; Lopez-Jaramillo et al. 2014; Leong et al. 2015; Spahillari et al. 2016; Steiber 2016; de Santana et al. 2021). Subjects in the tertile of higher muscle density, that is, with the lowest fat infiltration and therefore a higher muscle quality, showed up to a 70% lower risk of overall mortality than the worst tertile (Larsen et al. 2020).
In relation to more integrative evaluations of muscle performance, such as sitting repeatedly in a chair, or walking speed, poorer physical capacity is associated with higher mortality from all causes in both sexes (Cooper et al. 2010; Cooper et al. 2014). For example, people in the quartile with the lowest walking speed have 3 times the risk of death as the quartile with the highest speed (Cooper et al. 2010).
The findings cited in this section apply to people between the fourth and ninth decades of life, without the diagnosis of sarcopenia, and health conditions taken from the general population. In addition, the results are maintained after multiple adjustments and analyses in countries with ethnic, cultural, and socioeconomic diversity (Leong et al. 2015), confirming that muscle status is an independent risk factor for cardiometabolic and global morbidity and mortality.
The special case of sarcopenia
The population that is below 2.5 standard deviations of muscle strength (compared to the values in young people), accompanied by a reduction of muscle mass, meet the criteria of the most recent guidelines for the diagnosis of sarcopenia (Cruz-Jentoft et al. 2019). The reader is referred to a specialized bibliography given that the topic of sarcopenia is outside the scope of this review (Rubbieri et al. 2014; Cruz-Jentoft et al. 2019; de Santana et al. 2021).
Biological mechanisms of CRF and muscle status as protective against adverse outcomes
Protective cardiorespiratory mechanisms
The CRF expressed in terms of the maximal oxygen uptake (VO2max) reflects the integrative capacity of the organism to transport oxygen from the atmosphere to the mitochondria during physical activities (Balady et al. 2010). VO2 can be expressed in mL/kg/min (relative to body mass), L/min (absolute values), or in METs (Balady et al. 2010). The variability of VO2max depends on age, sex, physical activity, and genetic factors (Bouchard et al. 1999; Balady et al. 2010). VO2max decreases with age and is lower in women than in men and may improve after an exercise training program (Bouchard et al. 1999; Balady et al. 2010; Batacan et al. 2017; Su et al. 2019).
VO2max is considered the best measure of CRF and physical work capacity (Balady et al. 2010). From Fick’s principle (VO2 = Q × avO2), where VO2 is oxygen consumption, Q is cardiac output, and avO2 is the arteriovenous oxygen difference, it can be deduced that VO2max depends on the processes related to (i) ventilation and pulmonary diffusion; (ii) the functioning of the ventricles; (iii) ventriculoarterial coupling; (iv) the ability of blood vessels to efficiently transport blood from the heart to different organs according to oxygen requirements; (v) oxidative adaptations of skeletal muscle to extract oxygen from arterial circulation; and (vi) the integration of metabolic signals with the central commands of the cardiovascular system (Balady et al. 2010). In this sense, any alteration in one of the processes described could lead to a decrease in the CRF of an individual. Given the ability to integrate and demonstrate the condition of various systems, VO2max as a reflection of CRF is considered a robust indicator of human health (Kaminsky et al. 2013; Ross et al. 2016).
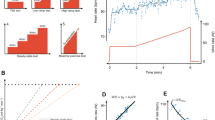
A few biological mechanisms can explain why a better CRF is a protective factor: (i) a cardioprotective cardiovascular risk profile, mediated in part by an associated greater level of physical activity; (ii) better body composition (less visceral fat and more muscle mass); (iii) more favorable lipid profile; (iv) lower blood pressure values; (v) increased sensitivity to insulin; (vi) less inflammation; (vii) a higher vagal tone, which lowers the risk of arrhythmias; (viii) lower risk of thrombotic events; and (ix) better endothelial function (Ravussin et al. 1988; Zurlo et al. 1990; Gallo et al. 2010; Wisloff et al. 2005; Fiuza-Luces et al. 2013, 2018; Fletcher et al. 2018; Pantiya et al. 2022) (Fig. 1).
Physiological mechanisms by which better cardiorespiratory fitness and muscle status protect against adverse outcomes. A better cardiorespiratory fitness (right), as reflected by a higher VO2max, associates with less arrhythmias, visceral fat, free fatty acids (FFA), inflammation and thus insulin resistance (IR). This profile also favors improved vascular reactivity (VR). A better muscle status (left), as indicated by a higher handgrip and muscle mass, increases energy expenditure, and endows with a healthy profile of myokines such as muscle-derived interleukins, irisin, chitinase-3-like protein 1 (CHI3-L1), apelin and myonectin. The result is a reduction in obesity risk, FFA, inflammation and IR and the associated improvement in VR. The reduction in musclin reinforces the reduction in IR. Together, good cardiorespiratory fitness and muscle status reduce the risk of developing cardiovascular and metabolic diseases, which directly leads to a reduction in mortality
Protective muscle mechanisms
Until recently, from the metabolic point of view, skeletal muscle was considered a passive energy store/consumer and the target of molecules produced by other tissues. However, during the last two decades, it was definitively recognized that the muscle itself is a primary determinant of the health-disease relationship and that it plays an active role in the pathophysiology of cardiometabolic diseases independent of other risk factors.
Given that the relationship between muscle and morbimortality is independent of the level of physical activity (see the previous section) and that muscle strength depends on the mass and fibre type composition, the three aspects that mediate the protective role of muscle are: (i) muscle mass, (ii) its composition of fibre types, and (iii) its function as an endocrine organ. These three variables can, in an articulated way, modulate metabolic and hemodynamic aspects relevant to multiple chronic cardiovascular and metabolic diseases.
Mass and types of muscle fibres
Muscles represent about 35–40% of body weight in a healthy adult and are composed mainly of type I, IIA, and IIX fibres (Bottinelli et al. 1999; Bottinelli and Reggiani 2000; Lee et al. 2000). The amount of muscle mass, as well as its metabolism, are determinants of energy expenditure in adults. In fact, muscle mass can explain 20–30% of total oxygen consumption at rest and therefore of body metabolism. Interestingly, differences in muscle metabolism can explain up to 50% of the variability in energy expenditure between individuals. Unlike other tissues with high energy expenditure, such as the kidney or the brain, total muscle energy expenditure is modifiable because both muscle mass and composition are modifiable (Zurlo et al. 1990). Promoting greater energy expenditure at baseline and over 24 h of the day prevents weight gain and the accumulation of adipose tissue, reducing the risk of obesity (Ravussin et al. 1988; Zurlo et al. 1990).
Muscle is the main carbohydrate store in the body, both in response to insulin and to contractile activity (DeFronzo 1988; Lund et al. 1995). In postprandial conditions, the muscle can capture up to 70% of ingested glucose (DeFronzo 1988), mainly in fibres type I and IIA, which are more oxidative, rich in mitochondria, with higher expression of the facilitated glucose transporter member 4 (GLUT-4) and greater sensitivity to insulin than fibres type IIX (Daugaard et al. 2000; Levin et al. 2007; Mackrell and Cartee 2012). A higher percentage of type IIX fibres is found in obese subjects with IR than in thin subjects. In adults, insulin sensitivity and the elasticity of arteries are positively associated with the proportion of type I fibres, but this proportion is negatively associated with blood pressure. Together, these results suggest a relationship between fibre types, obesity, IR, and vascular function (Tanner et al. 2002; de Courten et al. 2015; Stegen et al. 2015; Fisher et al. 2017).
The ability of the muscle to accumulate carbohydrates is so important that alterations in glucose uptake in the thigh cause IR in humans (Olsen et al. 2005). The fact that voluminous and mainly oxidative muscles (i.e., up to 75–85% of type I and IIA fibres) are present in this region makes it quantitatively important for glucose homeostasis (Staron et al. 2000; Olsen et al. 2005; Londono et al. 2012).
Type I and IIA fibres also have a greater capacity to store lipids than type IIX fibres (Daugaard et al. 2000; Levin et al. 2007; Mackrell and Cartee 2012). This ability of the muscle to store lipids, intra- and extramyocellularly (Goodpaster et al. 2001; Li et al. 2015), gives it a role in the distribution of body fat (Sinha et al. 2002). This muscle storage of fat is relevant because it can lead to the development of regional IR (Jacob et al. 1999; Virkamaki et al. 2001; Savage et al. 2019; Kim and Kim 2021) and alter muscle oxidative metabolism (Petersen et al. 2004; Li et al. 2016).
In summary, the reservoir capacity of muscle, which depends on the amount of muscle and its composition, regulates whole-body glycemic and lipid metabolism, substrate oxidation, and body energy expenditure. Thus, increased muscle mass, with a phenotype with more oxidative fibres (I and IIA) is metabolically and hemodynamically healthier.
The muscle is an endocrine organ which modulates cardiometabolic health
Myokines are mainly peptides and proteins produced and secreted by the muscle under different stimuli, with autocrine, paracrine, and endocrine functions that regulate the metabolism of various tissues (Ahima and Park 2015; Narvaez-Sanchez et al. 2019; Bay and Pedersen 2020). The myokine profile produced depends, among other factors, on muscle mass and composition. Although it has not been demonstrated, muscle quality is probably also a determinant of the circulating myokine profile of a human.
Several myokines have notable metabolic functions (e.g., modulation of glucose and lipid metabolism) and cardiovascular functions (e.g., regulation of vascular reactivity, production of nitric oxide, blood pressure, and angiogenesis), which give them an important role in the pathophysiology and development of some chronic cardiometabolic diseases.
Irisin: It reduces hyperglycemia, IR, and weight in mice fed a high-fat diet (Bostrom et al. 2012). Its metabolic functions are mediated in part by the induction of mitochondrial uncoupling in adipose tissue and muscle (Bostrom et al. 2012; Vaughan et al. 2014). In addition, it increases the expression of GLUT-4 and the mitochondrial content in myoblasts (Vaughan et al. 2014), favoring a more oxidative and healthier phenotype in this tissue.
Irisin is reduced in obese patients, in whom it was also positively associated with dysfunction of endothelium-dependent vasodilation (Hou et al. 2015). Irisin increased nitric oxide synthase phosphorylation and nitric oxide production in human and rat endothelial cells, and in aortic rings from obese mice, in a dose- and time-dependent manner (Han et al. 2015; Hou et al. 2016). The favorable vascular impact of irisin could also be because it improves the function of perivascular adipose tissue (Hou et al. 2016, 2017). Irisin has anti-inflammatory properties in many experimental models (Han et al. 2015; Hou et al. 2017; Mazur-Bialy et al. 2017), which can explain the metabolic improvement and vascular function induced by this myokine.
Although it is still debated, studies in humans tend to show an increase in serum irisin after continuous exercise at not less than 60% of the maximal physical capacity, interval training with peaks over 90% of the maximal physical capacity, or strength training over 60% of one maximal repetition, at least 3 times per week for more than 8 weeks (Dinas et al. 2017). However, the use of inadequately validated measurement techniques has limited the scope of the findings.
Therefore, the evidence suggests that irisin has beneficial metabolic and vascular effects in healthy humans and patients with chronic cardiometabolic conditions.
Myonectin: It is produced mostly, but not exclusively, by type I fibres. In murine models, it reduces circulating triglycerides and free fatty acids by increasing the expression of its transporters in the liver and fat tissue (Seldin et al. 2012). In addition, it increases in response to aerobic exercise in murines (Seldin et al. 2012). In agreement with these results, knockout mice for myonectin accumulated less triglycerides in the liver and showed an increase in lipid storage in hypertrophied adipocytes, as well as intolerance to an oral lipid load (Little et al. 2019).
The relevance of these results to humans is unclear. On the one hand, studies have shown a direct association between myonectin and IR markers, but contrary to expectations, no correlation was observed between myonectin and plasma lipids or total fat mass (Toloza et al. 2018; Mi et al. 2019). On the other hand, a negative association between serum myonectin and IR markers and visceral obesity was reported in another population (Li et al. 2021). Future studies should aim to better understand the role of myonectin in the pathophysiology of various diseases in humans.
Apelin: This myokine, also an adipokine, circulates in several isoforms. However, due to technical limitations, it is not always easy to know which isoform is responsible for the observed effects. In general, apelin is reduced with aging and with the loss of muscle mass in murines and humans (Vinel et al. 2018). Its supplementation in murine models increases muscle strength and mass, demonstrating an autocrine effect (Vinel et al. 2018). Therefore, it seems to have an important regulatory role in muscle status. Its expression increased in human muscle after an 8-week intervention consisting of cycling or running 5 times a week at 85% of the maximal physical capacity, and this increase was associated with an improvement in insulin sensitivity (Besse-Patin et al. 2014). Its effect is mediated by the activation of 5′-AMP-activated protein kinase (AMPK) and serine/threonine protein kinase akt (AKT) in muscle and adipose tissue (Dray et al. 2008; Yue et al. 2010). Given that apelin induces phosphorylation of nitric oxide synthase in several tissues, a vasodilator effect and therefore a beneficial cardiovascular effect have been observed (Tatemoto et al. 2001), confirming that this protein is beneficial for cardiometabolic and muscular health.
Interleukins: The skeletal muscle produces a wide panel of interleukins (Ostrowski et al. 1999; Steensberg et al. 2000, 2002; Quinn et al. 2002, 2011; Pedersen et al. 2003; Nielsen et al. 2007; Bay and Pedersen 2020). In general, this cocktail has beneficial effects on health, as it favors glucose metabolism, is lipolytic, anti-inflammatory, immunomodulatory, and favors muscle hypertrophy, opposing the induction of IR and indirectly improving vascular function.
Chitinase-3-like protein 1 (CHI3-L1): It is produced and secreted by immune, endothelial and muscle cells. It is expressed at lower levels in the muscle tissue of overweight subjects with glycemic alterations (Gorgens et al. 2016; Kwak et al. 2020), and increases in muscle and serum after a single bout of continuous exercise at 70% of the maximal physical capacity or strength training at 80% of one maximal repetition (Gorgens et al. 2016; Kwak et al. 2020). Its anti-inflammatory effects and its ability to induce the activation of AMPK and AKT as well as the translocation of GLUT-4 in muscle cell lines explain its beneficial effect on insulin sensitivity and myoblast proliferation (Gorgens et al. 2016; Kwak et al. 2020). In humans with coronary disease and in murine models, CHI3-L1 reduces and stabilizes the atherosclerotic plaque (Tsantilas et al. 2021), granting this myokine a clear cardiovascular and metabolic protective profile.
Musclin: It reduces glucose uptake and glycogen synthesis, both in the presence and in the absence of insulin, in animal models, and cultured myotubes (Nishizawa et al. 2004), inducing IR. In this sense, musclin reduces the phosphorylation of AKT induced by insulin (Liu et al. 2008). Elevated insulin activates AKT, which phosphorylates forkhead box protein O1 (FoxO1), producing a loss of suppression of the musclin gene, thus linking excess insulin, typical of an IR state, to an excess of musclin, favoring a vicious cycle that generates more IR (Nishizawa et al. 2004; Sierra et al. 2016). It is upregulated by palmitate, an inductor of IR, in cellular models (Gu et al. 2015; Guo et al. 2019) and is mostly expressed in type II fibres (Banzet et al. 2007), which predominate in obese and diabetic patients (Tanner et al. 2002). Musclin is increased in murine models fed a high-fat diet (Deng and Tang 2012; Yu et al. 2016; Chen et al. 2017) but is reduced in serum and muscle as a response to an exercise program based on ladder climbing or swimming during 8 to 13 weeks (Deng and Tang 2012; Shimomura et al. 2021).
In humans, we have shown a positive association between serum musclin and IR in adults with metabolic syndrome (Sanchez et al. 2021). In addition, musclin positively correlated with insulinemia and visceral fat (Sanchez et al. 2021) and was reduced after interventions with either continuous exercise on a treadmill at 60% of the maximal physical capacity or interval training with peaks over 90% of the maximal physical capacity for 12 weeks (Gallo-Villegas et al. 2022), validating the findings described above in murine models. In summary, musclin seems to be a key player in the induction of metabolic alterations.
Figure 1 summarizes the mechanisms by which a better muscle status protects from cardiometabolic diseases and mortality.
Methods to quantify the CRF and muscle status
Cardiorespiratory fitness
It can be assessed by measuring VO2max in maximum stress tests (direct maximum tests with gas analysis) or estimated with maximum stress tests (indirect maximum tests without gas analysis), submaximal field and clinical tests, and nonexercise prediction equations (Cooper 1968; Bruce et al. 1973; Jackson et al. 1990; Laboratories 2002; Maranhao Neto Gde et al. 2004; Balady et al. 2010; Nes et al. 2011; Jackson et al. 2012; Kaminsky et al. 2019b; Cuenca-Garcia et al. 2022).
The direct tests combine procedures of the conventional stress test with ventilatory analysis of expired gases, which allow the concomitant evaluation of three functional variables with prognostic value: (i) VO2; (ii) carbon dioxide production (VCO2); and (iii) minute ventilation (VE) (Balady et al. 2010). VO2max corrected for body mass is the gold-standard measure of physical cardiorespiratory capacity, while the slope of the VE/VCO2 ratio is a key indicator of ventilatory efficiency, which is abnormally high in most patients with cardiovascular or pulmonary diseases (Balady et al. 2010; Arena et al. 2014). Although the performance of the direct maximal stress test involves the participation of trained human resources, as well as expensive equipment, given the independent and additive information for the diagnosis and prognosis of many patients, its use may be justified (Balady et al. 2010; Arena et al. 2014). In many clinical environments, it is increasingly feasible to perform the direct stress test to quantify VO2max as a measure of CRF. There are reference values of VO2max calculated from population data of different countries (Peterman et al. 2020). Recently, a process was initiated to develop global reference standards for VO2max obtained from direct maximum stress tests (Peterman et al. 2020; Kaminsky et al. 2022).
Through indirect tests, CRF can be estimated from regression equations according to the speed, inclination, duration, and workload (in watts) reached on a treadmill or cycle ergometer (Bruce et al. 1973). When the CRF is estimated using a treadmill protocol, the tests should be performed without allowing the patients to lean on the handrails. Likewise, care must be taken in the appropriate selection of the protocol according to the functional capacity of each individual.
Submaximal tests on a treadmill, bicycle, or step can estimate the CRF from the response of the heart rate to the intensity of the exercise (Davies 1968). Additionally, there are field exercise tests or submaximal clinical tests to estimate the CRF with regression equations that take into account the distance covered in 12 min (Cooper test) and six minutes (six-minute walk test) or the time to travel 1.5 miles (Cooper 1968; Laboratories 2002). These tests are highly reliable in adults (intraclass correlation coefficient, ICC > 0.9) (Cuenca-Garcia et al. 2022) and provide valuable information for clinical practice and should be considered when evaluating large groups of resources are limited. However, they are not as accurate as the direct stress tests to estimate CRF.
Nonexercise prediction equations are an alternative to maximal and submaximal stress tests to estimate CRF in the health setting (Jackson et al. 1990, 2012; Maranhao Neto Gde et al. 2004; Nes et al. 2011; Kaminsky et al. 2019b; Arcila et al. 2022). These models include physiological variables commonly evaluated that indicate physical fitness, such as: (i) sex, (ii) age, (iii) body mass index, (iv) heart rate at rest, and (v) physical activity performed (objective and subjective) (Jackson et al. 1990; Maranhao Neto Gde et al. 2004; Arcila et al. 2022). The nonexercise prediction equations to estimate CRF have shown consistent associations with mortality from all causes and of cardiovascular origin and have good discriminatory power and excellent capacity for risk reclassification (Stamatakis et al. 2013; Qiu et al. 2021). They could be a practical, quick, economical, safe, first-line tool for evaluating CRF and predicting cardiovascular risk in primary health settings, which is why their use is suggested for follow-up in adults at least once a year (Ross et al. 2016; Kaminsky et al. 2019b). Different nonexercise prediction equations have been validated in different regions of the world (Jackson et al. 1990; Maranhao Neto Gde et al. 2004; Nes et al. 2011; Jackson et al. 2012; Ross et al. 2016; Kaminsky et al. 2019b; Arcila et al. 2022).
Care takers should consider the use of submaximal, clinical, or field tests as an alternative because these can tell them how the individual responds to exercise and what exercise to prescribe (Kaminsky et al. 2019b). Finally, patients with chronic diseases should undergo a direct maximal stress test (Ross et al. 2016). Table 1 shows the median and interquartile range for the distribution of VO2max values obtained from direct and indirect maximum tests on the treadmill for selected ages (Kaminsky et al. 2015).
Muscle status
The most common strength and functional tests in adults include handgrip strength, standing and sitting in a chair, isometric mid-thigh pull and gait speed. They are reliable (ICC > 0.85), inexpensive, fast and easy to apply (Bohannon et al. 2010; Cooper et al. 2014; Leong et al. 2016; Cruz-Jentoft et al. 2019; Ramirez-Velez et al. 2021; Cuenca-Garcia et al. 2022). Typically, men have grip values up to 50% higher than those of women, and there are notable differences between races. In general, grip strength increases up to age 25–30, stabilizes between 30 and 40 years, and then shows a curvilinear reduction (Schlussel et al. 2008; Dodds et al. 2014; Leong et al. 2016; Lee et al. 2019). The high variability has necessitated the adoption of different reference values for both sexes in different regions of the world over a wide age range (Leong et al. 2016; Martinez-Torres et al. 2022). The PURE study reported reference values for the general population of at least 21 countries of different levels of development. Table 2 shows the medians and the 25th and 75th percentiles of the data obtained in various regions of the world (Leong et al. 2016). In addition, multiple countries have seen recent specific studies that report normative values with selected percentiles of grip strength for adults between the fourth and ninth decades of life (Schlussel et al. 2008; Dodds et al. 2014; Wong 2016; Steiber 2016; Wang et al. 2018; Landi et al. 2020; Ramirez-Velez et al. 2021). Those patients below the 25th percentile are at greater risk. The ease of measurement, the availability of reference values, and their relevance to clinical outcomes make feasible the evaluation and routine follow-up of grip strength in primary healthcare settings. For instance, during the medical consultation of patients with cardiometabolic risk factors, or when registering participants in exercise, physical activity or conditioning interventions, nutritional programs or physiotherapy sessions.
The chair test consists of measuring the time required for the patient to stand and sit five times, without using the support of their hands or arms. The results depend on age and sex and are correlated with the strength of the lower limbs. The average times increase from 6 to 14 s between the third and ninth decades of life (Bohannon et al. 2010; Cruz-Jentoft et al. 2019; Landi et al. 2020; Gao et al. 2021). Values in percentiles for some populations are also available (Landi et al. 2020; Gao et al. 2021). Recent results suggest that both bilateral and unilateral isometric mid-thigh pull tests can be used to measure lower-limb strength with high reliability in healthy and obese subjects (Orange et al. 2019; Grgic et al. 2022). Isometric mid-thigh pull strength partially explains the performance of these populations in functional tasks (Orange et al. 2019; Grgic et al. 2022) and is expected to be increasingly used among middle-age and older adults in the near future. Gait speed is typically evaluated over 4 m. Walking velocities greater than 1.0 m/s are expected (Cruz-Jentoft et al. 2019).
Though nuclear magnetic resonance (NMR) and computed tomography are the reference methods, dual-energy X-ray absorptiometry (DXA), bioelectrical impedance analysis (BIA), and anthropometry are the most used methods to study muscle mass.
DXA is the first-choice method because provides accurate (low variability between measurements: ICC 0.97 and coefficient of variation, CV < 1.0%) and valid (comparable with the reference test) total and appendicular lean mass information in a noninvasive, fast, safe, and economical way (Rubbieri et al. 2014; Cruz-Jentoft et al. 2019; Walowski et al. 2020; Kawakami et al. 2021; Aristizabal et al. 2021). Fat-free adipose tissue (FFAT) corrections of the DXA lean mass measurements are currently necessary. They can be performed assuming that ~ 13–15% of the adipose tissue is a fat-free component (Abe et al. 2019, 2020a). The application of this correction increases validity when assessing muscle mass, without adding costs (Abe et al. 2019, 2020b), thus strengthening DXA as a tool for evaluating muscle status. The appendicular mass index seems to be the best indicator and the most used to assess muscle mass (Cruz-Jentoft et al. 2019; Walowski et al. 2020). Unfortunately, the normative values still lack the FFAT correction, and there are doubts about the feasibility of comparing data obtained in multiple countries: there are variations of up to 35% in the cutoff values between different populations using the same technique (Walowski et al. 2020).
Although simple, cheap and useful in large surveillance studies, classical BIA and anthropometry have limited validity for their application in muscle mass evaluation in individual cases (Rubbieri et al. 2014; Furushima et al. 2017). Their reduced reliability and the lack of normative values further limit their use for following up on a specific patient in most health settings (Walowski et al. 2020). In addition, complex anthropometric models (Londono et al. 2012) are out of the expertise of most health care practitioners and are discouraged from general use.
Fortunately, improved methods such as specific bioelectrical impedance vector analysis (BIVA), refined anthropometric equations or equations integrating both BIA results and anthropometric measurements have partially overcome these limitations. If DXA is not available even after referral or if contraindicated, these techniques can be used as second-line choices to assess muscle mass.
Specific BIVA is based on a corrected classical bioelectrical impedance vectorial approach. It is cheap and effective to distinguish adults with different muscle mass index values. Further, reference data for some populations is available (Buffa et al. 2014). Anthropometric equations with enhanced validity (R2 over 0.90 and standard errors of the estimate as low as 1–2 kg compared to DXA or NMR) have been developed for total and appendicular mass measurements in some populations, including middle-aged adults (Al-Gindan et al. 2014; Lee et al. 2017; Heymsfield et al. 2020; Kawakami et al. 2021). These equations rely on simple measurements such as height, body weight, and a few circumferences and skinfolds. Others have refined equations to estimate total and appendicular muscle mass starting from BIA measurements with high validity (ICC over 0.96 and errors within 2 kg compared to DXA) in adults and elder subjects (Lee et al. 2018). Enhanced-validity equations should now be developed for different populations so its use can be spread soon.
Muscle composition can be studied in tissue obtained by biopsy, but since this is an invasive method, its use is not recommended. An alternative is the use of proton magnetic resonance spectroscopy (1H NMRS), which has been used in healthy subjects and in patients with metabolic syndrome and diabetes (Stegen et al. 2015; Gallo-Villegas et al. 2022; Vega et al. 2022). However, its use is not yet widespread because it is a costly, highly specialized technique. Similarly, the assessment of muscle quality based on quantifying myosteatosis by computed tomography or 1H NMRS (Larsen et al. 2020; Kim and Kim 2021) is currently limited to research studies.
Application of concepts to clinical practice
History of the present illness: A 47-year-old Latino man who works in financial activities in an office is seen in an outpatient consultation. He comes for a biannual consultation as part of the cardiometabolic risk program, which he has participated in for 3 years. He reported deterioration in his functional capacity since the last consultation, which manifested as dyspnea and tachycardia when climbing stairs without angina or dizziness.
Past medical history: Overweight for 20 years. Four years ago, he started medication for fasting hyperglycemia (metformin 250 mg every 12 h) and arterial hypertension (losartan 50 mg every 24 h).
Habits: He is sedentary 6–8 h a day, has not performed physical activity in his free time for approximately 15 years, does not smoke, does not consume liquor, sleeps 7 h a day, and reports a high consumption of processed foods.
Physical examination reveals a good general condition. Vital signs sitting: heart rate 87 beats/min; respiratory rate 17 cycles/min; blood pressure 148/94 mmHg. Weight 89 kg; height 1.77 m; body mass index 28.4 kg/m2; waist circumference 98 cm. Normal heart sounds, conserved vesicular murmurs without superimposed sounds, and no carotid murmurs. Normal abdomen, normal peripheral pulses. Normal gait. Reduction of the force in the flexion–extension of the knee and hip. No myalgia.
Physical tests in the office: Grip strength of 33.5 kg by dynamometry in his dominant hand; 10.5 s to stand and sit in a chair five times; walking speed 1.2 m/s; estimated VO2max: 28.5 mL O2/kg/min (8.1 METs).
Paraclinical: Fasting glycemia 108 mg/dL, homeostatic model assessment (HOMA-IR) 2.8, glycosylated hemoglobin 6.1%, total cholesterol 210 mg/dL, HDL-cholesterol 40 mg/dL, LDL-cholesterol 172 mg/dL, triglycerides 175 mg/dL, creatinine 1.1 mg/dL, normal hemoleukogram. FFAT-corrected appendicular lean mass 18.9 kg, FFAT-corrected appendicular lean mass index (appendicular skeletal mass/height2) 6.1 kg/m2, visceral fat 820 g, and body fat percentage 42%, by DXA.
Diagnoses: Overweight and abdominal obesity (code XS7R, ICD-11), arterial hypertension grade 1 (BA00), fasting hyperglycemia (5A40), metabolic syndrome and insulin resistance (5A44), dyslipidemia (5C8Z), decreased muscle mass (FB329), low cardiorespiratory fitness (CM91), and sedentary lifestyle (QE20).
Decision: An endurance training program plan with cardiovascular components and muscle strengthening was prescribed under current guidelines. Referral to the nutritionist and psychologist. Pharmacological treatment was adjusted to achieve goals.
Follow-up: Six months later, the patient improved his VO2max by 12%, increased his grip strength by 7%, lost 2.6% of body fat, and gained 660 g of appendicular muscle mass and 1.0 kg of total lean mass. His fasting glycemia was 100 mg/dL, HbA1c 5.8%, HOMA-IR 2.5, and blood lipids were 10% better. He was recommended to continue with the multidisciplinary treatment.
Discussion of the clinical case: The patient has metabolic, cardiorespiratory, and muscular alterations (Fig. 2). Previous management was limited to a pharmacological approach, without considering lifestyle changes, such as nutritional and physical activity modifications. Basic evaluations were lacking at the time of patient admission to the cardiometabolic risk program, such as body composition, VO2max, grip strength, and gait speed. An adequate follow-up and an early intervention would have improved the health of the patient before.
General evolution of cardiorespiratory fitness and muscle status during lifetime. Both conditions rapidly increase during youth, then stabilize during early adulthood, and finally decrease with slow, more or less linear kinetics. There is always variability in the values found across all ages, from which those in the lowest quartile (below percentile 25th, P25, i.e., below the white, discontinuous line) are at increased risk of cardiometabolic morbidity and mortality. The white dot illustrates the position of the patient in the clinical case regarding the distribution of the population of the same age. The routine evaluation of cardiorespiratory fitness and muscle status in adults allows to identify those below the P25 and take effective actions to help them soon cross the line over the P25 (white arrow in the insert), notably reducing their cardiometabolic risk
His CRF is poor since his VO2max is within the lowest quartile according to that expected for his age and sex (10 METs; 35 mL O2/kg/min) (Kaminsky et al. 2015). The low CRF observed may be related to the lack of physical activity in his free time and his sedentary lifestyle. Taking into account that VO2max was estimated by a nonexercise prediction equation, it is recommended to perform a supervised maximal or submaximal stress test to estimate VO2max and evaluate the cardiorespiratory response to physical effort and thereby make a more precise exercise prescription.
The patient showed a grip strength in the lowest quartile according to the PURE study and also according to recent normative tables for the Latino population (Leong et al. 2016; Ramirez-Velez et al. 2021). His muscle mass is lower than the mean values expected for Latino men (Aleman-Mateo and Ruiz Valenzuela 2014; Aristizabal et al. 2021). This puts him at greater risk of developing cardiometabolic diseases compared to the general population, although he does not meet the criteria for the formal diagnosis of sarcopenia (Cruz-Jentoft et al. 2019).
This analysis highlights that appropriate cutoff values should be applied to each individual, that the objective of the evaluation of strength and muscle mass goes beyond seeking the diagnosis of sarcopenia, and that this middle-aged patient can reduce his cardiometabolic risk through interventions aimed at improving his CRF and muscle status.
The main actions that increase the CRF and protect the muscle are a healthy diet, well-prescribed exercise, and prevention of chronic or inflammatory conditions (Argiles et al. 2016; Cruz-Jentoft et al. 2019). The first item was addressed by referring the man to a nutritionist, who may suggest him a low-energy diet. Low-energy (i.e., caloric restriction and intermittent fasting) or very low-carbohydrate diets improve cardiometabolic health and extend healthspan and lifespan in humans and model organisms (Stekovic et al. 2019; Cipryan et al. 2022). These diets are characterized by their provision of no more than 1200 kcal/day or 50 g/day of carbohydrates, respectively, to induce rapid weight (1.0–2.5 kg/week) and fat loss. To preserve muscle mass and function under these conditions, it is recommended to keep: (i) slower rates of weight loss; (ii) higher protein intakes; (iii) incorporate strength training as part of the exercise program. Indeed, the prescribed exercise for this patient included an endurance intensity component and a strength component, following international recommendations (Bull et al. 2020). Considering that a low-energy diet and exercise have an anti-inflammatory effect, both interventions can be used in the prevention and treatment of chronic and inflammatory conditions (Pedersen 2017; Stekovic et al. 2019; Cipryan et al. 2022). Pharmacological treatments were adjusted to achieve the clinical objectives. Finally, a psychologist addressed the personal and occupational psychosocial factors of the patient linked to his sedentary lifestyle and unhealthy chronic behaviors (Seefeldt et al. 2002; Sassen et al. 2010; Godoy-Izquierdo et al. 2021). Patient’s exposure to health-damaging behaviors and, potentially, chronic stressful experiences, may have led him to an increased allostatic load. A quantitative assessment of allostatic load can be done based on biomarkers and clinical (physical and psychosocial) signs and symptoms. This will allow to understand if the patient is coping well enough with his life situations or may have reached an allostatic overload condition, further increasing his cardiometabolic risk (Fava et al. 2019; Guidi et al. 2021). The aim is to offer the patient a multidisciplinary, comprehensive, personalized evaluation and intervention, which assures long-lasting improvements.
These improvements are gradual and take 3 to 6 months, as observed in the follow-up. The beneficial change in body composition (less fat mass, more lean mass) and in the biochemical profile, and the gains in VO2max, strength, and muscle mass guarantee a reduction in his risk of morbidity and mortality. The observed increase in METs, as part of the CRF of the patient, is in agreement with what was expected (Batacan et al. 2017; Su et al. 2019) and lowers his risk of death from cardiovascular causes by 15% (Kodama et al. 2009). The greatest benefits are reached by people with greater physical cardiorespiratory deconditioning (< 5 METs) when they begin to be physically active (Eijsvogels et al. 2016). Similarly, the patient exceeded the goal of a ≥ 1% increase in global and appendicular lean mass, enough to improve his lipid and glycemic control and reduce his risk of developing metabolic syndrome (Oh et al. 2021).
Proposal for translation
The feasibility of applying several techniques in non-specialized health contexts, along with the possibility offered by them to classify the patients above or below the percentile 25th (P25) based on the published normative data, allow us to propose a simple flowchart to guide the implementation of the routine evaluation of the CRF and muscle status in adults (Fig. 3). The external validity of the epidemiological evidence presented along this review makes this approach suitable for any patient or individual older than 30 years of age from the general population who contacts a primary health carer.
Proposed flowchart for the implementation of the routine evaluation of the cardiorespiratory fitness and muscle status in adults. A wealth of epidemiological, mechanistic and methodological evidence supports that patients or participants from the general population seeking assistance in different primary health scenarios such as clinics, nutrition centres, training and conditioning centres, and personalized health programmes, who are ≥ 30 years old, should be evaluated for their cardiorespiratory fitness (right) and muscle status (left). Start by estimating the VO2max using nonexercise equations and assess muscle status through handgrip and standing and sitting tests. Compare results with normative values according to the subject`s population. If above percentile 25th (P25), reevaluate in one year. If the performance was below P25, complement the evaluation and refer to a medical specialist, who should take action to improve both cardiorespiratory fitness and muscle status so that they surpass P25
Limitations and perspectives
Given the lack of longitudinal studies to generate normative values and given that the benefit is greater than the risk, it is sensible to use cross-sectional studies to evaluate and monitor physical capacity. Validation studies of the nonexercise prediction equations should be done to estimate VO2max in different populations, with a view to using them in the first level of health care. Unfortunately, the emphasis of the literature on the diagnosis of sarcopenia has caused much information from population groups to be reported only with means and cutoff values of 2 or more standard deviations (Wigodski et al. 2019; Lee et al. 2019; Alrashdan et al. 2021; Gonzalez et al. 2021), losing the utility for assessment, monitoring, and early intervention in the general population. The way to measure grip strength should still be better standardized and unified around the world. Great efforts should be made to generate FFAT-corrected normative values of lean mass, particularly of the appendicular mass index, and they should be presented in percentiles. More studies on muscle quality should be carried out to generate simple measurement protocols and reference values for different populations worldwide. The future development and standardization of the biochemical measurement of some myokines will open the door to the evaluation of muscle endocrine function as part of the battery tests to assess the muscle status more comprehensively. It is necessary to estimate in longer longitudinal studies the impact of a gain in physical capacity on cardiometabolic outcomes and mortality. People living with disabilities (physical, mental, sensory, or intellectual) deserve special attention because they are at a greater risk of injury and of developing non-communicable chronic diseases and age-related health conditions at earlier ages. Those people have lower physical fitness and poorer health than the general population. In these adults, it is a priority to assess their CRF and muscle status, closely follow them up and implement intervention strategies to improve their condition (Martin Ginis et al. 2021). Health schools around the world are invited to include training modules on the importance of evaluating CRF and muscle status in the general population. This should be accompanied by a strategy to boost practitioners’ skills at assessing the results of paraclinical tests and adequately prescribing measures that improve the altered parameters.
In health and care settings, a recent study of the implementation of routine measurement of grip strength in the elderly showed that it is cheap and can be successful when there are highly motivated advisors and instructors who are integrated with health personnel with whom they have a shared commitment. High turnover of personnel involved in the implementation strategy should be avoided (Ibrahim et al. 2018). Despite this progress, it is clear that the loss of functional reserve and the deterioration in CRF and muscle status begin decades before a person reaches old age. The knowledge derived from these pioneering experiences will allow us to extend the implementation of the measures discussed here to other healthcare settings from early adulthood (Fig. 3), improving the morbidity and mortality of the general population.
Conclusions
Many high-quality studies show that CRF and muscle status are determinants of morbidity and mortality in multiple population groups over a wide range of ages in both sexes, justifying the generalization and applicability of the results to those ≥ 30 years old. Because they are robust indicators of health status, with prognostic value, CRF and muscle status should be routinely evaluated in primary health practice. The main parameter that reflects CRF is VO2max. The best and more practical indicators of muscle status are grip strength, appendicular mass, and performance in functional tests such as sit-and-stand test. The availability of protocols and techniques that are easy to apply allows us to start evaluating CRF and muscle status at the first level of health care without further delay. Epidemiological evidence permits us to propose P25 as a cutoff to take actions. The evaluation of the muscle status must not be limited to the assessment of the diagnostic criteria for sarcopenia.
Data availability
Not applicable.
Code availability
Not applicable.
Abbreviations
- AKT:
-
Serine/threonine protein kinase akt
- AMPK:
-
5′-AMP-activated protein kinase
- avO2 :
-
Arteriovenous oxygen difference
- BIA:
-
Bioelectrical impedance analysis
- CHI3-L1:
-
Chitinase-3-like protein 1
- CV:
-
Coefficient of variation
- DXA:
-
Dual-energy X-ray absorptiometry
- FoxO1:
-
Forkhead box protein O1
- GLUT-4:
-
Solute carrier family 2, facilitated glucose transporter member 4
- ICC:
-
Intraclass correlation coefficient
- IR:
-
Insulin resistance
- MET:
-
Metabolic equivalents
- NMR:
-
Nuclear magnetic resonance
- Q:
-
Cardiac output
- VCO2 :
-
Carbon dioxide production
- VE:
-
Minute ventilation
- VO2max :
-
Maximal oxygen uptake
- 1H NMRS:
-
Nuclear magnetic resonance spectroscopy
References
Abe T, Bell ZW, Dankel SJ, Wong V, Spitz RW, Loenneke JP (2020a) The water-fat separation method for determining the fat-free component of subcutaneous adipose tissue in humans: a brief review. J Clin Densitom 23(3):390–394. https://doi.org/10.1016/j.jocd.2018.12.007
Abe T, Dankel SJ, Bell ZW, Fujita E, Yaginuma Y, Akamine T, Spitz RW, Wong V, Viana RB, Loenneke JP (2020b) Impact of fat-free adipose tissue on the prevalence of low muscle mass estimated using calf circumference in middle-aged and older adults. J Frailty Aging 9(2):90–93. https://doi.org/10.14283/jfa.2019.34
Abe T, Loenneke JP, Thiebaud RS, Fujita E, Akamine T (2019) The impact of DXA-derived fat-free adipose tissue on the prevalence of low muscle mass in older adults. Eur J Clin Nutr 73(5):757–762. https://doi.org/10.1038/s41430-018-0213-z
Ahima RS, Park HK (2015) Connecting myokines and metabolism. Endocrinol Metab (seoul) 30(3):235–245. https://doi.org/10.3803/EnM.2015.30.3.235
Al-Gindan YY, Hankey CR, Leslie W, Govan L, Lean ME (2014) Predicting muscle mass from anthropometry using magnetic resonance imaging as reference: a systematic review. Nutr Rev 72(2):113–126. https://doi.org/10.1111/nure.12096
Aleman-Mateo H, Ruiz Valenzuela RE (2014) Skeletal muscle mass indices in healthy young Mexican adults aged 20–40 years: implications for diagnoses of sarcopenia in the elderly population. ScientificWorld J 2014:672158. https://doi.org/10.1155/2014/672158
Alrashdan A, Ghaleb AM, Almobarek M (2021) Normative static grip strength of Saudi Arabia’s population and influences of numerous factors on grip strength. Healthcare (basel) 9(12):1647. https://doi.org/10.3390/healthcare9121647
Arcila E, Restrepo C, Valbuena L, Quintero MA, Marino F, Osorio JA, Gallo-Villegas J, Saldarriaga JF (2022) Validity and reproducibility of a method to estimate cardiorespiratory fitness in college adults. Biomedica 42(4):611–622. https://doi.org/10.7705/biomedica.6404
Arena R, Guazzi M, Cahalin LP, Myers J (2014) Revisiting cardiopulmonary exercise testing applications in heart failure: aligning evidence with clinical practice. Exerc Sport Sci Rev 42(4):153–160. https://doi.org/10.1249/JES.0000000000000022
Argiles JM, Campos N, Lopez-Pedrosa JM, Rueda R, Rodriguez-Manas L (2016) Skeletal muscle regulates metabolism via interorgan crosstalk: roles in health and disease. J Am Med Dir Assoc 17(9):789–796. https://doi.org/10.1016/j.jamda.2016.04.019
Aristizabal JC, Montoya E, Sanchez YL, Yepes-Calderon M, Narvaez-Sanchez R, Gallo-Villegas JA, Calderón JC (2021) Effects of low-volume, high-intensity interval training compared with continuous training on regional and global body composition in adults with metabolic syndrome: a post hoc analysis of a randomized clinical trial. Ann Nutr Metab 77(5):279–288. https://doi.org/10.1159/000518909
Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA, Members of the Florey Adelaide Male Ageing S (2009) Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism 58(7):1013–1022. https://doi.org/10.1016/j.metabol.2009.02.027
Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV, American Heart Association Exercise CR, Prevention Committee of the Council on Clinical C, Council on E, Prevention, Council on Peripheral Vascular D, Interdisciplinary Council on Quality of C, Outcomes R (2010) Clinician’s Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation 122(2):191–225. https://doi.org/10.1161/CIR.0b013e3181e52e69
Banzet S, Koulmann N, Sanchez H, Serrurier B, Peinnequin A, Bigard AX (2007) Musclin gene expression is strongly related to fast-glycolytic phenotype. Biochem Biophys Res Commun 353(3):713–718. https://doi.org/10.1016/j.bbrc.2006.12.074
Batacan RB Jr, Duncan MJ, Dalbo VJ, Tucker PS, Fenning AS (2017) Effects of high-intensity interval training on cardiometabolic health: a systematic review and meta-analysis of intervention studies. Br J Sports Med 51(6):494–503. https://doi.org/10.1136/bjsports-2015-095841
Bay ML, Pedersen BK (2020) Muscle-organ crosstalk: focus on immunometabolism. Front Physiol 11:567881. https://doi.org/10.3389/fphys.2020.567881
Besse-Patin A, Montastier E, Vinel C, Castan-Laurell I, Louche K, Dray C, Daviaud D, Mir L, Marques MA, Thalamas C, Valet P, Langin D, Moro C, Viguerie N (2014) Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. Int J Obes (lond) 38(5):707–713. https://doi.org/10.1038/ijo.2013.158
Blair SN, Kohl HW 3rd, Barlow CE, Paffenbarger RS Jr, Gibbons LW, Macera CA (1995) Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA 273(14):1093–1098
Blair SN, Kohl HW 3rd, Paffenbarger RS Jr, Clark DG, Cooper KH, Gibbons LW (1989) Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262(17):2395–2401
Bohannon RW (2008) Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther 31(1):3–10. https://doi.org/10.1519/00139143-200831010-00002
Bohannon RW, Bubela DJ, Magasi SR, Wang YC, Gershon RC (2010) Sit-to-stand test: performance and determinants across the age-span. Isokinet Exerc Sci 18(4):235–240. https://doi.org/10.3233/IES-2010-0389
Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM (2012) A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481(7382):463–468. https://doi.org/10.1038/nature10777
Bottinelli R, Pellegrino MA, Canepari M, Rossi R, Reggiani C (1999) Specific contributions of various muscle fibre types to human muscle performance: an in vitro study. J Electromyogr Kinesiol 9(2):87–95. https://doi.org/10.1016/s1050-6411(98)00040-6
Bottinelli R, Reggiani C (2000) Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol 73(2–4):195–262. https://doi.org/10.1016/s0079-6107(00)00006-7
Bouchard C, An P, Rice T, Skinner JS, Wilmore JH, Gagnon J, Perusse L, Leon AS, Rao DC (1999) Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. J Appl Physiol (1985) 87(3):1003–1008. https://doi.org/10.1152/jappl.1999.87.3.1003
Bruce RA, Kusumi F, Hosmer D (1973) Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J 85(4):546–562. https://doi.org/10.1016/0002-8703(73)90502-4
Buffa R, Mereu E, Comandini O, Ibanez ME, Marini E (2014) Bioelectrical impedance vector analysis (BIVA) for the assessment of two-compartment body composition. Eur J Clin Nutr 68(11):1234–1240. https://doi.org/10.1038/ejcn.2014.170
Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, Carty C, Chaput JP, Chastin S, Chou R, Dempsey PC, DiPietro L, Ekelund U, Firth J, Friedenreich CM, Garcia L, Gichu M, Jago R, Katzmarzyk PT, Lambert E, Leitzmann M, Milton K, Ortega FB, Ranasinghe C, Stamatakis E, Tiedemann A, Troiano RP, van der Ploeg HP, Wari V, Willumsen JF (2020) World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 54(24):1451–1462. https://doi.org/10.1136/bjsports-2020-102955
Chen WJ, Liu Y, Sui YB, Zhang B, Zhang XH, Yin XH (2017) Increased circulating levels of musclin in newly diagnosed type 2 diabetic patients. Diab Vasc Dis Res 14(2):116–121. https://doi.org/10.1177/1479164116675493
Chin SO, Rhee SY, Chon S, Hwang YC, Jeong IK, Oh S, Ahn KJ, Chung HY, Woo JT, Kim SW, Kim JW, Kim YS, Ahn HY (2013) Sarcopenia is independently associated with cardiovascular disease in older Korean adults: the Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PLoS ONE 8(3):e60119. https://doi.org/10.1371/journal.pone.0060119
Church TS, Cheng YJ, Earnest CP, Barlow CE, Gibbons LW, Priest EL, Blair SN (2004) Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care 27(1):83–88. https://doi.org/10.2337/diacare.27.1.83
Cipryan L, Litschmannova M, Maffetone PB, Plews DJ, Dostal T, Hofmann P, Laursen PB (2022) Very low-carbohydrate high-fat diet improves risk markers for cardiometabolic health more than exercise in men and women with overfat constitution: secondary analysis of a randomized controlled clinical trial. Front Nutr 9:867690. https://doi.org/10.3389/fnut.2022.867690
Cohen DD, Gomez-Arbelaez D, Camacho PA, Pinzon S, Hormiga C, Trejos-Suarez J, Duperly J, Lopez-Jaramillo P (2014) Low muscle strength is associated with metabolic risk factors in Colombian children: the ACFIES study. PLoS ONE 9(4):e93150. https://doi.org/10.1371/journal.pone.0093150
Cooper KH (1968) A means of assessing maximal oxygen intake. Correlation between field and treadmill testing. JAMA 203(3):201–204
Cooper R, Kuh D, Hardy R, Mortality Review G, Falcon, Teams HAS (2010) Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ 341:c4467. https://doi.org/10.1136/bmj.c4467
Cooper R, Strand BH, Hardy R, Patel KV, Kuh D (2014) Physical capability in mid-life and survival over 13 years of follow-up: British birth cohort study. BMJ 348:g2219. https://doi.org/10.1136/bmj.g2219
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M, Writing Group for the European Working Group on Sarcopenia in Older P, the Extended Group for E (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48(1):16–31. https://doi.org/10.1093/ageing/afy169
Cuenca-Garcia M, Marin-Jimenez N, Perez-Bey A, Sanchez-Oliva D, Camiletti-Moiron D, Alvarez-Gallardo IC, Ortega FB, Castro-Pinero J (2022) Reliability of field-based fitness tests in adults: a systematic review. Sports Med 52(8):1961–1979. https://doi.org/10.1007/s40279-021-01635-2
Daugaard JR, Nielsen JN, Kristiansen S, Andersen JL, Hargreaves M, Richter EA (2000) Fiber type-specific expression of GLUT4 in human skeletal muscle: influence of exercise training. Diabetes 49(7):1092–1095. https://doi.org/10.2337/diabetes.49.7.1092
Davies CT (1968) Limitations to the prediction of maximum oxygen intake from cardiac frequency measurements. J Appl Physiol 24(5):700–706. https://doi.org/10.1152/jappl.1968.24.5.700
de Courten B, Kurdiova T, de Courten MP, Belan V, Everaert I, Vician M, Teede H, Gasperikova D, Aldini G, Derave W, Ukropec J, Ukropcova B (2015) Muscle carnosine is associated with cardiometabolic risk factors in humans. PLoS ONE 10(10):e0138707. https://doi.org/10.1371/journal.pone.0138707
de Santana FM, Premaor MO, Tanigava NY, Pereira RMR (2021) Low muscle mass in older adults and mortality: A systematic review and meta-analysis. Exp Gerontol 152:111461. https://doi.org/10.1016/j.exger.2021.111461
DeFronzo RA (1988) Lilly lecture 1987 The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37(6):667–687
Deng Y, Tang Z (2012) Study on the effect of an intervention with aerobic exercise on the musclin content in serum of rats with diabetes type 2 [Translated from Chinese]. Zhejiang Sport Sci 34(2):3
Dinas PC, Lahart IM, Timmons JA, Svensson PA, Koutedakis Y, Flouris AD, Metsios GS (2017) Effects of physical activity on the link between PGC-1a and FNDC5 in muscle, circulating Iotarisin and UCP1 of white adipocytes in humans: a systematic review. F1000Res 6:286. https://doi.org/10.12688/f1000research.11107.2
Dodds RM, Syddall HE, Cooper R, Benzeval M, Deary IJ, Dennison EM, Der G, Gale CR, Inskip HM, Jagger C, Kirkwood TB, Lawlor DA, Robinson SM, Starr JM, Steptoe A, Tilling K, Kuh D, Cooper C, Sayer AA (2014) Grip strength across the life course: normative data from twelve British studies. PLoS ONE 9(12):e113637. https://doi.org/10.1371/journal.pone.0113637
Dray C, Knauf C, Daviaud D, Waget A, Boucher J, Buleon M, Cani PD, Attane C, Guigne C, Carpene C, Burcelin R, Castan-Laurell I, Valet P (2008) Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab 8(5):437–445. https://doi.org/10.1016/j.cmet.2008.10.003
Eijsvogels TM, Molossi S, Lee DC, Emery MS, Thompson PD (2016) Exercise at the extremes: the amount of exercise to reduce cardiovascular events. J Am Coll Cardiol 67(3):316–329. https://doi.org/10.1016/j.jacc.2015.11.034
Ezzatvar Y, Izquierdo M, Nunez J, Calatayud J, Ramirez-Velez R, Garcia-Hermoso A (2021a) Cardiorespiratory fitness measured with cardiopulmonary exercise testing and mortality in patients with cardiovascular disease: a systematic review and meta-analysis. J Sport Health Sci 10(6):609–619. https://doi.org/10.1016/j.jshs.2021.06.004
Ezzatvar Y, Ramirez-Velez R, Saez de Asteasu ML, Martinez-Velilla N, Zambom-Ferraresi F, Lobelo F, Izquierdo M, Garcia-Hermoso A (2021b) Cardiorespiratory fitness and all-cause mortality in adults diagnosed with cancer systematic review and meta-analysis. Scand J Med Sci Sports 31(9):1745–1752. https://doi.org/10.1111/sms.13980
Faselis C, Doumas M, Pittaras A, Narayan P, Myers J, Tsimploulis A, Kokkinos P (2014) Exercise capacity and all-cause mortality in male veterans with hypertension aged >/=70 years. Hypertension 64(1):30–35. https://doi.org/10.1161/HYPERTENSIONAHA.114.03510
Fava GA, McEwen BS, Guidi J, Gostoli S, Offidani E, Sonino N (2019) Clinical characterization of allostatic overload. Psychoneuroendocrinology 108:94–101. https://doi.org/10.1016/j.psyneuen.2019.05.028
Fisher G, Windham ST, Griffin P, Warren JL, Gower BA, Hunter GR (2017) Associations of human skeletal muscle fiber type and insulin sensitivity, blood lipids, and vascular hemodynamics in a cohort of premenopausal women. Eur J Appl Physiol 117(7):1413–1422. https://doi.org/10.1007/s00421-017-3634-9
Fiuza-Luces C, Garatachea N, Berger NA, Lucia A (2013) Exercise is the real polypill. Physiology (bethesda) 28(5):330–358. https://doi.org/10.1152/physiol.00019.2013
Fiuza-Luces C, Santos-Lozano A, Joyner M, Carrera-Bastos P, Picazo O, Zugaza JL, Izquierdo M, Ruilope LM, Lucia A (2018) Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol 15(12):731–743. https://doi.org/10.1038/s41569-018-0065-1
Fletcher GF, Landolfo C, Niebauer J, Ozemek C, Arena R, Lavie CJ (2018) Promoting physical activity and exercise: JACC Health promotion series. J Am Coll Cardiol 72(14):1622–1639. https://doi.org/10.1016/j.jacc.2018.08.2141
Furushima T, Miyachi M, Iemitsu M, Murakami H, Kawano H, Gando Y, Kawakami R, Sanada K (2017) Development of prediction equations for estimating appendicular skeletal muscle mass in Japanese men and women. J Physiol Anthropol 36(1):34. https://doi.org/10.1186/s40101-017-0150-x
Gallo J, Saldarriaga J, Clavijo M, Arango E, Rodríguez N, Osorio J (2010) Actividad física y salud cardiovascular: en búsqueda de la relación dosis respuesta. 1 edn. Corporación para investigaciones biológicas (CIB), Medellín
Gallo-Villegas J, Castro-Valencia LA, Perez L, Restrepo D, Guerrero O, Cardona S, Sanchez YL, Yepes-Calderon M, Valbuena LH, Pena M, Milan AF, Trillos-Almanza MC, Granados S, Aristizabal JC, Estrada-Castrillon M, Narvaez-Sanchez R, Osorio J, Aguirre-Acevedo DC, Calderón JC (2022) Efficacy of high-intensity interval- or continuous aerobic-training on insulin resistance and muscle function in adults with metabolic syndrome: a clinical trial. Eur J Appl Physiol 122(2):331–344. https://doi.org/10.1007/s00421-021-04835-w
Gao SY, Xia Y, Wu QJ, Chang Q, Zhao YH (2021) Reference values for five-repetition chair stand test among middle-aged and elderly community-dwelling Chinese adults. Front Med (lausanne) 8:659107. https://doi.org/10.3389/fmed.2021.659107
Godoy-Izquierdo D, Lara R, Ogallar A, Rodriguez-Tadeo A, Ramirez MJ, Navarron E, Arbinaga F (2021) Psychosocial and diet-related lifestyle clusters in overweight and obesity. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph18126461
Gonzalez MC, Mehrnezhad A, Razaviarab N, Barbosa-Silva TG, Heymsfield SB (2021) Calf circumference: cutoff values from the NHANES 1999–2006. Am J Clin Nutr 113(6):1679–1687. https://doi.org/10.1093/ajcn/nqab029
Goodpaster BH, He J, Watkins S, Kelley DE (2001) Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86(12):5755–5761. https://doi.org/10.1210/jcem.86.12.8075
Gorgens SW, Hjorth M, Eckardt K, Wichert S, Norheim F, Holen T, Lee S, Langleite T, Birkeland KI, Stadheim HK, Kolnes KJ, Tangen DS, Kolnes AJ, Jensen J, Drevon CA, Eckel J (2016) The exercise-regulated myokine chitinase-3-like protein 1 stimulates human myocyte proliferation. Acta Physiol (oxf) 216(3):330–345. https://doi.org/10.1111/apha.12579
Grgic J, Scapec B, Mikulic P, Pedisic Z (2022) Test-retest reliability of isometric mid-thigh pull maximum strength assessment: a systematic review. Biol Sport 39(2):407–414. https://doi.org/10.5114/biolsport.2022.106149
Gu N, Guo Q, Mao K, Hu H, Jin S, Zhou Y, He H, Oh Y, Liu C, Wu Q (2015) Palmitate increases musclin gene expression through activation of PERK signaling pathway in C2C12 myotubes. Biochem Biophys Res Commun 467(3):521–526. https://doi.org/10.1016/j.bbrc.2015.10.005
Guidi J, Lucente M, Sonino N, Fava GA (2021) Allostatic load and its impact on health: a systematic review. Psychother Psychosom 90(1):11–27. https://doi.org/10.1159/000510696
Guo Q, Hu H, Liu X, Yang D, Yin Y, Zhang B, He H, Oh Y, Wu Q, Liu C, Gu N (2019) C/EBPbeta mediates palmitate-induced musclin expression via the regulation of PERK/ATF4 pathways in myotubes. Am J Physiol Endocrinol Metab 316(6):E1081–E1092. https://doi.org/10.1152/ajpendo.00478.2018
Haines MS, Leong A, Porneala BC, Meigs JB, Miller KK (2022) Association between muscle mass and diabetes prevalence independent of body fat distribution in adults under 50 years old. Nutr Diabetes 12(1):29. https://doi.org/10.1038/s41387-022-00204-4
Han F, Zhang S, Hou N, Wang D, Sun X (2015) Irisin improves endothelial function in obese mice through the AMPK-eNOS pathway. Am J Physiol Heart Circ Physiol 309(9):H1501-1508. https://doi.org/10.1152/ajpheart.00443.2015
Han M, Qie R, Shi X, Yang Y, Lu J, Hu F, Zhang M, Zhang Z, Hu D, Zhao Y (2022) Cardiorespiratory fitness and mortality from all causes, cardiovascular disease and cancer: dose-response meta-analysis of cohort studies. Br J Sports Med. https://doi.org/10.1136/bjsports-2021-104876
Harber MP, Kaminsky LA, Arena R, Blair SN, Franklin BA, Myers J, Ross R (2017) Impact of cardiorespiratory fitness on all-cause and disease-specific mortality: advances since 2009. Prog Cardiovasc Dis 60(1):11–20. https://doi.org/10.1016/j.pcad.2017.03.001
Heymsfield SB, Stanley A, Pietrobelli A, Heo M (2020) Simple skeletal muscle mass estimation formulas: what we can learn from them. Front Endocrinol (lausanne) 11:31. https://doi.org/10.3389/fendo.2020.00031
Hou N, Du G, Han F, Zhang J, Jiao X, Sun X (2017) Irisin regulates heme oxygenase-1/adiponectin axis in perivascular adipose tissue and improves endothelial dysfunction in diet-induced obese mice. Cell Physiol Biochem 42(2):603–614. https://doi.org/10.1159/000477864
Hou N, Han F, Sun X (2015) The relationship between circulating irisin levels and endothelial function in lean and obese subjects. Clin Endocrinol (oxf) 83(3):339–343. https://doi.org/10.1111/cen.12658
Hou N, Liu Y, Han F, Wang D, Hou X, Hou S, Sun X (2016) Irisin improves perivascular adipose tissue dysfunction via regulation of the heme oxygenase-1/adiponectin axis in diet-induced obese mice. J Mol Cell Cardiol 99:188–196. https://doi.org/10.1016/j.yjmcc.2016.09.005
Ibrahim K, May CR, Patel HP, Baxter M, Sayer AA, Roberts HC (2018) Implementation of grip strength measurement in medicine for older people wards as part of routine admission assessment: identifying facilitators and barriers using a theory-led intervention. BMC Geriatr 18(1):79. https://doi.org/10.1186/s12877-018-0768-5
Israel A, Kivity S, Sidi Y, Segev S, Berkovitch A, Klempfner R, Lavi B, Goldenberg I, Maor E (2016) Use of exercise capacity to improve SCORE risk prediction model in asymptomatic adults. Eur Heart J 37(29):2300–2306. https://doi.org/10.1093/eurheartj/ehw053
Jackson AS, Blair SN, Mahar MT, Wier LT, Ross RM, Stuteville JE (1990) Prediction of functional aerobic capacity without exercise testing. Med Sci Sports Exerc 22(6):863–870. https://doi.org/10.1249/00005768-199012000-00021
Jackson AS, Sui X, O’Connor DP, Church TS, Lee DC, Artero EG, Blair SN (2012) Longitudinal cardiorespiratory fitness algorithms for clinical settings. Am J Prev Med 43(5):512–519. https://doi.org/10.1016/j.amepre.2012.06.032
Jacob S, Machann J, Rett K, Brechtel K, Volk A, Renn W, Maerker E, Matthaei S, Schick F, Claussen CD, Haring HU (1999) Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes 48(5):1113–1119. https://doi.org/10.2337/diabetes.48.5.1113
Jefferis BJ, Whincup PH, Papacosta O, Wannamethee SG (2014) Protective effect of time spent walking on risk of stroke in older men. Stroke 45(1):194–199. https://doi.org/10.1161/STROKEAHA.113.002246
Kaminsky LA, Arena R, Beckie TM, Brubaker PH, Church TS, Forman DE, Franklin BA, Gulati M, Lavie CJ, Myers J, Patel MJ, Pina IL, Weintraub WS, Williams MA, American Heart Association Advocacy Coordinating Committee CoCC, Council on Nutrition PA, Metabolism (2013) The importance of cardiorespiratory fitness in the United States: the need for a national registry: a policy statement from the American Heart Association. Circulation 127(5):652–662. https://doi.org/10.1161/CIR.0b013e31827ee100
Kaminsky LA, Arena R, Ellingsen O, Harber MP, Myers J, Ozemek C, Ross R (2019a) Cardiorespiratory fitness and cardiovascular disease - the past, present, and future. Prog Cardiovasc Dis 62(2):86–93. https://doi.org/10.1016/j.pcad.2019.01.002
Kaminsky LA, Arena R, Myers J (2015) Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: data from the fitness registry and the importance of exercise national database. Mayo Clin Proc 90(11):1515–1523. https://doi.org/10.1016/j.mayocp.2015.07.026
Kaminsky LA, Arena R, Myers J, Peterman JE, Bonikowske AR, Harber MP, Medina Inojosa JR, Lavie CJ, Squires RW (2022) Updated reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: data from the fitness registry and the importance of exercise national database (FRIEND). Mayo Clin Proc 97(2):285–293. https://doi.org/10.1016/j.mayocp.2021.08.020
Kaminsky LA, Myers J, Arena R (2019b) Determining cardiorespiratory fitness with precision: compendium of findings from the FRIEND registry. Prog Cardiovasc Dis 62(1):76–82. https://doi.org/10.1016/j.pcad.2018.10.003
Kawakami R, Miyachi M, Tanisawa K, Ito T, Usui C, Midorikawa T, Torii S, Ishii K, Suzuki K, Sakamoto S, Higuchi M, Muraoka I, Oka K (2021) Development and validation of a simple anthropometric equation to predict appendicular skeletal muscle mass. Clin Nutr 40(11):5523–5530. https://doi.org/10.1016/j.clnu.2021.09.032
Kim HK, Kim CH (2021) Quality matters as much as quantity of skeletal muscle: clinical implications of myosteatosis in cardiometabolic health. Endocrinol Metab (seoul) 36(6):1161–1174. https://doi.org/10.3803/EnM.2021.1348
Kim YH, So WY (2016) A low arm and leg muscle mass to total body weight ratio is associated with an increased prevalence of metabolic syndrome: The Korea National Health and Nutrition Examination Survey 2010–2011. Technol Health Care 24(5):655–663. https://doi.org/10.3233/THC-161162
Kligfield P, Lauer MS (2006) Exercise electrocardiogram testing: beyond the ST segment. Circulation 114(19):2070–2082. https://doi.org/10.1161/CIRCULATIONAHA.105.561944
Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H (2009) Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 301(19):2024–2035. https://doi.org/10.1001/jama.2009.681
Kokkinos PF, Faselis C, Myers J, Panagiotakos D, Doumas M (2013) Interactive effects of fitness and statin treatment on mortality risk in veterans with dyslipidaemia: a cohort study. Lancet 381(9864):394–399. https://doi.org/10.1016/S0140-6736(12)61426-3
Kondamudi N, Mehta A, Thangada ND, Pandey A (2021) Physical activity and cardiorespiratory fitness: vital signs for cardiovascular risk assessment. Curr Cardiol Rep 23(11):172. https://doi.org/10.1007/s11886-021-01596-y
Kujala UM (2009) Evidence on the effects of exercise therapy in the treatment of chronic disease. Br J Sports Med 43(8):550–555. https://doi.org/10.1136/bjsm.2009.059808
Kwak SY, Seo IH, Chung I, Kim SA, Lee JO, Lee HJ, Kim SE, Han JA, Kang MJ, Kim SJ, Lim S, Kim KM, Chung JH, Lim E, Hwang JI, Kim HS, Shin MJ (2020) Effect of chitinase-3-like protein 1 on glucose metabolism: In vitro skeletal muscle and human genetic association study. FASEB J 34(10):13445–13460. https://doi.org/10.1096/fj.202000925R
Laboratories ATSCoPSfCPF (2002) ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 166(1):111–117. https://doi.org/10.1164/ajrccm.166.1.at1102
Landi F, Calvani R, Martone AM, Salini S, Zazzara MB, Candeloro M, Coelho-Junior HJ, Tosato M, Picca A, Marzetti E (2020) Normative values of muscle strength across ages in a “real world” population: results from the longevity check-up 7+ project. J Cachexia Sarcopenia Muscle 11(6):1562–1569. https://doi.org/10.1002/jcsm.12610
Larsen B, Bellettiere J, Allison M, McClelland RL, Miljkovic I, Vella CA, Ouyang P, De-Guzman KR, Criqui M, Unkart J (2020) Muscle area and density and risk of all-cause mortality: the multi-ethnic study of atherosclerosis. Metabolism 111:154321. https://doi.org/10.1016/j.metabol.2020.154321
Lee J (2021) Influence of cardiorespiratory fitness on risk of dementia and dementia mortality: a systematic review and meta-analysis of prospective cohort studies. J Aging Phys Act 29(5):878–885. https://doi.org/10.1123/japa.2019-0493
Lee DC, Sui X, Church TS, Lee IM, Blair SN (2009) Associations of cardiorespiratory fitness and obesity with risks of impaired fasting glucose and type 2 diabetes in men. Diabetes Care 32(2):257–262. https://doi.org/10.2337/dc08-1377
Lee DH, Keum N, Hu FB, Orav EJ, Rimm EB, Sun Q, Willett WC, Giovannucci EL (2017) Development and validation of anthropometric prediction equations for lean body mass, fat mass and percent fat in adults using the National Health and Nutrition Examination Survey (NHANES) 1999–2006. Br J Nutr 118(10):858–866. https://doi.org/10.1017/S0007114517002665
Lee RC, Wang Z, Heo M, Ross R, Janssen I, Heymsfield SB (2000) Total-body skeletal muscle mass: development and cross-validation of anthropometric prediction models. Am J Clin Nutr 72(3):796–803. https://doi.org/10.1093/ajcn/72.3.796
Lee SY, Ahn S, Kim YJ, Ji MJ, Kim KM, Choi SH, Jang HC, Lim S (2018) Comparison between dual-energy X-ray absorptiometry and bioelectrical impedance analyses for accuracy in measuring whole body muscle mass and appendicular skeletal muscle mass. Nutrients 10(6):738. https://doi.org/10.3390/nu10060738
Lee YL, Lee BH, Lee SY (2019) Handgrip strength in the Korean population: normative data and cutoff values. Ann Geriatr Med Res 23(4):183–189. https://doi.org/10.4235/agmr.19.0042
Leong DP, Teo KK, Rangarajan S, Kutty VR, Lanas F, Hui C, Quanyong X, Zhenzhen Q, Jinhua T, Noorhassim I, AlHabib KF, Moss SJ, Rosengren A, Akalin AA, Rahman O, Chifamba J, Orlandini A, Kumar R, Yeates K, Gupta R, Yusufali A, Dans A, Avezum A, Lopez-Jaramillo P, Poirier P, Heidari H, Zatonska K, Iqbal R, Khatib R, Yusuf S (2016) Reference ranges of handgrip strength from 125,462 healthy adults in 21 countries: a prospective urban rural epidemiologic (PURE) study. J Cachexia Sarcopenia Muscle 7(5):535–546. https://doi.org/10.1002/jcsm.12112
Leong DP, Teo KK, Rangarajan S, Lopez-Jaramillo P, Avezum A Jr, Orlandini A, Seron P, Ahmed SH, Rosengren A, Kelishadi R, Rahman O, Swaminathan S, Iqbal R, Gupta R, Lear SA, Oguz A, Yusoff K, Zatonska K, Chifamba J, Igumbor E, Mohan V, Anjana RM, Gu H, Li W, Yusuf S, Prospective Urban Rural Epidemiology Study i (2015) Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 386(9990):266–273. https://doi.org/10.1016/S0140-6736(14)62000-6
Levin MC, Monetti M, Watt MJ, Sajan MP, Stevens RD, Bain JR, Newgard CB, Farese RV Sr, Farese RV Jr (2007) Increased lipid accumulation and insulin resistance in transgenic mice expressing DGAT2 in glycolytic (type II) muscle. Am J Physiol Endocrinol Metab 293(6):E1772-1781. https://doi.org/10.1152/ajpendo.00158.2007
Li R, Steyn FJ, Stout MB, Lee K, Cully TR, Calderón JC, Ngo ST (2016) Development of a high-throughput method for real-time assessment of cellular metabolism in intact long skeletal muscle fibre bundles. J Physiol 594(24):7197–7213. https://doi.org/10.1113/JP272988
Li Y, Xu S, Zhang X, Yi Z, Cichello S (2015) Skeletal intramyocellular lipid metabolism and insulin resistance. Biophys Rep 1:90–98. https://doi.org/10.1007/s41048-015-0013-0
Li Z, Yang YL, Zhu YJ, Li CG, Tang YZ, Ni CL, Chen LM, Niu WY (2021) Circulating serum myonectin levels in obesity and type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 129(7):528–534. https://doi.org/10.1055/a-0896-8548
Little HC, Rodriguez S, Lei X, Tan SY, Stewart AN, Sahagun A, Sarver DC, Wong GW (2019) Myonectin deletion promotes adipose fat storage and reduces liver steatosis. FASEB J 33(7):8666–8687. https://doi.org/10.1096/fj.201900520R
Liu Y, Huo X, Pang XF, Zong ZH, Meng X, Liu GL (2008) Musclin inhibits insulin activation of Akt/protein kinase B in rat skeletal muscle. J Int Med Res 36(3):496–504
Londono FJ, Calderón JC, Gallo J (2012) Association between thigh muscle development and the metabolic syndrome in adults. Ann Nutr Metab 61(1):41–46. https://doi.org/10.1159/000339267
Lopez-Jaramillo P, Cohen DD, Gomez-Arbelaez D, Bosch J, Dyal L, Yusuf S, Gerstein HC, Investigators OT (2014) Association of handgrip strength to cardiovascular mortality in pre-diabetic and diabetic patients: a subanalysis of the ORIGIN trial. Int J Cardiol 174(2):458–461. https://doi.org/10.1016/j.ijcard.2014.04.013
Lund S, Holman GD, Schmitz O, Pedersen O (1995) Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc Natl Acad Sci USA 92(13):5817–5821
Mackrell JG, Cartee GD (2012) A novel method to measure glucose uptake and myosin heavy chain isoform expression of single fibers from rat skeletal muscle. Diabetes 61(5):995–1003. https://doi.org/10.2337/db11-1299
Maranhao Neto Gde A, Lourenco PM, Farinatti Pde T (2004) Prediction of aerobic fitness without stress testing and applicability to epidemiological studies: a systematic review. Cad Saude Publica 20(1):48–56. https://doi.org/10.1590/s0102-311x2004000100018
Martin Ginis KA, van der Ploeg HP, Foster C, Lai B, McBride CB, Ng K, Pratt M, Shirazipour CH, Smith B, Vasquez PM, Heath GW (2021) Participation of people living with disabilities in physical activity: a global perspective. Lancet 398(10298):443–455. https://doi.org/10.1016/S0140-6736(21)01164-8
Martinez-Torres J, Gallo-Villegas JA, Aguirre-Acevedo DC (2022) Normative values for handgrip strength in Colombian children and adolescents from 6 to 17 years of age: estimation using quantile regression. J Pediatr (Rio J) 98(6):590–598. https://doi.org/10.1016/j.jped.2022.02.004
Mazur-Bialy AI, Bilski J, Pochec E, Brzozowski T (2017) New insight into the direct anti-inflammatory activity of a myokine irisin against proinflammatory activation of adipocytes. Implication for exercise in obesity. J Physiol Pharmacol 68(2):243–251
Mi Q, Li Y, Wang M, Yang G, Zhao X, Liu H, Zheng H, Li L (2019) Circulating C1q/TNF-related protein isoform 15 is a marker for the presence of metabolic syndrome. Diabetes Metab Res Rev 35(1):e3085. https://doi.org/10.1002/dmrr.3085
Mora S, Redberg RF, Sharrett AR, Blumenthal RS (2005) Enhanced risk assessment in asymptomatic individuals with exercise testing and Framingham risk scores. Circulation 112(11):1566–1572. https://doi.org/10.1161/CIRCULATIONAHA.105.542993
Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE (2002) Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 346(11):793–801. https://doi.org/10.1056/NEJMoa011858
Narvaez-Sanchez R, Calderón JC, Vega G, Trillos MC, Ospina S (2019) Skeletal muscle as a protagonist in the pregnancy metabolic syndrome. Med Hypotheses 126:26–37. https://doi.org/10.1016/j.mehy.2019.02.049
Nes BM, Janszky I, Vatten LJ, Nilsen TI, Aspenes ST, Wisloff U (2011) Estimating V.O 2peak from a nonexercise prediction model: the HUNT Study, Norway. Med Sci Sports Exerc 43(11):2024–2030. https://doi.org/10.1249/MSS.0b013e31821d3f6f
Nielsen AR, Mounier R, Plomgaard P, Mortensen OH, Penkowa M, Speerschneider T, Pilegaard H, Pedersen BK (2007) Expression of interleukin-15 in human skeletal muscle effect of exercise and muscle fibre type composition. J Physiol 584(Pt 1):305–312. https://doi.org/10.1113/jphysiol.2007.139618
Nishizawa H, Matsuda M, Yamada Y, Kawai K, Suzuki E, Makishima M, Kitamura T, Shimomura I (2004) Musclin, a novel skeletal muscle-derived secretory factor. J Biol Chem 279(19):19391–19395. https://doi.org/10.1074/jbc.C400066200
Oh YH, Choi S, Lee G, Son JS, Kim KH, Park SM (2021) Changes in body composition are associated with metabolic changes and the risk of metabolic syndrome. J Clin Med 10(4):745. https://doi.org/10.3390/jcm10040745
Olsen DB, Sacchetti M, Dela F, Ploug T, Saltin B (2005) Glucose clearance is higher in arm than leg muscle in type 2 diabetes. J Physiol 565(Pt 2):555–562. https://doi.org/10.1113/jphysiol.2004.081356
Orange ST, Marshall P, Madden LA, Vince RV (2019) Can sit-to-stand muscle power explain the ability to perform functional tasks in adults with severe obesity? J Sports Sci 37(11):1227–1234. https://doi.org/10.1080/02640414.2018.1553500
Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK (1999) Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol 515(Pt 1):287–291. https://doi.org/10.1111/j.1469-7793.1999.287ad.x
Pantiya P, Thonusin C, Sumneang N, Ongnok B, Chunchai T, Kerdphoo S, Jaiwongkam T, Arunsak B, Siri-Angkul N, Sriwichaiin S, Chattipakorn N, Chattipakorn SC (2022) High cardiorespiratory fitness protects against molecular impairments of metabolism, heart, and brain with higher efficacy in obesity-induced premature aging. Endocrinol Metab (seoul) 37(4):630–640. https://doi.org/10.3803/EnM.2022.1430
Park BS, Yoon JS (2013) Relative skeletal muscle mass is associated with development of metabolic syndrome. Diabetes Metab J 37(6):458–464. https://doi.org/10.4093/dmj.2013.37.6.458
Pedersen BK (2017) Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Invest 47(8):600–611. https://doi.org/10.1111/eci.12781
Pedersen BK, Saltin B (2006) Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports 16(Suppl 1):3–63. https://doi.org/10.1111/j.1600-0838.2006.00520.x
Pedersen BK, Steensberg A, Keller P, Keller C, Fischer C, Hiscock N, van Hall G, Plomgaard P, Febbraio MA (2003) Muscle-derived interleukin-6: lipolytic, anti-inflammatory and immune regulatory effects. Pflugers Arch 446(1):9–16. https://doi.org/10.1007/s00424-002-0981-z
Peterman JE, Arena R, Myers J, Marzolini S, Ross R, Lavie CJ, Wisloff U, Stensvold D, Kaminsky LA (2020) Development of global reference standards for directly measured cardiorespiratory fitness: a report from the fitness registry and importance of exercise national database (FRIEND). Mayo Clin Proc 95(2):255–264. https://doi.org/10.1016/j.mayocp.2019.06.013
Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI (2004) Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350(7):664–671. https://doi.org/10.1056/NEJMoa031314
Qiu S, Cai X, Sun Z, Wu T, Schumann U (2021) Is estimated cardiorespiratory fitness an effective predictor for cardiovascular and all-cause mortality? A meta-analysis. Atherosclerosis 330:22–28. https://doi.org/10.1016/j.atherosclerosis.2021.06.904
Quinn LS, Anderson BG, Conner JD, Pistilli EE, Wolden-Hanson T (2011) Overexpression of interleukin-15 in mice promotes resistance to diet-induced obesity, increased insulin sensitivity, and markers of oxidative skeletal muscle metabolism. Int J Interferon Cytokine Mediat Res 3:29–42. https://doi.org/10.2147/IJICMR.S19007
Quinn LS, Anderson BG, Drivdahl RH, Alvarez B, Argiles JM (2002) Overexpression of interleukin-15 induces skeletal muscle hypertrophy in vitro: implications for treatment of muscle wasting disorders. Exp Cell Res 280(1):55–63. https://doi.org/10.1006/excr.2002.5624
Rabiee R, Agardh E, Kjellberg K, Falkstedt D (2015) Low cardiorespiratory fitness in young adulthood and future risk of disability pension: a follow-up study until 59 years of age in Swedish men. J Epidemiol Community Health 69(3):266–271. https://doi.org/10.1136/jech-2014-204851
Ramirez-Velez R, Rincon-Pabon D, Correa-Bautista JE, Garcia-Hermoso A, Izquierdo M (2021) Handgrip strength: Normative reference values in males and females aged 6–64 Years old in a Colombian population. Clin Nutr ESPEN 44:379–386. https://doi.org/10.1016/j.clnesp.2021.05.009
Ravussin E, Lillioja S, Knowler WC, Christin L, Freymond D, Abbott WG, Boyce V, Howard BV, Bogardus C (1988) Reduced rate of energy expenditure as a risk factor for body-weight gain. N Engl J Med 318(8):467–472. https://doi.org/10.1056/NEJM198802253180802
Ross R, Blair SN, Arena R, Church TS, Despres JP, Franklin BA, Haskell WL, Kaminsky LA, Levine BD, Lavie CJ, Myers J, Niebauer J, Sallis R, Sawada SS, Sui X, Wisloff U (2016) Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: A Scientific Statement From the American Heart Association. Circulation 134(24):e653–e699. https://doi.org/10.1161/CIR.0000000000000461
Rubbieri G, Mossello E, Di Bari M (2014) Techniques for the diagnosis of sarcopenia. Clin Cases Miner Bone Metab 11(3):181–184
Sanchez YL, Yepes-Calderon M, Valbuena L, Milan AF, Trillos-Almanza MC, Granados S, Pena M, Estrada-Castrillon M, Aristizabal JC, Narvez-Sanchez R, Gallo-Villegas J, Calderón JC (2021) Musclin is related to insulin resistance and body composition, but not to body mass index or cardiorespiratory capacity in adults. Endocrinol Metab (seoul) 36(5):1055–1068. https://doi.org/10.3803/EnM.2021.1104
Sassen B, Kok G, Schaalma H, Kiers H, Vanhees L (2010) Cardiovascular risk profile: cross-sectional analysis of motivational determinants, physical fitness and physical activity. BMC Public Health 10:592. https://doi.org/10.1186/1471-2458-10-592
Savage DB, Watson L, Carr K, Adams C, Brage S, Chatterjee KK, Hodson L, Boesch C, Kemp GJ, Sleigh A (2019) Accumulation of saturated intramyocellular lipid is associated with insulin resistance. J Lipid Res 60(7):1323–1332. https://doi.org/10.1194/jlr.M091942
Schlussel MM, dos Anjos LA, de Vasconcellos MT, Kac G (2008) Reference values of handgrip dynamometry of healthy adults: a population-based study. Clin Nutr 27(4):601–607. https://doi.org/10.1016/j.clnu.2008.04.004
Schmid D, Leitzmann MF (2015) Cardiorespiratory fitness as predictor of cancer mortality: a systematic review and meta-analysis. Ann Oncol 26(2):272–278. https://doi.org/10.1093/annonc/mdu250
Seefeldt V, Malina RM, Clark MA (2002) Factors affecting levels of physical activity in adults. Sports Med 32(3):143–168. https://doi.org/10.2165/00007256-200232030-00001
Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW (2012) Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem 287(15):11968–11980. https://doi.org/10.1074/jbc.M111.336834
Shimomura M, Horii N, Fujie S, Inoue K, Hasegawa N, Iemitsu K, Uchida M, Iemitsu M (2021) Decreased muscle-derived musclin by chronic resistance exercise is associated with improved insulin resistance in rats with type 2 diabetes. Physiol Rep 9(9):e14823. https://doi.org/10.14814/phy2.14823
Sierra A, Subbotina E, Zhu Z, Gao Z, Koganti SR, Coetzee WA, Goldhamer DJ, Hodgson-Zingman DM, Zingman LV (2016) Disruption of ATP-sensitive potassium channel function in skeletal muscles promotes production and secretion of musclin. Biochem Biophys Res Commun 471(1):129–134. https://doi.org/10.1016/j.bbrc.2016.01.166
Silventoinen K, Magnusson PK, Tynelius P, Batty GD, Rasmussen F (2009) Association of body size and muscle strength with incidence of coronary heart disease and cerebrovascular diseases: a population-based cohort study of one million Swedish men. Int J Epidemiol 38(1):110–118. https://doi.org/10.1093/ije/dyn231
Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ, Savoye M, Rothman DL, Shulman GI, Caprio S (2002) Assessment of skeletal muscle triglyceride content by (1)H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes 51(4):1022–1027. https://doi.org/10.2337/diabetes.51.4.1022
Smith TB, Stonell C, Purkayastha S, Paraskevas P (2009) Cardiopulmonary exercise testing as a risk assessment method in non cardio-pulmonary surgery: a systematic review. Anaesthesia 64(8):883–893. https://doi.org/10.1111/j.1365-2044.2009.05983.x
Spahillari A, Mukamal KJ, DeFilippi C, Kizer JR, Gottdiener JS, Djousse L, Lyles MF, Bartz TM, Murthy VL, Shah RV (2016) The association of lean and fat mass with all-cause mortality in older adults: The Cardiovascular Health Study. Nutr Metab Cardiovasc Dis 26(11):1039–1047. https://doi.org/10.1016/j.numecd.2016.06.011
Srikanthan P, Karlamangla AS (2011) Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab 96(9):2898–2903. https://doi.org/10.1210/jc.2011-0435
Stamatakis E, Hamer M, O’Donovan G, Batty GD, Kivimaki M (2013) A non-exercise testing method for estimating cardiorespiratory fitness: associations with all-cause and cardiovascular mortality in a pooled analysis of eight population-based cohorts. Eur Heart J 34(10):750–758. https://doi.org/10.1093/eurheartj/ehs097
Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, Ragg KE, Toma K (2000) Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem 48(5):623–629. https://doi.org/10.1177/002215540004800506
Steensberg A, Keller C, Starkie RL, Osada T, Febbraio MA, Pedersen BK (2002) IL-6 and TNF-alpha expression in, and release from, contracting human skeletal muscle. Am J Physiol Endocrinol Metab 283(6):E1272-1278. https://doi.org/10.1152/ajpendo.00255.2002
Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund Pedersen B (2000) Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol 529(Pt 1):237–242. https://doi.org/10.1111/j.1469-7793.2000.00237.x
Stegen S, Everaert I, Deldicque L, Vallova S, de Courten B, Ukropcova B, Ukropec J, Derave W (2015) Muscle histidine-containing dipeptides are elevated by glucose intolerance in both rodents and men. PLoS ONE 10(3):e0121062. https://doi.org/10.1371/journal.pone.0121062
Steiber N (2016) Strong or weak handgrip? Normative reference values for the german population across the life course stratified by sex, age, and body height. PLoS ONE 11(10):e0163917. https://doi.org/10.1371/journal.pone.0163917
Stekovic S, Hofer SJ, Tripolt N, Aon MA, Royer P, Pein L, Stadler JT, Pendl T, Prietl B, Url J, Schroeder S, Tadic J, Eisenberg T, Magnes C, Stumpe M, Zuegner E, Bordag N, Riedl R, Schmidt A, Kolesnik E, Verheyen N, Springer A, Madl T, Sinner F, de Cabo R, Kroemer G, Obermayer-Pietsch B, Dengjel J, Sourij H, Pieber TR, Madeo F (2019) Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab 30(3):462–476. https://doi.org/10.1016/j.cmet.2019.07.016
Su L, Fu J, Sun S, Zhao G, Cheng W, Dou C, Quan M (2019) Effects of HIIT and MICT on cardiovascular risk factors in adults with overweight and/or obesity: a meta-analysis. PLoS ONE 14(1):e0210644. https://doi.org/10.1371/journal.pone.0210644
Sui X, LaMonte MJ, Laditka JN, Hardin JW, Chase N, Hooker SP, Blair SN (2007) Cardiorespiratory fitness and adiposity as mortality predictors in older adults. JAMA 298(21):2507–2516. https://doi.org/10.1001/jama.298.21.2507
Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PR, Swanson MS, Houmard JA (2002) Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab 282(6):E1191-1196. https://doi.org/10.1152/ajpendo.00416.2001
Tatemoto K, Takayama K, Zou MX, Kumaki I, Zhang W, Kumano K, Fujimiya M (2001) The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept 99(2–3):87–92
Toloza FJK, Mantilla-Rivas JO, Perez-Matos MC, Ricardo-Silgado ML, Morales-Alvarez MC, Pinzon-Cortes JA, Perez-Mayorga M, Arevalo-Garcia ML, Tolosa-Gonzalez G, Mendivil CO (2018) Plasma levels of myonectin but not myostatin or fibroblast-derived growth factor 21 are associated with insulin resistance in adult humans without diabetes mellitus. Front Endocrinol (lausanne) 9:5. https://doi.org/10.3389/fendo.2018.00005
Tsantilas P, Lao S, Wu Z, Eberhard A, Winski G, Vaerst M, Nanda V, Wang Y, Kojima Y, Ye J, Flores A, Jarr KU, Pelisek J, Eckstein HH, Matic L, Hedin U, Tsao PS, Paloschi V, Maegdefessel L, Leeper NJ (2021) Chitinase 3 like 1 is a regulator of smooth muscle cell physiology and atherosclerotic lesion stability. Cardiovasc Res 117(14):2767–2780. https://doi.org/10.1093/cvr/cvab014
Vaughan RA, Gannon NP, Barberena MA, Garcia-Smith R, Bisoffi M, Mermier CM, Conn CA, Trujillo KA (2014) Characterization of the metabolic effects of irisin on skeletal muscle in vitro. Diabetes Obes Metab 16(8):711–718. https://doi.org/10.1111/dom.12268
Vega G, Ricaurte G, Estrada-Castrillon M, Reyngoudt H, Cardona OM, Gallo-Villegas JA, Narvaez-Sanchez R, Calderón JC (2023) In vivo absolute quantification of carnosine in the vastus lateralis muscle with (1)H MRS using a surface coil and water as internal reference. Skeletal Radiol 52(2):157–165. https://doi.org/10.1007/s00256-022-04149-8
Vinel C, Lukjanenko L, Batut A, Deleruyelle S, Pradere JP, Le Gonidec S, Dortignac A, Geoffre N, Pereira O, Karaz S, Lee U, Camus M, Chaoui K, Mouisel E, Bigot A, Mouly V, Vigneau M, Pagano AF, Chopard A, Pillard F, Guyonnet S, Cesari M, Burlet-Schiltz O, Pahor M, Feige JN, Vellas B, Valet P, Dray C (2018) The exerkine apelin reverses age-associated sarcopenia. Nat Med 24(9):1360–1371. https://doi.org/10.1038/s41591-018-0131-6
Virkamaki A, Korsheninnikova E, Seppala-Lindroos A, Vehkavaara S, Goto T, Halavaara J, Hakkinen AM, Yki-Jarvinen H (2001) Intramyocellular lipid is associated with resistance to in vivo insulin actions on glucose uptake, antilipolysis, and early insulin signaling pathways in human skeletal muscle. Diabetes 50(10):2337–2343. https://doi.org/10.2337/diabetes.50.10.2337
Walowski CO, Braun W, Maisch MJ, Jensen B, Peine S, Norman K, Muller MJ, Bosy-Westphal A (2020) Reference values for skeletal muscle mass - current concepts and methodological considerations. Nutrients 12(3):755. https://doi.org/10.3390/nu12030755
Wang YC, Bohannon RW, Li X, Sindhu B, Kapellusch J (2018) Hand-grip strength: normative reference values and equations for individuals 18 to 85 years of age residing in the United States. J Orthop Sports Phys Ther 48(9):685–693. https://doi.org/10.2519/jospt.2018.7851
Wen Y, Liao J, Yin Y, Liu C, Gong R, Wu D (2021) Risk of falls in 4 years of follow-up among Chinese adults with diabetes: findings from the China Health and Retirement Longitudinal Study. BMJ Open 11(6):e043349. https://doi.org/10.1136/bmjopen-2020-043349
Wigodski S, Carrasco F, Bunout D, Barrera G, Hirsch S, de la Maza MP (2019) Sarcopenia: the need to establish different cutoff points of fat-free mass for the Chilean population. Nutrition 57:217–224. https://doi.org/10.1016/j.nut.2018.05.031
Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL (2005) Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 307(5708):418–420. https://doi.org/10.1126/science.1108177
Wong SL (2016) Grip strength reference values for Canadians aged 6 to 79: Canadian Health Measures Survey, 2007 to 2013. Health Rep 27(10):3–10
Yi DW, Khang AR, Lee HW, Son SM, Kang YH (2018) Relative handgrip strength as a marker of metabolic syndrome: the Korea National Health and Nutrition Examination Survey (KNHANES) VI (2014–2015). Diabetes Metab Syndr Obes 11:227–240. https://doi.org/10.2147/DMSO.S166875
Yu J, Zheng J, Liu XF, Feng ZL, Zhang XP, Cao LL, Zhou ZP (2016) Exercise improved lipid metabolism and insulin sensitivity in rats fed a high-fat diet by regulating glucose transporter 4 (GLUT4) and musclin expression. Braz J Med Biol Res 49(5):e5129. https://doi.org/10.1590/1414-431X20165129
Yue P, Jin H, Aillaud M, Deng AC, Azuma J, Asagami T, Kundu RK, Reaven GM, Quertermous T, Tsao PS (2010) Apelin is necessary for the maintenance of insulin sensitivity. Am J Physiol Endocrinol Metab 298(1):E59-67. https://doi.org/10.1152/ajpendo.00385.2009
Zurlo F, Larson K, Bogardus C, Ravussin E (1990) Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest 86(5):1423–1427. https://doi.org/10.1172/JCI114857
Acknowledgements
The authors thank the support received from Centro Clínico y de Investigación SICOR (Soluciones Integrales en Riesgo Cardiovascular), IPS-Universitaria, CODI and Minciencias, Colombia.
Funding
Open Access funding provided by Colombia Consortium. This work was funded by grants from IPS-Universitaria (project IN08-2021 from 2021), CODI (project 40430 from 2021), CODI (project 34909 from 2020), Minciencias (project 763 from 2018) and Centro Clínico y de Investigación SICOR (Soluciones Integrales en Riesgo Cardiovascular).
Author information
Authors and Affiliations
Contributions
JGV and JCC conceived and further developed the idea. Both of them prepared the manuscript, figures and tables. Both read and approved the last version to be submitted and are fully accountable for the content of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Michael I Lindinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gallo-Villegas, J.A., Calderón, J.C. Epidemiological, mechanistic, and practical bases for assessment of cardiorespiratory fitness and muscle status in adults in healthcare settings. Eur J Appl Physiol 123, 945–964 (2023). https://doi.org/10.1007/s00421-022-05114-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-022-05114-y