Abstract
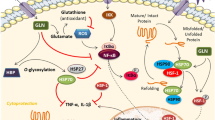
The aim of the current study was to investigate the effect of glutamine supplementation on the expression of HSP70 and the calcium-binding proteins from the S100 superfamily in the recovering extensor digitorum longus (EDL) muscle after injury. Two-month-old Wistar rats were subjected to cryolesion of the EDL muscle and then randomly divided into two groups (with or without glutamine supplementation). Starting immediately after the injury, the supplemented group received daily doses of glutamine (1 g/kg/day, via gavage) for 3 and 10 days orally. Then, muscles were subjected to histological, molecular, and functional analysis. Glutamine supplementation induced an increase in myofiber size of regenerating EDL muscles and prevented the decline in maximum tetanic strength of these muscles evaluated 10 days after injury. An accelerated upregulation of myogenin mRNA levels was detected in glutamine-supplemented injured muscles on day 3 post-cryolesion. The HSP70 expression increased only in the injured group supplemented with glutamine for 3 days. The increase in mRNA levels of NF-κB, the pro-inflammatory cytokines IL-1β and TNF-α, and the calcium-binding proteins S100A8 and S100A9 on day 3 post-cryolesion in EDL muscles was attenuated by glutamine supplementation. In contrast, the decrease in S100A1 mRNA levels in the 3-day-injured EDL muscles was minimized by glutamine supplementation. Overall, our results suggest that glutamine supplementation accelerates the recovery of myofiber size and contractile function after injury by modulating the expression of myogenin, HSP70, NF-κB, pro-inflammatory cytokines, and S100 calcium-binding proteins.




Similar content being viewed by others
Availability of data and material
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Alderton JM, Steinhardt RA (2000) How calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. Trends Cardiovasc Med 10:268–272
Aoki MS, Soares AG, Miyabara EH, Baptista IL, Moriscot AS (2009) Expression of genes related to myostatin signaling during rat skeletal muscle longitudinal growth. Muscle Nerve 40:992–999
Antonio J, Street C (1999) Glutamine: a potentially useful supplement for athletes. Canadian J Appl Physiol 24:1–14
Ardawi MS, Newsholme EA (1985) Metabolism in lymphocytes and its importance in the immune response. Essays Biochem 21:1–44
Bentzinger CF, Wang YX, Dumont NA, Rudnicki MA (2013) Cellular dynamics in the muscle satellite cell niche. EMBO Rep 14:1062–1072
Boyd JH, Kan B, Roberts H, Wang Y, Walley KR (2008) S100A8 and S100A9 mediate endotoxin-induced cardiomyocyte dysfunction via the receptor for advanced glycation end products. Circ Res 102:1239–1246
Chen G, Shi J, Qi M, Yin H, Hang C (2008) Glutamine decreases intestinal nuclear factor kappa B activity and pro-inflammatory cytokine expression after traumatic brain injury in rats. Inflamm Res 57:57–64
Castell L (2003) Glutamine supplementation in vitro and in vivo in exercise and in immuno depression. Sports Med 33:323–345
Cruzat VF, Rogero MM, Tirapegui J (2010) Effects of supplementation with free glutamine and the dipeptide alanyl-glutamine on parameters of muscle damage and inflammation in rats submitted to prolonged exercise. Cell Biochem Funct 28:24–30
Cruzat V, Macedo Rogero M, Noel Keane K, Curi R, Newsholme P (2018) Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients 10:1564
Chang TW, Goldberg AL (1978) The metabolic fates of amino acids and theformation of glutamine in skeletal muscle. J Biol Chem 253:3685–3693
Curi TC, De Melo MP, De Azevedo RB, Zorn TM, Curi R (1997) Glutamine utilization by rat neutrophils: presence of phosphate-dependent glutaminase. Am J Physiol Cell Physiol 273:C1124–C1129
Curi R, Lagranha CJ, Doi SQ, Sellitti DF, Procopio J, Pithon-Curi TC, Corless M, Newsholme P (2005) Molecular mechanisms of glutamine action. J Cellular Physiol 204:392–401
de Melo JO, Soto SF, Katayama IA, Wenceslau CF, Pires AG, Veras MM, Furukawa LN, de Castro I, Saldiva PH, Heimann JC (2015) Inhalation of fine particulate matter during pregnancy increased IL-4 cytokine levels in the fetal portion of the placenta. Toxicol Lett 232:475–480
Dort J, Fabre P, Molina T, Dumont NA (2019) Macrophages are key regulators of stem cells during skeletal muscle regeneration and diseases. Stem Cells Int 2019:4761427
Feige P, Brun CE, Ritso M, Rudnicki MA (2019) Orienting muscle stem cells for regeneration in homeostasis, aging, and disease. Cell Stem Cell 23:653–664
Fläring UB, Rooyackers OE, Wernerman J, Hammarqvist F (2003) Glutamine attenuates post-traumatic glutathione depletion in human muscle. Clin Sci 104:275–282
Golding JD, Rigley MacDonald ST, Juurlink BH, Rosser BW (2006) The effect of glutamine on locomotor performance and skeletal muscle myosins following spinal cord injury in rats. J Appl Physiol (1985) 101:1045–1052
Guzhova IV, Darieva ZA, Melo AR, Margulis BA (1997) Major stress protein Hsp70 interacts with NF-kB regulatory complex in human T-lymphoma cells. Cell Stress Chaperones 2:132–139
Hardy D, Besnard A, Latil M, Jouvion G, Briand D, Thepenier C, Pascal Q, Guguin A, Gayraud-Morel B, Cavaillon JM, Tajbakhsh S, Rocheteau P, Chretien F (2016) Comparative study of injury models for studying muscle regeneration in mice. PLoS One 11:e0147198
Hawke TJ, Garry DJ (2001) Myogenic satellite cells: physiology to molecular biology. J Appl Physiol (1985) 91:534–551
Heizmann CW, Ackermann GE, Galichet A (2007) Pathologies involving the S100 proteins and RAGE. Subcell Biochem 45:93–138
Jacquier-Sarlin MR, Fuller K, Dinh-Xuan AT, Richard MJ, Polla BS (1994) Protective effects of hsp70 in inflammation. Experientia 50:1031–1038
Kamikawa Y, Ikeda S, Harada K, Ohwatashi A, Yoshida A (2013) Passive repetitive stretching for a short duration within a week increases myogenic regulatory factors and myosin heavy chain mRNA in rats’ skeletal muscles. ScientificWorldJournal 2013:493656
Koike TE, Dell Aquila RA, Silva KS, Aoki MS, Miyabara EH (2022) Glutamine supplementation improves contractile function of regenerating soleus muscles from rats. J Muscle Res Cell Motil 43:87–97
Kozakowska M, Pietraszek-Gremplewicz K, Jozkowicz A, Dulak J (2015) The role of oxidative stress in skeletal muscle injury and regeneration: focus on antioxidant enzymes. J Muscle Res Cell Motil 36:377–393
Kuhn KS, Schuhmann K, Stehle P, Darmaun D, Fürst P (1999) Determination of glutamine in muscle protein facilitates accurate assessment of proteolysis and de novo synthesis–derived endogenous glutamine production. Am J Clin Nutri 70:484–489
Landar A, Rustandi RR, Weber DJ, Zimmer DB (1998) S100A1 utilizes different mechanisms for interacting with calcium-dependent and calcium-independent target proteins. Biochemistry 37:17429–17438
Lambertucci AC, Lambertucci RH, Hirabara SM, Curi R, Moriscot AS, Alba-Loureiro TC, Guimarães-Ferreira L, Levada-Pires AC, Vasconcelos DAA, Sellitti DF, Pithon-Curi TC (2012) Glutamine supplementation stimulates protein-synthetic and inhibits protein-degradative signaling pathways in skeletal muscle of diabetic rats. PLoS ONE 7:e50390
Leclerc E, Fritz G, Weibel M, Heizmann CW, Galichet A (2007) S100B and S100A6 differentially modulate cell survival by interacting with distinct RAGE (receptor for advanced glycation end products) immunoglobulin domains. J Biol Chem 282:31317–31331
Lacey JM, Wilmore DW (1990) Is glutamine a conditionally essential amino acid? Nutri Rev 48(8):297–309
Marelli G, Sica A, Vannucci L, Allavena P (2017) Inflammation as target in cancer therapy. Curr Opin Pharmacol 35:57–65
Matés JM, Pérez-Gómez C, Núñez de Castro I, Asenjo M, Márquez J (2002) Glutamine and its relationship with intracellular redox status, oxidative stressand cell proliferation/death. Int J Biochem Cell Biol 34:439–458
McRae MP (2017) Therapeutic benefits of glutamine: An umbrella review of meta-analyses. Biomed Rep 6:576–584
Miyabara EH, Aoki MS, Soares AG, Moriscot AS (2005) Expression of tropism-related genes in regenerating skeletal muscle of rats treated with cyclosporin-A. Cell Tissue Res 319:479–489
Miyabara EH, Martin JL, Griffin TM, Moriscot AS, Mestril R (2006) Overexpression of inducible 70-kDa heat shock protein in mouse attenuates skeletal muscle damage induced by cryolesioning. Am J Physiol Cell Physiol 290:C1128–1138
Moore BW, McGregor D (1965) Chromatographic and electrophoretic fractionation of soluble proteins of brain and liver. J Biol Chem 240:1647–1653
Morris CR, Hamilton-Reeves J, Martindale RG, Sarav M, Ochoa Gautier JB (2017) Acquired amino acid deficiencies: a docus on arginine and glutamine. Nutrition in Clinical Practice 32:30S–47S
Mourkioti F, Kratsios P, Luedde T, Song YH, Delafontaine P, Adami R, Parente V, Bottinelli R, Pasparakis M, Rosenthal N (2006) Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J Clin Invest 116:2945–2954
Mukai M, Uchida K, Inoue G, Satoh M, Miyagi M, Yokozeki Y, Hirosawa N, Matsuura Y, Ohtori S, Takaso M (2022) Nerve decompression surgery suppresses TNF-a expression and T cell infiltration in a rat sciatic nerve chronic constriction injury model. J Orthop Res 40:2537–2545
Munn LL (2017) Cancer and inflammation. Wiley Interdiscip Rev Syst Biol Med 9:e1370
Nascimento TL, Silva MT, Miyabara EH (2018) BGP-15 improves contractile function of regenerating soleus muscle. J Muscle Res Cell Motil 39:25–34
Newsholme P (2001) Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J Nutr 131:2515S-2522S (discussion 2523S-2514S)
Newsholme P, Lima MMR, Procopio J, Pithon-Curi TC, Doi SQ, Bazotte RB, Curi R (2003) Glutamine and glutamate as vital metabolites. Brazilian J Med Biol Res 36:153–163
Nishikawa Y, Kajiura Y, Lew JH, Kido JI, Nagata T, Naruishi K (2017) Calprotectin induces IL-6 and MCP-1 production via toll-like receptor 4 signaling in human gingival fibroblasts. J Cell Physiol 232:1862–1871
Novak F, Heyland DK, Avenell A, Drover JW, Su X (2002) Glutamine supplementation in serious illness: a systematic review of the evidence. Crit Care Med 30:2022–2029
Nukui T, Ehama R, Sakaguchi M, Sonegawa H, Katagiri C, Hibino T, Huh NH (2008) S100A8/A9, a key mediator for positive feedback growth stimulation of normal human keratinocytes. J Cell Biochem 104:453–464
Oki K, Wiseman RW, Breedlove SM, Jordan CL (2013) Androgen receptors in muscle fibers induce rapid loss of force but not mass: implications for spinal bulbar muscular atrophy. Muscle Nerve 47:823–834
Oppenheim JJ, Yang D (2005) Alarmins: chemotactic activators of immune responses. Curr Opin Immunol 17:359–365
Parry-Billings M, Evans J, Calder PC, Newsholme EA (1990) Does glutamine contribute to immunosuppression after major burns? Lancet 336:523–525
Pereira MG, Silva MT, Carlassara EO, Goncalves DA, Abrahamsohn PA, Kettelhut IC, Moriscot AS, Aoki MS, Miyabara EH (2014) Leucine supplementation accelerates connective tissue repair of injured tibialis anterior muscle. Nutrients 6:3981–4001
Pereira MG, Silva MT, da Cunha FM, Moriscot AS, Aoki MS, Miyabara EH (2015) Leucine supplementation improves regeneration of skeletal muscles from old rats. Exp Gerontol 72:269–277
Petry ER, Cruzat VF, Heck TG, Leite JS, de Bittencourt PIH Jr, Tirapegui J (2014) Alanyl-glutamine and glutamine plus alanine supplements improve skeletal redox status in trained rats: involvement of heat shock protein pathways. Life Sci 94:130–136
Petry ER, Cruzat VF, Heck TG, de Bittencourt PIH Jr, Tirapegui J (2015) L-glutamine supplementations enhance liver glutamine-glutathione axis and heat shock factor-1 expression in endurance-exercise trained rats. Int J Sport Nutr Exerc Metab 25:188–197
Prosser BL, Wright NT, Hernandez-Ochoa EO, Varney KM, Liu Y, Olojo RO, Zimmer DB, Weber DJ, Schneider MF (2008) S100A1 binds to the calmodulin-binding site of ryanodine receptor and modulates skeletal muscle excitation-contraction coupling. J Biol Chem 283:5046–5057
Prosser BL, Hernandez-Ochoa EO, Lovering RM, Andronache Z, Zimmer DB, Melzer W, Schneider MF (2010) S100A1 promotes action potential-initiated calcium release flux and force production in skeletal muscle. Am J Physiol Cell Physiol 299:C891–902
Rogero MM, Tirapegui J, Pedrosa RG, Castro IA, Pires IS (2006) Effect of alanyl-glutamine supplementation on plasma and tissue glutamine concentrations in rats submitted to exhaustive exercise. Nutrition 22:564–571
Rohde T, MacLean DA, Hartkopp A, Pedersen BK (1996) The immune system and serum glutamine during a triathlon. Euro J Appl Physiol Occupat Physiol 74:428–434
Roth E, Oehler R, Manhart N, Exner R, Wessner B, Strasser E, Spittler A (2002) Regulative potential of glutamine–relation to glutathione metabolism. Nutrition 18:217–221
Senf SM (2013) Skeletal muscle heat shock protein 70: diverse functions and therapeutic potential for wasting disorders. Front Physiol 4:330
Shabani F, Farasat A, Mahdavi M, Gheibi N (2018) Calprotectin (S100A8/S100A9): a key protein between inflammation and cancer. Inflamm Res 67:801–812
Shahsavari A, Azad M, Mobarra N, Chegini KG, Gheibi N (2016) Calprotectin pegylation enhanced its physical and structural properties. Protein J 35:363–370
Shang M, Cappellesso F, Amorim R, Serneels J, Virga F, Eelen G, Carobbio S, Rincon MY, Maechler P, De Bock K, Ho PC, Sandri M, Ghesquiere B, Carmeliet P, Di Matteo M, Berardi E, Mazzone M (2020) Macrophage-derived glutamine boosts satellite cells and muscle regeneration. Nature 587:626–631
Silva MT, Wensing LA, Brum PC, Camara NO, Miyabara EH (2014) Impaired structural and functional regeneration of skeletal muscles from beta2-adrenoceptor knockout mice. Acta Physiol (Oxf) 211:617–633
Takano APC, Munhoz CD, Moriscot AS, Gupta S, Barreto-Chaves MLM (2017) S100A8/MYD88/NF-қB: a novel pathway involved in cardiomyocyte hypertrophy driven by thyroid hormone. J Mol Med (berl) 95:671–682
Tanaka T, Shibazaki A, Ono R, Kaisho T (2014) HSP70 mediates degradation of the p65 subunit of nuclear factor kappaB to inhibit inflammatory signaling. Sci Signal 7:ra119
Tomaz da Silva M, Santos AR, Koike TE, Nascimento TL, Rozanski A, Bosnakovski D, Pereira LV, Kumar A, Kyba M, Miyabara EH (2023) The fibrotic niche impairs satellite cell function and muscle regeneration in mouse models of Marfan syndrome. Acta Physiol (Oxf) 237:e13889
Tsubuku S, Hatayama K, Mawatari K, Smriga M, Kimura T (2004) Thirteen-week oral toxicity study of L-glutamine in rats. Int J Toxicol 23:107–112
Wagenmakers AJ (1998) Muscle amino acid metabolism at rest and during exercise: role in human physiology and metabolism. Exerc Sport Sci Rev 26:287–314
Wang C, Zhu Y, Wu D, Wang Z, Xu X, Shi Y, Yang G, Yu Y, Peng X (2020) The role of PDIA3 in myogenesis during muscle regeneration. Exp Mol Med 52:105–117
Wernerman J, Hammarqvist F (1999) Modulation of endogenous glutathione availability. Curr Opin Clin Nutri Metabol Care 2:487–492
Wirén M, Magnusson KE, Larsson J (1998) The role of glutamine, serum and energy factors in growth of enterocyte-like cell lines. Int J Biochem Cell Biol 30:1331–1336
Wischmeyer PE (2002) Glutamine and heat shock protein expression. Nutrition 18:225–228
Yin H, Price F, Rudnicki MA (2013) Satellite cells and the muscle stem cell niche. Physiol Rev 93:23–67
Zhang X, Mosser DM (2008) Macrophage activation by endogenous danger signals. J Pathol 214:161–178
Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA (2008) Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab 28:53–63
Zhou X, Thompson JR (1997) Regulation of protein turnover by glutamine in heat-shocked skeletal myotubes. Biochim Biophys Acta 1357:234–242
Acknowledgements
We are thankful to Ajinomoto for providing us with L-glutamine and Bianca R. Baggio (M.S. fellowship from CAPES) for technical assistance.
Funding
This study was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (Grants No. 18/24946-4 and No. 20/15351-7) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Fellowship/Grant No. 314857/2021-4). A.R.S. and T.E.K. received Ph.D. fellowships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and FAPESP (Grant No. 17/09069-4), respectively. T.C.S. received an M.S. fellowship from CAPES. A.M.S. and N.C.M. received scientific initiation fellowships from Programa Unificado de Bolsas (PUB) of University of São Paulo. A.M.S. also received scientific initiation fellowship from FAPESP (Grant No. 23/00289-2).
Author information
Authors and Affiliations
Contributions
MSA and EHM: designed the study; ARS, TEK, AMS, NCM, RADA, TCS, MSA and EHM: performed the experiments and/or analyzed the data; ARS, TEK, AMS, NCM, RADA, TCS, MSA and EHM: interpreted the data; ARS, TEK, AMS, NCM, RADA, TCS, MSA and EHM: wrote or revised the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All animal experiments were approved by the Animal Research Ethics Committee of the Institute of Biomedical Sciences of the University of São Paulo (Permit No. 71/2017).
Code availability
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors have read and agreed to the publication of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santos, A.R., Koike, T.E., Santana, A.M. et al. Glutamine supplementation accelerates functional recovery of EDL muscles after injury by modulating the expression of S100 calcium-binding proteins. Histochem Cell Biol 160, 135–146 (2023). https://doi.org/10.1007/s00418-023-02194-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-023-02194-5