Abstract
Purpose
To investigate whether there is a differential response at rest and following exercise to conditions of genuine high altitude (GHA), normobaric hypoxia (NH), hypobaric hypoxia (HH), and normobaric normoxia (NN).
Method
Markers of sympathoadrenal and adrenocortical function [plasma normetanephrine (PNORMET), metanephrine (PMET), cortisol], myocardial injury [highly sensitive cardiac troponin T (hscTnT)], and function [N-terminal brain natriuretic peptide (NT-proBNP)] were evaluated at rest and with exercise under NN, at 3375 m in the Alps (GHA) and at equivalent simulated altitude under NH and HH. Participants cycled for 2 h [15-min warm-up, 105 min at 55% Wmax (maximal workload)] with venous blood samples taken prior (T0), immediately following (T120) and 2-h post-exercise (T240).
Results
Exercise in the three hypoxic environments produced a similar pattern of response with the only difference between environments being in relation to PNORMET. Exercise in NN only induced a rise in PNORMET and PMET.
Conclusion
Biochemical markers that reflect sympathoadrenal, adrenocortical, and myocardial responses to physiological stress demonstrate significant differences in the response to exercise under conditions of normoxia versus hypoxia, while NH and HH appear to induce broadly similar responses to GHA and may, therefore, be reasonable surrogates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Performing field research at high altitude (HA) is challenging and is often compounded by an austere environment and confounders such as variable environmental temperature, sleep, and exercise intensity. To mitigate these factors, the hypoxia of HA may be simulated. This may be done by reducing barometric pressure while maintaining the inspired fraction of oxygen at 20.9% (hypobaric hypoxia, HH) or by maintaining barometric pressure at 760 mmHg and reducing the inspired fraction of oxygen (normobaric hypoxia, NH). The modality employed will, pragmatically, often be determined by institutional history and availability.
Whether NH and HH are interchangeable means of simulating HA is not clearly understood, and yet, the literature is populated by studies examining the response to NH and HH with little consideration for whether there is any differential effect (Lundby et al. 2005; Woods et al. 2011b; Wille et al. 2012; Li et al. 2016). However, as illness at HA is relatively common and the pathophysiology of acute mountain sickness poorly understood (Schmerbach and Patzak 2013), there remains a need for both field and simulation studies. In 2012 (Millet et al. 2012a, b; Mounier and Brugniaux 2012), it was suggested that HH represented a more severe environmental condition that induces different physiological responses to NH (Millet et al. 2012a, b). This perspective was vigorously contested (Mounier and Brugniaux 2012) and stimulated significant debate within the field (Girard et al. 2012; Millet et al. 2012b). This debate highlighted a significant knowledge gap and the limited evidence-base on which any firm conclusions could be based. Among the very few studies comparing NH and HH, this journal has reported that HH may elicit a lower arterial carbon dioxide, lower oxygen saturation, and greater alkalosis than NH during an acute (30 and 40 min) exposure to an equivalent of 4500 m (Savourey et al. 2003, 2007), an effect thought to be due to increased dead space ventilation. However, there remains little direct comparison between the effects of NH, HH, and a “real-world” equivalent altitude, particularly in relation to the physiological stress induced. This was emphasized again when a recent review of studies utilizing NH and HH reiterated the limitations of the existing literature (Coppel et al. 2015).
We, therefore, chose an array of biochemical markers that would reflect a breadth of physiological responses to the environment at rest and with exercise allowing comparison between those environments. We, and others, have previously shown that highly sensitive cardiac troponin T (hscTnT), N-terminal pro-brain natriuretic peptide (NT-proBNP), and cortisol are influenced by hypoxia and exercise (Rostrup 1998; Banfi et al. 2010; Richalet et al. 2010; Woods et al. 2011a, 2012a, b; Mellor et al. 2014; Heinonen et al. 2014; Pagé et al. 2015; Li et al. 2016). An increased sympathoadrenal response has been reported with both acute and more prolonged HA exposure (Mazzeo et al. 1995) that is further stimulated by exercise (Mazzeo et al. 1991, 1995, 2000; Rostrup 1998). While assay of the traditional catecholamines dominates the literature, they have a very short half-life of 1–2 min (Peaston and Weinkove 2004) and are highly unstable and so labile they alter in response to a change in posture (Raber et al. 2000). Plasma normetanephrine (PNORMET, also known as normetadrenaline) and plasma metanephrine (PMET, also known as metadrenaline) are the more stable O-methylated extraneuronal metabolites of noradrenaline and adrenaline respectively. PNORMET and PMET, while being non-functional in themselves, both correlate with their respective catecholamines (Roden et al. 2001) and have the added advantage that they are not significantly influenced by the time of day, the phase of the menstrual cycle, or the act of venepuncture, and are stable, once separated, at 4 °C for 72 h (Deutschbein et al. 2010). Although they do not reflect global sympathoadrenal activity, they are sufficiently sensitive to increase in response to brief (2 min) high intensity exercise in normoxia (Bracken and Brooks 2010) and in view of their advantages for field work were chosen for assay rather than catecholamines.
These markers were then studied to investigate whether there was a differential response following exercise under conditions of normobaric normoxia (NN), NH, HH, and genuine high altitude (GHA).
Materials and methods
Ethical information
The study was approved by the Ministry of Defence Research Ethics Committee and was conducted according to the standards of the declaration of Helsinki. All participants gave written informed consent.
Participants
Participants were 14 healthy British military servicemen and women aged 22–35 years. Participant health status was confirmed by clinical history, examination, ECG, and transthoracic echocardiography.
Study protocol
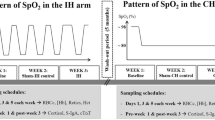
All participants underwent a standard incremental cycle test to volitional exhaustion at sea level (absolute altitude ~113 m) under NN conditions to determine maximal oxygen uptake and maximal workload (Wmax [watts]) on a bicycle affixed to a bicycle trainer (Compu Trainer Pro Lab, Racer Mate, USA). The bicycle trainer was calibrated following the manufacturer’s instructions. At least 7 days later, this was repeated under NH {FiO2 considering water vapour partial pressure and daily fluctuations of barometric pressure equivalent to 3375 m (PiO2 ~95 mmHg)} in an environmental chamber (TISS, Alton, UK and Sporting Edge, Sherfield on Loddon, UK) at Leeds Beckett University. This established and ensured that equivalent workloads were used for the hypoxic experimental trials.
Participants were then assessed under four different environmental conditions. They were first assessed at GHA in order that conditions experienced there could then be replicated under NH and HH. GHA was at 3375 m (barometric pressure 506.4 ± 1.4 mmHg) at the Torino Hut in Northern Italy. Access to the hut required rapid ascent by cable car to 3375 m. Participants slept in the valley floor the night before, only ascending to 3375 m on the morning of their testing. Participants then remained at the hut for 24 h, descending the following day after testing. At least 7 weeks later, participants underwent the same protocol under conditions of NN. Eight weeks after being at GHA, the participants underwent the same protocol under conditions of NH in an environmental chamber. Finally, at least 3 weeks following NH exposure, participants completed the protocol under conditions of HH at the Centre of Aviation Medicine, RAF Henlow, UK. This sequence ensured that the PiO2 experienced breathing ambient air during GHA (PiO2 = 96.3 ± 0.4 mmHg) could be replicated for each individual during subsequent NH and HH exposures.
During each exposure participants had 30 min of altitude acclimatization. This was followed by a standardized warm-up which included the calibration of the bicycle trainer (Compu Trainer Pro Lab, Racer Mate, USA) to the manufacturer’s instructions. Participants then completed 120 min of cycling: 5 min at 40% Wmax, 5 min at 45% Wmax, 5 min at 50% Wmax, and 105 min at 55% Wmax. The protocol was always performed following an overnight 12-h fast and energy intake was kept uniform by the ingestion of a fixed carbohydrate solution (glucose–fructose) throughout. Participants repeated their trials at the same time of day in each environmental condition to avoid any influence of circadian variance. The temperature range across the exposures was 18–23 °C.
Blood samples
Venous blood samples were drawn from an indwelling venous catheter in the arm following the acclimatization process, immediately prior to cycling (T0), immediately at the end of the 120 min cycling (T120), and then 2-h post-exercise (T240). At GHA, a further sample was taken 22 h later. Samples were drawn into EDTA (plasma metanephrine and normetanephrine, NT-proBNP) and SST (hscTnT, cortisol) tubes. Samples were then centrifuged and plasma and serum separated before being stored briefly at −20 °C and then at −80 °C before analysis.
Biochemical assays
Highly sensitive cardiac troponin T (hscTnT) and N-terminal pro-brain natriuretic peptide (NT-proBNP) were assayed by the biochemistry department at Poole Hospital, Poole, UK. Cortisol and plasma metanephrines were assayed in the Department of Blood Sciences, Royal Victoria Infirmary, Newcastle upon Tyne.
NT-proBNP was measured using the Roche NT-proBNP assay (Roche Diagnostics, Mannheim, Germany) with a range from 5 to 35,000 pg/ml and a coefficient of variation (CV) at a mean NT-proBNP of 474 pg/ml of 5.8%.
HscTNT was measured using an electro-chemiluminescence immunoassay (ECLIA) on a Cobas Analyser (Roche Diagnostics). The upper reference limit (99th percentile) for this assay in healthy volunteers is 14 ng/l (pg/ml). This assay has a range from 3 to 10,000 ng/l. The CV at a mean hscTnT level of 13.5 ng/l is 5.2, 8.5% at 5.3 ng/l, and 1.8% at 28.5 ng/l.
Cortisol was measured using the Roche Elecsys Cortisol I assay which is a competitive chemiluminescence immunoassay (CLIA) that is fully automated and run on the Roche modular E unit (Roche Diagnostics, Burgess Hill, UK). The analytical range of the assay is 0.5–1750 nmol/l with an intra-assay CV of 9.3–11.7%.
Plasma metanephrine assay was performed by LC–MSMS. Following off-line solid phase using weak ion exchange extraction in a 96-well plate format, plasma metanephrines are separated using rapid, hydrophilic interaction liquid chromatography. Mass spectrometry detection is performed in multiple-reaction monitoring mode using a tandem quadrupole mass spectrometer (Waters UK, Hertfordshire, UK) with positive electrospray ionization. As well as the sample, a quality control (QC) and internal standard are used. The lower limit of quantification is between 40 and 50 pmol/l with a CV of 13–16%.
Physiological measurements
Peripheral oxygen saturation (SpO2) in the finger was recorded using a Nellcor N-20P pulse oximeter (Nellcor Puritan Bennett, Coventry, UK) and heart rate was measured form a polar heart rate monitor. Measurements were taken at rest, 15 min into the acclimatization process, and then 15- and 120-min post-exercise.
Statistical methods
Data were analysed using GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla California USA, http://www.graphpad.com. The Shapiro–Wilk normality test and inspection of the data were undertaken to assess normality of all continuous data. All data are presented as mean ± standard deviations. Time-dependent changes within each group were assessed using repeated-measures ANOVA with Bonferroni post-test for parametric data and Friedman Test with Dunn Post-test for non-parametric data. To compare the hypoxic environments, a two-way ANOVA was performed using data only from the three hypoxic environments. A two tailed p value <0.05 was considered statistically significant for all comparisons.
Results
Of the original 14 participants (age: 25.9 ± 3.8 years, height: 174 ± 10 cm, weight: 71.5 ± 10 kg, BMI: 23.4 ± 1.8 kg/m2, 8 M, 6 F) who completed the protocol at GHA, 13 completed the protocol under NN and NH conditions. Eight completed the protocol under HH conditions. There were no significant differences in baseline demographics between the groups. The HH exposure took place during the autumn/early winter and several participants were unable to take part due to upper respiratory tract infections and an inability to clear their ears which are exclusions to entering the hypobaric chamber.
Heart rate (beats per minute) increased from T0 to T120 in all environments with no difference between environments: NN from 67 ± 11 to 163 ± 12; GHA from 74 ± 13 to 164 ± 11; NH from 73 ± 11 to 161 ± 9; and HH from 71 ± 9 to 142 ± 24. SpO2 (%) dropped significantly (p < 0.01) with exercise: NN from 99 ± 1 to 96 ± 1; GHA from 89 ± 3 to 82 ± 4; NH from 93 ± 2 to 87 ± 4; and HH from 90 ± 2 to 83 ± 5, before returning to baseline at T240.
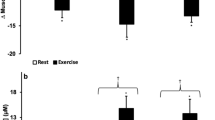
Data for PNORMET, PMET, cortisol, hscTnT, and NT-proBNP under conditions of NN, GHA, NH, and HH are shown in Table 1. The only significant difference in resting values (T0) was for PNORMET between NN and GHA (p < 0.01). NN differed from the three hypoxic environments in that only PNORMET and PMET showed a significant rise with exercise. Only at GHA was a sample taken 22-h post-exercise. PNORMET, PMET, hscTnT, and cortisol had returned to baseline 22-h post-exercise, but NT-proBNP was significantly elevated compared to baseline (36.75 ± 39 vs. 48.2 ± 61, pg/ml, p < 0.01).
Comparing the three hypoxic environments by two-way ANOVA (Table 2) revealed a significant (p < 0.0001) main effect of time on PNORMET, PMET, hscTnT, and cortisol but not NT-proBNP. The only significant effect for mode of hypoxia was with PNORMET (p = 0.0017) with no interaction between time and mode of hypoxia.
Discussion
To our knowledge, this is the first study to compare the effect of NN, NH, HH, and GHA in the same participants at rest and following exercise on a range of biochemical markers that might broadly reflect physiological stress. As might be expected, the hypoxic conditions induced a different response to NN with significant rises in all studied biomarkers, whereas only PNORMET and PMET rose with exercise in NN.
Plasma metanephrines are stable markers of catecholamine secretion and have previously been reported to reflect physiological stress and the sympathoadrenal response to cycling exercise (Raber et al. 2003). The fact that PNORMET increased under all experimental conditions with exercise suggests that the protocol was sufficiently provocative to induce the required physiological stress. When comparing hypoxic environments, the only significantly different response was in that of PNORMET, suggesting that there may be subtle differences between environments in the sympathoadrenal response.
While PNORMET and PMET reflect the sympathoadrenal response, cortisol is an adrenal marker of physiological stress. We found no difference in resting cortisol between the altitude environments and NN. This is consistent with our previous data showing no change in resting cortisol at moderate altitude (Woods et al. 2012b) and others at both 3000 m (Bouissou et al. 1988) and 4300 m (Maher et al. 1975), although it is in contrast with others that have shown an increase with rapid ascent to higher altitudes at 4760 m (Sutton et al. 1977) and 6000 m (Okazaki et al. 1984). We found no rise in cortisol with exercise in NN, and while some report a rise in cortisol with exercise at SL (Bouissou et al. 1988; Wahl et al. 2014), this is not a universal finding (Hough et al. 2011; Ros et al. 2011). We did find cortisol increased significantly immediately following exercise under all hypoxic conditions.
The fact hscTnT rose 2 h following exercise under all hypoxic conditions but not in NN indicates that the hypoxic protocols were also provocative enough to induce significant myocardial TnT release. We have previously reported a rise in cTnT with exercise at HA (Boos et al. 2014; Mellor et al. 2014), as have others (Li et al. 2016). cTnT is a highly specific marker of myocardial cell damage which is released in a biphasic fashion following myocardial ischaemia with an initial small rise about 2 h after the event, thought to reflect release from the cytosolic pool, and a subsequent larger rise 12–24 h later, thought to reflect release of bound cTnT and myocardial damage (Wu et al. 1998). The fact that hscTnT had returned to baseline 22 h after exercise at GHA suggests that no myocardial necrosis had occurred and that the rise at T240 most likely reflects cytosolic release. This would be consistent with the data from our previous studies at HA that showed that while cTnT elevation was associated with higher pulmonary artery systolic pressure, there was no overt deleterious cardiac function detected by cardiac echo (Boos et al. 2014; Mellor et al. 2014).
NT-proBNP rose immediately following exercise and stayed significantly elevated 2 h later under conditions of GHA and NH, but it did not change significantly in NN. While a rise in NT-proBNP with exercise at SL has been reported, this has generally been following prolonged endurance exercise (such as running an ultramarathon) (Scharhag et al. 2005; Hew-Butler et al. 2008) rather than after moderate or short duration exercise (Nishikimi et al. 1997; Hew-Butler et al. 2008; Woods et al. 2011a) such as employed here.
We have previously reported a rise in BNP following exercise at GHA (Woods et al. 2011a, 2012a; Mellor et al. 2014). Myocardial hypoxia, intracellular acidosis, and cardiomyocyte stretch all contribute to increase NT-proBNP (Nishikimi et al. 2011). When NT-proBNP is elevated due to myocardial ischaemia and necrosis, the peak (similar to hscTnT) occurs 12–24 h later (Nishikimi et al. 2011). Interestingly, while hscTnT fell back to baseline at 22-h post-exercise, NT-proBNP remained elevated, though did not rise further. Pressure overload in the right atrium and ventricle stimulates NT-proBNP regardless of aetiology (Nishikimi et al. 2011) and we have also shown an association between NT-proBNP and high pulmonary artery systolic pressure (PASP) at HA (Banfi et al. 2010; Woods et al. 2013). The lack of a rise under conditions of HH warrants further comment. We have previously examined the BNP response to acute HH on two occasions: first, at a simulated altitude of 5334 m for 40 min involving a brief 1-min step-test (Woods et al. 2011b) and, second, at a simulated 4800 m over 3 h with a brief 5-min step-test (Boos et al. 2013). On neither occasion did we find a significant rise in BNP despite significant rises in PASP which we consider to be due to the brevity of the exercise stimulus. We have recently published the echocardiographic arm of this study (Boos et al. 2016) which reported that the right ventricular systolic pressure (RVSP) and pulmonary vascular resistance were higher in HH compared to NH. It would, therefore, be reasonable to expect that this would be associated with an elevation in NT-proBNP that we were unable to detect in HH. It is with great regret that we failed to get all the participants from the GHA through the HH exposure and we suspect that the lack of a rise in NT-proBNP under these conditions may simply reflect a type II error.
Although there appears, on the initial inspection of the data, to be some baseline variability in the parameters studied on statistical analysis, the only significant difference in resting values between environments was for PNORMET between NN and GHA. We suspect the higher resting PNORMET at GHA compared to NN relates to a degree of sympathoadrenal activation as a result of the cable car ascent to 3375 m on the morning of testing. As PMET and cortisol did not show any difference at baseline between NN and GHA and the fact there was still a significant increment in PNORMET with exercise at GHA, we suspect this was a minor confounder only. In support of this is the fact that all parameters at baseline in all environments were within the normal range expected for a healthy adult.
In summary, our exercise protocol produced significantly different results under conditions of NN versus the hypoxic environments. Exercise in the hypoxic environments successfully induced a measureable rise in several markers of physiological stress. From two-way ANOVA analysis, the changes seen between hypoxic environments were very similar with the only difference being for PNORMET. The previous comparisons between NH and HH have often focussed on respiratory or cardiovascular variables (Coppel et al. 2015), and while our data cannot be definitive, they have at least begun to evaluate equivalence of the environments from a perspective of biochemical and physiological stress.
Strengths of our study include the use of the same cohort of participants for each exposure with an adequate wash-out period between stimuli, with accurate reporting of barometric pressure to ensure homogeneity across environments. The major limitation was our inability, despite multiple attempts, to get all participants through the final HH exposure.
Conclusion
To our knowledge, this is the first study to compare the effect of NN, NH, HH, and GHA in the same participants at rest and following exercise on a range of biochemical markers that reflect physiological stress. Responses under hypoxic conditions were different to NN, but the similarities between HH and NH suggest that they may be valid surrogates for GHA.
Abbreviations
- ANOVA:
-
Analysis of variance
- BNP:
-
Brain natriuretic peptide
- CV:
-
Coefficient of variation
- CLIA:
-
Chemiluminescence immunoassay
- ECG:
-
Electrocardiograph
- ECLIA:
-
Electro-chemiluminescence immunoassay
- FiO2 :
-
Fraction of inspired oxygen
- GHA:
-
Genuine high altitude
- HA:
-
High altitude
- HH:
-
Hypobaric hypoxia
- hscTnT:
-
Highly sensitive cardiac troponin T
- LC–MSMS:
-
Liquid chromatography mass spectrometry
- NH:
-
Normobaric hypoxia
- NN:
-
Normobaric normoxia
- NT-proBNP:
-
N-terminal pro-brain natriuretic peptide
- PASP:
-
Pulmonary artery systolic pressure
- PiO2 :
-
Partial pressure of inspired oxygen
- PMET:
-
Plasma metanephrine
- PNORMET:
-
Plasma normetanephrine
- RVSP:
-
Right ventricular systolic pressure
- SpO2 :
-
Peripheral oxygen saturation
- QC:
-
Quality control
- Wmax:
-
Maximal workload
References
Banfi G, Lippi G, Susta D, Barassi A, D’Eril GM, Dogliotti G, Corsi MM (2010) NT-proBNP concentrations in mountain marathoners. J Strength Cond Res 24:1369–1372
Boos CJ, Hodkinson PD, Mellor A, Green NP, Bradley D, Greaves K, Woods DR (2013) The effects of prolonged acute hypobaric hypoxia on novel measures of biventricular performance. Echocardiography 30:534–541
Boos CJ, Mellor A, Begley J, Stacey M, Smith C, Hawkins A, Woods DR (2014) The effects of exercise at high altitude on high-sensitivity cardiac troponin release and associated biventricular cardiac function. Clin Res Cardiol 103:291–299
Boos CJ, O’Hara JP, Mellor A, Hodkinson PD, Tsakirides C, Reeve N, Gallagher L, Green ND, Woods DR (2016) A four-way comparison of cardiac function with normobaric normoxia, normobaric hypoxia, hypobaric hypoxia and genuine high altitude. PLoS ONE 21(4):e0152868
Bouissou P, Fiet J, Guezennec CY, Pesquies PC (1988) Plasma adrenocorticotrophin and cortisol responses to acute hypoxia at rest and during exercise. Eur J Appl Physiol Occup Physiol 57:110–113
Bracken RM, Brooks S (2010) Plasma catecholamine and nephrine responses following 7 weeks of sprint cycle training. Amino Acids 38:1351–1359
Coppel J, Hennis P, Gilbert-Kawai E, Grocott MP (2015) The physiological effects of hypobaric hypoxia versus normobaric hypoxia: a systematic review of crossover trials. Extrem Physiol Med 26: 4:1–20
Deutschbein T, Unger N, Jaeger A, Broecker-Preuss M, Mann K, Petersenn S (2010) Influence of various confounding variables and storage conditions on metanephrine and normetanephrine levels in plasma. Clin Endocrinol 73: 153–160
Girard O, Koehle MS, MacInnis MJ, Guenette JA, Koehle MS, Verges S, Rupp T, Jubeau M, Perrey S, Millet GY, Chapman RF, Levine BD, Conkin J, Wessel JH 3rd, Nespoulet H, Wuyam B, Tamisier R, Verges S, Levy P, Casey DP, Taylor BJ, Snyder EM, Johnson BD, Laymon AS, Stickford JL, Weavil JC, Loeppky JA, Pun M, Schommer K, Bartsch P, Vagula MC, Nelatury CF (2012) Comments on point: counterpoint: hypobaric hypoxia induces/does not induce different responses from normobaric hypoxia. J Appl Physiol 112:1788–1794
Heinonen I, Luotolahti M, Vuolteenaho O, Nikinmaa M, Saraste A, Hartiala J, Koskenvuo J, Knuuti J, Arjamaa O (2014) Circulating N-terminal brain natriuretic peptide and cardiac function in response to acute systemic hypoxia in healthy humans. J Transl Med 12:189. doi:10.1186/1479-5876-12-189
Hew-Butler T, Noakes TD, Soldin SJ, Verbalis JG (2008) Acute changes in endocrine and fluid balance markers during high intensity, steady-state, and prolonged endurance running: unexpected increases in oxytocin and brain natriuretic peptide during exercise. Eur J Endocrinol 159:729–737
Hough JP, Papacosta E, Wraith E, Gleeson M (2011) Plasma and salivary steroid hormone responses of men to high-intensity cycling and resistance exercise. J Strength Cond Res 25:23–31
Li F, Hu Y, Nie J, Fu FH (2016) Effects of acute, intermittent exercise in hypoxic environments on the release of cardiac troponin. Scand J Med Sci Sports 26:397–403
Lundby C, Nielsen TK, Dela F, Damsgaard R (2005) The influence of intermittent altitude exposure to 4100 m on exercise capacity and blood variables. Scand J Med Sci Sports 15:182–187
Maher JT, Jones LG, Hartley LH, Williams GH, Rose LI (1975) Aldosterone dynamics during graded exercise at sea level and high altitude. J Appl Physiol 1:18–22
Mazzeo RS, Bender PR, Brooks GA, Butterfield GE, Groves BM, Sutton JR, Wolfel EE, Reeves JT (1991) Arterial catecholamine responses during exercise with acute and chronic high altitude exposure. Am J Physiol Endocrinol Metab 261:E419–E424
Mazzeo RS, Brooks GA, Butterfield GE, Podolin DA, Wolfel EE, Reves JT (1995) Acclimatization to high altitude increases muscle sympathetic activity both at rest and during exercise. Am J Physiol Regul Integr Comp Physiol 269:R201–R207
Mazzeo RS, Child A, Butterfield GE, Braun B, Rock PB, Wolfel EE, Zamudio S, Moore LG (2000) Sympathoadrenal responses to submaximal exercise in women after acclimatization to 4,000 m. Metabolism 49:1036–1042
Mellor A, Boos C, Holdsworth D, Begley J, Hall D, Lumley A, Burnett A, Hawkins A, O’Hara J, Ball S, Woods DR (2014) Cardiac biomarkers at high altitude. High Alt Med Biol 15:452–458
Millet GP, Faiss R, Pialoux V (2012a) Point: hypobaric hypoxia induces different physiological responses from normobaric hypoxia. J Appl Physiol 112:1783–1784
Millet GP, Faiss R, Pialoux V (2012b) Last word on point: counterpoint: hypobaric hypoxia induces different responses from normobaric hypoxia. J Appl Physiol 112:1795
Mounier R, Brugniaux JV (2012) Counterpoint: hypobaric hypoxia does not induce different physiological responses from normobaric hypoxia. J Appl Physiol 112:1784–1786
Nishikimi T, Morimoto A, Ishikawa K, Saito Y, Kangawa K, Matsuo H, Kitamura K, Takishita S, Matsuoka H (1997) Different secretion patterns of adrenomedullin, brain natriuretic peptide, and atrial natriuretic peptide during exercise in hypertensive and normotensive subjects. Clin Exp Hypertens 19:503–518
Nishikimi T, Kuwahara K, Nakao K (2011) Current biochemistry, molecular biology, and clinical relevance of natriuretic peptides. J Cardiol 57:131–140
Okazaki S, Tamura Y, Hatano T, Matsui N (1984) Hormonal disturbances of fluid-electrolyte metabolism under altitude exposure in man. Aviat Space Environ Med 55:200–205
Pagé M, Henri C, Pagé P, Sauvé C, Schampaert E (2015) Brain natriuretic peptide levels and the occurrence of subclinical pulmonary edema in healthy lowlanders at high altitude. Can J Cardiol 31:1025–1031
Peaston RT, Weinkove C (2004) Measurement of catecholamines and their metabolites. Ann Clin Biochem 41:17–38
Raber W, Raffesberg W, Bischof M, Scheuba C, Niederle B, Gasic S, Waldhäusl W, Roden M (2000) Diagnostic efficacy of unconjugated plasma metanephrines for the detection of pheochromocytoma. Arch Intern Med 160:2957–2963
Raber W, Raffesberg W, Waldhäusl W, Gasic S, Roden M (2003) Exercise induces excessive normetanephrine responses in hypertensive diabetic patients. Eur J Clin Invest 33:480–487
Richalet JP, Letournel M, Souberbielle JC (2010) Effects of high-altitude hypoxia on the hormonal response to hypothalamic factors. Am J Physiol Regul Integr Comp Physiol 299:1685–1692
Roden M, Raffesberg W, Raber W, Bernroider E, Niederle B, Waldhäusl W, Gasic S (2001) Quantification of unconjugated metanephrines in human plasma without interference by acetaminophen. Clin Chem 47:1061–1067
Ros G, Dantas EHM, de Mello DB (2011) The response of serum leptin, cortisol and zinc concentrations to concurrent training. Hormones 10:215–221
Rostrup M (1998) Catecholamines, hypoxia and high altitude. Acta Physiol Scand 162:389–399
Savourey G, Launay JC, Besnard Y, Guinet A, Travers S (2003) Normo- and hypobaric hypoxia: are there any physiological differences? Eur J Appl Physiol 89:122–126
Savourey G, Launay JC, Besnard Y, Guinet-Lebreton A, Alonso A, Sauvet F, Bourrilhon C (2007) Normo or hypobaric hypoxic tests: propositions for the determination of the individual susceptibility to altitude illnesses. Eur J Appl Physiol 100:193–205
Scharhag J, Herrmann M, Urhausen A, Haschke M, Herrmann W, Kindermann W (2005) Independent elevations of N-terminal pro-brain natriuretic peptide and cardiac troponins in endurance athletes after prolonged strenuous exercise. Am Heart J 150:1128–1134
Schmerbach K, Patzak A (2013) Pathophysiological mechanisms in acute mountain sickness. Acta Physiol 209:246–249
Sutton JR, Viol GW, Gray GW, McFadden M, Keane PM (1977) Renin, aldosterone, electrolyte, and cortisol responses to hypoxic decompression. J Appl Physiol 43:421–424
Wahl P, Hein M, Achtzehn S, Bloch W, Mester J (2014) Acute metabolic, hormonal and psychological responses to cycling with superimposed electromyostimulation. Eur J Appl Physiol 114:2331–2339
Wille M, Gatterer H, Mairer K, Philippe M, Schwarzenbacher H, Faulhaber M, Burtscher M (2012) Short-term intermittent hypoxia reduces the severity of acute mountain sickness. Scand J Med Sci Sports 22:e79–e85
Woods D, Hooper T, Hodkinson P Ball S, Wakeford R, Peaston B, Bairsto C, Green N, Mellor A (2011a) Effects of altitude exposure on brain natriuretic peptide in humans. Eur J Appl Physiol 111:2687–2693
Woods D, Hooper T, Mellor A, Hodkinson P, Wakeford R, Peaston B, Ball S, Green N (2011b) Brain natriuretic peptide and acute hypobaric hypoxia in humans. J Physiol Sci 61:217–220
Woods DR, Begley J, Stacey M, Smith C, Boos CJ, Hooper T, Hawkins A, Hodkinson P, Green N, Mellor A (2012a) Severe acute mountain sickness, brain natriuretic peptide and NT-proBNP in humans. Acta Physiol 205:349–355
Woods DR, Davison A, Stacey M, Smith C, Hooper T, Neely D, Turner S, Peaston R, Mellor A (2012b) The cortisol response to hypobaric hypoxia at rest and post-exercise. Horm Metab Res 44:302–305
Woods DR, Mellor A, Begley J, Stacey M, O’Hara J, Hawkins A, Yarker J, Foxen S, Smith C, Boos C (2013) Brain natriuretic peptide and NT-proBNP levels reflect pulmonary artery systolic pressure in trekkers at high altitude. Physiol Res 6:597–603
Wu AH, Feng YJ, Moore R, Apple FS, McPherson PH, Buechler KF, Bodor G (1998) Characterization of cardiac troponin subunit release into serum after acute myocardial infarction and comparison of assays for troponin T and I: American Association for Clinical Chemistry Subcommittee on cTnI Standardization. Clin Chem 44:1198–1208
Acknowledgements
The authors would like to acknowledge the efforts of the participants. Research reported in this study was supported by the Surgeon General and Joint Medical Command, UK, the Drummond Foundation, and Leeds Beckett University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Defence Medical Services.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest or financial ties to disclose.
Additional information
Communicated by Guido Ferretti.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Woods, D.R., O’Hara, J.P., Boos, C.J. et al. Markers of physiological stress during exercise under conditions of normoxia, normobaric hypoxia, hypobaric hypoxia, and genuine high altitude. Eur J Appl Physiol 117, 893–900 (2017). https://doi.org/10.1007/s00421-017-3573-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-017-3573-5