Abstract
Background
Glutamine plays a key role in several essential metabolic processes and is an important modulator of the heat shock protein (HSP) response, a crucial mechanism to maintain cellular homeostasis and to promote cell resistance to injury and death. This review summarized the effects of free l-glutamine or the dipeptide l-alanyl-l-glutamine upon muscle injury and inflammation, as well as muscle recovery from resistance training.
Main body of the abstract
The 70-kDa HSP (HSP70) expression is enhanced by glutamine, via the hexosamine biosynthetic pathway, which inhibits the NF-κB pathway regenerating and recovering myofibers through the regulation of the early inflammatory response to muscle injury, which may be impaired by local and systemic inflammatory injury due to reduced intracellular levels of HSP70.
Short conclusion
Studies show that chronic oral administration of free l-glutamine or the dipeptide can attenuate the injury and inflammation induced by intense aerobic and exhaustive exercise. However, the effects on muscle recovery from resistance training are unclear.
Similar content being viewed by others
Background
Glutamine is a versatile amino acid, abundant in the plasma and skeletal muscle, accounting for most of the intramuscular free amino acid content. It is synthesized from glutamate and ammonia by the enzyme glutamine synthetase, and is stored and released predominantly by the skeletal muscle [1]. This amino acid is also synthesized by adipocytes, liver, and lung, and after its release into the bloodstream, glutamine is transported to be metabolized in several tissues [2]. As a precursor for purines and pyrimidines, glutamine enables the synthesis of DNA and RNA, for mRNA synthesis and DNA repair of nucleotide and nucleic acids [3,4,5]. This amino acid is also used as the main oxidative fuel to replenish intermediates of the tricarboxylic acid cycle in rapidly dividing cells [1], such as enterocytes and colonocytes [6], fibroblasts, and immune cells such as lymphocytes [7, 8], macrophages, and neutrophils [9,10,11,12]. The members of the solute carrier (SLC) 38 gene family are the principal transporters of glutamine in mammalian cells, allowing the extremely rapid cellular turnover rates of glutamine flux [13, 14] and the redox control [15].
Glutamine is the major inter-organ nitrogen transporter and regulator of acid-base balance. In the kidneys, glutamine is used by the tubular epithelial cells providing NH3 for urea synthesis and elimination of the excess acid [1, 16]. The skeletal muscle amino acid metabolism generates glutamine to detoxify the ammonia produced [17, 18]. Additionally, glutamine contributes to the intermediary metabolism [19] in the synthesis of amino sugars and proteins [20,21,22], promotes insulin secretion from pancreatic beta cells, and is the precursor of key molecules such as the excitatory neurotransmitter glutamate, the inhibitory neurotransmitter γ-aminobutyrate (GABA), and the antioxidant glutathione (GSH) [1, 23, 24], considered a powerful marker of the cellular redox potential [25, 26]. It has also been demonstrated that glutamine enhances the tight junction protein abundance, maintaining the integrity of the intestinal mucosal barrier and improving its function [27, 28]. Recently, glutamine was suggested to reduce the intestinal catabolism of amino acids, which may improve their bioavailability in the systemic circulation [29]. Notwithstanding, glutamine is also a potent inducer of the heat shock protein (HSP) response to maintain homeostasis, facilitating repair from injury and cell death [30, 31].
Despite being originally classified as a non-essential amino acid [32] in healthy individuals [33], abundant evidence suggests that glutamine is essential in specific stress situations such as severe illness, trauma, and overtraining [9, 34,35,36,37,38]. In hypercatabolic states, when the elevated demand exceeds the capacity to produce adequate amounts of this amino acid [39,40,41], the impairment of immune function may occur [37, 42]. Due to the important pleiotropic roles in metabolism and tissue homeostasis, glutamine is one of the most studied amino acids in exercise immunology [33].
Given the high consumption of free glutamine by intestinal cells, glutamine dipeptides have been studied as an alternative for transposing the intestinal barrier and increasing the bioavailability of this amino acid to cells of the immune system. Studies in animal models from our laboratory have shown that oral supplementation with l-glutamine and l-alanine administered in its free form or as dipeptide (l-alanyl-l-glutamine), during 8 weeks, can attenuate the tissue injury, inflammation, and immune suppression induced by intense aerobic and exhaustive exercise [43,44,45,46]. Conversely, the effects of these supplements on muscle recovery from resistance training are poorly elucidated. Thus, the aim of this review was to summarize the evidence regarding the effects of free l-glutamine or the dipeptide l-alanyl-l-glutamine upon muscle injury and inflammation, as well as on muscle recovery from resistance training.
Main text
Exercise-induced stress response
In sports, high metabolic stress followed by the short recovery period makes the athlete’s training routine exhaustive [47]. Muscle contractions from mechanical loading induce microtrauma in muscle fibers, resulting in the rupture of the extracellular matrix, basal lamina, and sarcolemma, in addition to the alteration of calcium homeostasis, which promotes changes in the cell membrane structure and permeability [48, 49]. Following structural damage and functional impairment of the muscle tissue, myofibrillar rupture and extravasation of intracellular proteins such as myoglobin, creatine kinase (CK), and lactate dehydrogenase (LDH), into the extracellular medium, trigger the local inflammatory response [47, 50,51,52]. Hence, exercise-induced stress response in the skeletal muscle is triggered by damage to protein structure and might be further increased by the secondary induced damage in addition to inflammatory processes [49].
The local inflammatory response involves muscle protein degradation systems that are orchestrated by a network of signalling pathways, activated or suppressed by hormones and cytokines [50, 53]. Protein degradation in muscle tissue is accompanied by a systemic acute phase response that may vary according to the type of exercise and its frequency, duration, and intensity [54]. Local inflammation is characterized by an increased number of infiltrating and resident immune cells, such as mast cells [55], neutrophils and T regulatory lymphocytes [56], eosinophils [57], and CD8 T lymphocytes [58] at the injury site, thereby releasing pro-inflammatory effectors. Macrophages are the predominant leucocytes observed during the regeneration phase of the stretch-injured skeletal muscle, exerting specific roles throughout the whole process. After muscle injury, tissue-resident macrophages migrate to the injured area, and part of them prevents complete monocytes’ recruitment from the circulation [59]. Briefly, exercise-induced inflammatory processes include the release of cytokines and chemokines driving a rapid influx of neutrophils, followed by the differentiation of monocytes into macrophages that promote the phagocytosis of necrotic muscle debris. These cells switch then into anti-inflammatory macrophages and proliferate during the regeneration process of the damaged skeletal muscle [60].
During local inflammation occurs the synthesis and release of molecules such as monocyte chemotactic protein (MCP)-1, chemokine derived from macrophage (MDC), tumour necrosis factor (TNF)-α, interleukin (IL)-8, vascular endothelial growth factor (VEGF), leukaemia inhibitory factor (LIF), fractalkine, and urokinase plasminogen activator (uPA) [53, 61]. Most of these proteins act as chemotactic factors at the site of inflammation, promoting the initial recruitment of satellite cells, neutrophils, monocytes, and, later, lymphocytes for tissue repair [52, 62,63,64,65]. Eccentric exercise is acknowledged for the generation of a local inflammatory response in the skeletal muscle with the timing and peak of neutrophil infiltration linked to the magnitude of muscle function decrements [66, 67]. Intense exercise stimulates a well-defined systemic cytokine response, associated with the exercise-induced metabolic stress responses [68, 69]. The systemic response initiates with a rapid increase of pro-inflammatory components (IL-6, IL-8), which in turn generates an anti-inflammatory feedback by increasing the release of interleukin (IL)-10 and interleukin (IL)-1 receptor antagonist [70].
The resolution of inflammation characterizes a shift from a pro-inflammatory state to the anti-inflammatory phase, followed by repair and regeneration of injured tissues, processes markedly played by macrophages that include angiogenesis, matrix remodelling, and establishment of homeostasis [71]. This process is vital for the recovery of injured muscle; however, continuous muscle injury triggers a chronic inflammatory response, which can aggravate the underlying lesions by degrading intact proteins, implying reduced performance and compromised health [52, 72, 73]. Systemic inflammation is associated with reduced rates of protein synthesis in addition to an enhanced protein breakdown [74]. In this regard, pro-inflammatory cytokines may account for the loss of muscle mass by activating catabolic and downregulating the anabolic pathways [75].
Intense training with continuous rest deprivation increases the release of pro-inflammatory indicators, which may induce fatigue and overtraining syndrome in athletes [53]. The effects exerted by pro-inflammatory cytokines on muscle mass are partially mediated by the induction of the transcription factor NF-κB signalling [48, 76, 77]. In a single bout of intense resistance exercise and in an acute bout of treadmill run, NF-κB activity is increased in the skeletal muscle of humans and rats, respectively [78, 79], in addition to an increase in genic expression of the interleukins IL-6, IL-8, IL-1β, and IL-15 and of TNF-α, MCP-1, LIF, and TG-β [53]. It also observed increased levels of anti-inflammatory agents, such as IL-10 and MCP-1, 24 h after eccentric exercise, in an endeavor to contain inflammation [78].
It has been proposed that cytokines (e.g. IL-10, IL-13, and IL-15) may have anabolic effects and modify the contractile function of the skeletal muscle. Cytokine secretion by the skeletal muscle involves several intracellular factors such as MCP-1, heat shock factor (HSF)-1, and histone deacetylases, besides nuclear factor of activated T cells and NF-κB [53, 61, 76, 80, 81]. The NF-κB signalling pathway acts as the central regulator of the stress-induced mechanical, oxidative, and inflammatory responses [52, 77]. However, its persistent activation, as well as increased synthesis of inflammatory molecules, may excessively recruit immune cells, consequently promoting additional tissue damage [73]. Under these conditions, protective systems such as HSP are activated against excessive inflammatory damage induced by exercise, in order to restore homeostasis and ensure cell survival [30, 31].
Physical exercise and heat shock proteins
One of the most basic mechanisms of cellular defence includes the expression of HSP to neutralize harmful agents and events, induce cell protection and tolerance to injury, and warrant maximum cell survival in the skeletal muscle [82]. HSP is a highly conserved family of stress-inducible proteins, essential for cellular homeostasis, protecting against a variety of stress stimulus [83, 84], injury, and death and modulating the early inflammatory response to muscle injury [30, 31]. These proteins are named according to the molecular weight as follows: HSP90, HSP70, HSP60, and HSP27, and the upregulation under stress conditions provides cytoprotection by re-establishing protein homeostasis against several stressors, including exercise [85]. Under normal physiological conditions, HSP acts as a chaperone protein helping the protein folding (mainly unfolded, misfolded, and partially folded new peptide chains) and translocation into the endoplasmic reticulum lumen [86]. When the body is under excessive stress, these proteins exert a protective role by lessening oxidative action of the reactive oxygen species (ROS) and a wide range of metabolic stress, including structural and functional myodamage [87, 88]. Despite the fact that exercise is a potent inductor of the HSP response [89], local and systemic inflammatory lesion leads to a reduction in intracellular HSP70 levels, which may impair tissue readjustment [30, 89, 90].
Modifications in gene expression occur to yield an increase in the content of HSP [30, 31], proteins that act as molecular chaperones, being crucial in helping the cellular remodelling processes of denatured proteins, independent of the training response [89, 91]. A reduction in pro-inflammatory cytokine release has been observed following the initiation of a heat shock response [85], and this process may be related to the binding of HSP to the heat shock element (HSE) found in the promoters of cytokine genes (e.g. IL-1β) [92, 93]. During stress, the latent monomer of heat shock factor (HSF)-1 is rapidly converted to a trimeric form active in the nucleus to bind to the promoters of HSF-responsive heat shock genes and activate their transcription [94, 95]. HSF-1 has been demonstrated to perform this function by repressing the transcription of cytokine genes, including TNF-α and IL-1β, antagonizing the acute phase response [92, 96]. Because IL-1β immediately responds to a wide diversity of pro-inflammatory insults and affects the function of many targets, it is essential to limit the potentially harmful aspects of inflammation by negatively regulating IL-1β expression [92].
HSP27 and HSP70 are considered the most robust and recognized induced chaperones; both play important cytoprotective roles acting at multiple apoptotic pathway control points to ensure that stress-induced injury does not inappropriately trigger cell death, thus disabling apoptosis [97]. The induction of HSP is characterized by low transient regulation of most cellular proteins and the expression of the 70-kDa protein family (HSP70). A 72-kDa stress-inducible Hsp72, a prominent member of the HSP70 family, is one of the largest inducible HSP isoforms interacting with other proteins in a way dependent of ATP and has been extensively studied in the mammalian skeletal muscle [31]. The Hsp72 expression is more abundant in slow-oxidative than fast-glycolytic skeletal muscle fibres, and the expression is elevated by the increased contractile activity of the muscles with exercise, as well as heat stress [98].
HSP70 has been involved in the regulation steps of skeletal muscle plasticity [30, 31, 99, 100], apoptosis, and cell death, affecting protein refolding processes, signalling for ubiquitin degradation, and translocation of proteins [101]. Both exhaustive endurance exercise and resistance exercise with maximal eccentric repetitions have been shown to increase the level of Hsp72 expression [102]. In general, HSP70 is induced by diversified stimuli such as hypoxia, acidosis, increased muscle temperature, and ischaemia-reperfusion, most of them are by-products of resistance exercise associated with elevated levels of metabolic stress [30, 89]. Conversely, in exercise, HSP facilitates mitochondrial biogenesis, despite regulating the signalling pathways associated with apoptosis [49, 103].
HSP70 is also involved in the control of the primary response to muscle injury [30] and inhibition of the NF-κB signalling pathway by modulating the inflammatory response and attenuating pro-inflammatory cytokine release [104,105,106]. The chaperone equilibrium hypothesis proposes that NF-κB activation may decrease intracellular levels of HSP70, releasing extracellular HSP70 as a pro-inflammatory component, which may be linked to reduced oxidative stress in target cells. Nonetheless, when extracellular HSP70 is continuously elevated, it stimulates inflammation, oxidative stress, reduced expression of HSF-1, and possibly reduced intracellular HSP70 [91].
Stress conditions promoting instability and denaturation of proteins induce the release of HSF-1, which the activity is associated with the expression of HSP70 in the myocardium, skeletal muscle, and human leucocytes [76, 107]. Evidence suggests that the increased HSP expression on the leucocyte surface after acute intense training signals excessive stress [108]. In general, the expression of HSP70 has become a major interest of studies because of its role in modulating inflammatory immune response and cytoprotection under stress conditions in a wide diversity of experimental injury models [73, 109]. In this sense, the effect of glutamine as a potential therapeutic element has been observed. Glutamine improves HSP70 and HSP27 expression [43, 44, 110] and acts not only as a modulator of the heat shock response but also as a competent inducer of HSF-1 expression, activating its transcription [93, 111, 112].
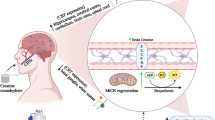
The hexosamine biosynthetic pathway (HBP) has been shown to induce HSP70 expression, and glutamine is an essential substrate for this pathway. Its activity is enhanced by glutamine via O-glycosylation, leading to the translocation and transcriptional stimulation of key transcription factors (HSF-1 and Sp1) required for maximal HSP70 induction [113]. The expression of the HSF-1, HSP70, and HSP27 all depend on Sp1 for optimal transcriptional activity [114]. Considering the effect of glutamine on Sp1 and in the modulation of the HSP response [93, 109, 111, 115], this amino acid has been considered an important therapeutic element [33]. Hence, glutamine could be used to induce a beneficial stress response and prevent tissue damage under disturbing conditions (Fig. 1). Moreover, HSP synthesis has shown to be dependent on adequate concentrations of glutamine [93].
Glutamine modulates the exercise-induced heat shock and inflammatory responses. The heat shock response is induced by stress signals produced during exercise. HSP70 and HSP27 are upregulated under stress conditions providing cytoprotection by remodeling misfolded and unfolded proteins and limiting damage induced by reactive oxygen species (ROS) and inflammatory stimulus. The latent heat shock factor (HSF)-1 monomer is converted to a trimeric active form in the nucleus during stress to bind to the promoters of heat shock genes and activate transcription. HSP70 also regulates the primary response to muscle lesion and inhibits the NF-κB signalling pathway by modulating the inflammatory response and attenuating pro-inflammatory cytokines release. Glutamine improves HSP70 and HSP27 expression and acts not only as a modulator of the heat shock response but also as a competent inducer of HSF-1 expression, activating its transcription. Glutamine enhances the hexosamine biosynthetic pathway (HBP), via O-glycosylation, contributing to the translocation and induction of HSF-1 transcription (→, stimulation; ⊥, inhibition; --›, translocation)
Roles of glutamine in response to exercise
Glutamine is endogenously synthesized from α-ketoglutarate, an intermediary metabolite of the citric acid cycle, in two steps mediated by the enzymes glutamate dehydrogenase and glutamine synthetase, that convert α-ketoglutarate into glutamate using NADPH and glutamate into glutamine using NH3, respectively [1, 22, 50]. This amino acid is essential for function and proliferation of cells that are rapidly dividing (e.g. enterocytes), as well as for the phagocytic activity of macrophages and production of GSH, which is the most potent antioxidant in the body [20, 33, 35]. The small non-protein thiol, GSH, plays a key role in maintaining the redox balance. The glutathione system (NADPH, glutathione reductase, and GSH), one of the major cellular thiol-dependent antioxidant mechanisms, participates in the synthesis and repair of DNA [116]. Accordingly, the elevation of GSH levels by dipeptides of glutamine enhances the antioxidant capacity reducing cell damage [117, 118].
Glutamine improves the production of HSP and activates the degradation of the NF-κB p65 subunit in the nucleus, protecting against excessive inflammatory states and cell death by apoptosis [23, 119,120,121,122]. HSP72 is indicated to participate as a stress-stimulated inducer of the microbicide activity of neutrophils during moderate exercise [123]. Thus, the modulatory effect of glutamine on the heat shock response may affect neutrophil function [124]. Moreover, glutamine might decrease the synthesis of IL-8 (the major neutrophil chemoattractant) in athletes [33].
Glutamine supplementation is a strategy used in situations of intense catabolism, in which there is a decrease in the synthesis and release of glutamine by the skeletal muscle and increased uptake by other organs (kidneys and liver), such as in prolonged and intense exercises, as well as in overtraining syndrome [125, 126]. This condition may be associated with immunosuppression, in view of the lower availability of this amino acid for the immune cell metabolism [1]. The decrease in glutamine concentration mediated by exhaustive exercise is often concomitant with a decrease in the number of circulating lymphocyte and immune cell function as seen in both lymphocytes and NK cells [36] since these cells present glutaminase, the major degradation enzyme of glutamine [34, 124]. Moreover, the maintenance or increase in skeletal muscle glutamine concentration might be fundamental for muscle protein synthesis [127,128,129], to prevent muscle atrophy [38, 130] and to increase glycogen synthesis [131], particularly under catabolic conditions.
Blomstrand and Essen-Gustavsson [132] observed significant reductions in vastus lateralis muscle glutamine concentrations of male subjects 2 h following 40 leg press repetitions at 80% of their maximum, indicating glutamine homeostasis disturbance following eccentric exercise, as already demonstrated in the literature [133].
A study with l-glutamine supplementation (0.3 g kg−1 day−1 + 0.3 g kg−1 day−1 maltodextrin), following eccentric exercise, has found attenuated strength loss, shorter strength recovery, and muscle soreness at 24, 48, and 72 h postexercise in the quadriceps muscle of healthy participants [67]. An enhancement of protein synthesis and attenuation of catabolic responses induced by heavy resistance training, which were related to increased muscular hypertrophy and reduced exercise-induced immunosuppression, have additionally been observed after glutamine supplementation [134]. It has been suggested that the effects of glutamine are mixed, and studies fail to demonstrate positive benefits on buffering capacity, time to exhaustion, protein balance, and other ergogenic effects regarding muscle recovery from exercise [135]. While a study showed that glutamine supplementation inhibits total body proteolysis by increasing leucine flux [136], other did not find any effect of glutamine on protein degradation markers [137].
Although glutamine supplementation in exercise has shown benefits, the effectiveness of oral administration has been questioned because approximately 50% of glutamine is metabolized by cells of the intestinal mucosa and liver before the peripheral circulation and skeletal muscle are achieved [138]. Hence, the bioavailability of this amino acid to cells of the immune system may be compromised [45]. Nonetheless, the alternative for transposing the intestinal barrier has been the utilization of glutamine dipeptides [2], such as l-alanyl-l-glutamine (DIP), due to the higher stability during heat sterilization and storage and higher solubility compared to free glutamine [139]. Lima et al. [140] reported high stability of alanine and glutamine together, possibly via an improvement in intestinal ion transporters, which may be responsible for the lower fatigue feelings in soccer players [141].
A previous study carried out for us investigated the acute and chronic effects of oral supplementation with DIP on glutamine concentrations in plasma and tissues. Rogero et al. [142] demonstrated that acutely, DIP supplementation increased plasma glutamine concentration, and chronically, DIP administration promoted increased glutamine stores in the muscle and hepatic tissues of healthy rats. In 2006 [45], it was demonstrated that chronic DIP supplementation increased glutamine concentrations in the gastrocnemius and soleus muscles immediately after an exhaustion test, compared to chronic glutamine supplementation. In the long-term exercise, oral supplementation with DIP or l-glutamine associated with l-alanine, both in the free form, represents an efficient alternative for the supply of glutamine and glutamate to the organism, promoting higher muscular and hepatic stocks of glutathione and influencing the cellular redox state [46].
The efficacy of DIP is due to the intestinal transporter (PepT)-1, which facilitates a wide absorption of dipeptides and tripeptides, behaving as a facilitated diffusion peptide transporter. The mechanism underlying the clearance of dipeptides is suggested to be exerted by hydrolysis through the membrane-bound peptide hydrolases [143]. DIP also warrants the supply of more glutamine molecules in the osmolality required for physiological fluids [43, 144]. Hence, the combination of glutamine and alanine allows the enhancement of electrolyte and fluid absorption compared to glutamine alone, and this effect is likely due to the specific ion transporters, increasing the absorption rate in intestinal epithelia [140]. Recently, the dipeptide l-alanylglutamine was suggested to inhibit signalling proteins that trigger protein degradation following an acute bout of resistance exercise [145].
In a study that evaluated rehydration with DIP in a sports drink during an hour of endurance exercise at submaximal intensity, the authors observed an increase in the reaction time of athletes to visual stimuli [146]. The intake of DIP during a moderate intensity run was also investigated, and the results indicated a significant improvement in performance during a subsequent exhaustion test. The authors of both studies attributed the results to an improvement in the intestinal absorption of fluids and electrolytes and possibly increased skeletal muscle uptake causing greater neuromuscular performance, besides a possible gluconeogenic effect of alanine, sparing muscle glycogen, and retarding fatigue [144, 146]. Although glutamine is a major gluconeogenic substrate, mainly in the kidney, alanine also contributes donating carbon for gluconeogenesis, being essentially confined to the liver [147].
In 2010, Hoffman et al. [148] found that the dipeptide l-alanyl-l-glutamine administration provided a beneficial ergogenic effect by increasing time to exhaustion following a mild hydration stress, and the effects were linked to an enhanced fluid and electrolyte absorption. In 2012, Hoffman et al. [149] demonstrated that rehydration with the dipeptide contributes in maintaining basketball skill performance and visual reaction time. The authors also suggested an enhanced intestinal fluid and electrolyte uptake, thus preserving the neural commands for fine motor control during physical activities. Investigations with animal model induced to intense and exhaustive aerobic exercise protocols or, in situations of high catabolism, such as sepsis, show that chronic supplementation with DIP or with glutamine and alanine in their free forms is efficient for the supply of glutamine to the body, which can attenuate biomarkers of injury and inflammation after periods of intense training, as well as attenuate the inflammatory response induced by long-term exercise [142].
In 2016 [150], we evaluated the effect of glutamine and alanine supplementation in their free forms or as DIP in rats subjected to intense resistance exercise and compared with the effects of free alanine. It was found that animals supplemented with l-glutamine presented increased glutamine concentration in plasma and muscle tissues, in addition to a reduction in the GSSG/GSH ratio, TBARS, and CK rates. The contents of HSF-1 and HSP-27 were elevated in all supplemented groups. The authors concluded that supplementations with l-glutamine and l-alanine either in free form or as DIP improved the GLN-GSH axis and promoted cytoprotective effects against oxidative stress caused by resistance exercise [150]. In a study that also used an intense resistance exercise protocol during 8 weeks, we showed that plasma and muscle glutamine levels were restored in trained rats receiving supplements containing glutamine in both forms. Additionally, there was an increase in HSP70 content in the skeletal muscle and peripheral blood mononuclear cells, concomitant with reduced activation of NF-κB and decreased concentration of cytokines [151].
Unlike skeletal muscle, leucocytes are largely dependent of the glutamine synthetized and released into the blood by the skeletal muscle, to satisfy their metabolic requirements [5, 152]. Raizel et al. also found muscle protection, shown by reduced plasma levels of CK, LDH, TNF-α, and IL-1β, in addition to the increased concentration of IL-6, IL-10, and MCP-1. Thus, oral supplementation with l-glutamine (administered with l-alanine or as DIP) has been shown to induce HSP70-mediated cytoprotective effects in response to muscle injury and inflammation [151]. In addition to these results, our group has recently demonstrated that the form of glutamine administration (free along l-alanine or as l-alanyl-l-glutamine) is an important factor determining improvement or impairment of central fatigue parameters in rats submitted to 8 weeks of heavy resistance training [153].
Conclusions
Although studies are contradictory regarding the effect of free glutamine supplementation on muscle injury and inflammation, due to the high intestinal and hepatic metabolism with consequently decreased availability of consuming organs and cells of the immune system, current evidence indicate that oral supplementation with free l-glutamine or the dipeptide provides an effective alternative for increasing plasma and muscle glutamine concentrations. Thus, cytoprotective systems, such as the heat shock response, and the body antioxidant system appear to be preserved and effectively activated in response to muscle injury and inflammation induced by intense resistance training.
Studies show that chronic oral administration of free l-glutamine or the dipeptide can attenuate the injury and inflammation induced by intense aerobic and exhaustive exercise. However, the effects on muscle recovery from resistance training are unclear.
Abbreviations
- CK:
-
Creatine kinase
- DIP:
-
l-alanyl-l-glutamine
- GABA:
-
γ-Aminobutyrate
- GSH:
-
Glutathione
- HSE:
-
Heat shock element
- HSF:
-
Heat shock factor
- HSP:
-
Heat shock proteins
- HSP70:
-
70-kDa protein family
- IL:
-
Interleukin
- LDH:
-
Lactate dehydrogenase
- LIF:
-
Leukaemia inhibitory factor
- MCP:
-
Monocyte chemotactic protein
- MDC:
-
Chemokine derived from macrophage
- PepT:
-
Intestinal transporter
- ROS:
-
Reactive oxygen species
- SLC:
-
Solute carrier
- TNF:
-
Tumour necrosis factor
- uPA:
-
Fractalkine and urokinase plasminogen activator
- VEGF:
-
Vascular endothelial growth factor
References
Newsholme P, Procopio J, Lima MM, Pithon-Curi TC, Curi R. Glutamine and glutamate--their central role in cell metabolism and function. Cell Biochem Funct. 2003;21(1):1–9.
Curi R, Lagranha CJ, Doi SQ, Sellitti DF, Procopio J, Pithon-Curi TC. Glutamine-dependent changes in gene expression and protein activity. Cell Biochem Funct. 2005;23(2):77–84.
Szondy Z, Newsholme EA. The effect of various concentrations of nucleobases, nucleosides or glutamine on the incorporation of [3H]thymidine into DNA in rat mesenteric-lymph-node lymphocytes stimulated by phytohaemagglutinin. Biochem J. 1990;270(2):437–40.
Szondy Z, Newsholme EA. The effect of glutamine concentration on the activity of carbamoyl-phosphate synthase II and on the incorporation of [3H]thymidine into DNA in rat mesenteric lymphocytes stimulated by phytohaemagglutinin. Biochem J. 1989;261(3):979–83.
Ardawi MS. Glutamine and glucose metabolism in human peripheral lymphocytes. Metabolism. 1988;37(1):99–103.
Windmueller HG. Glutamine utilization by the small intestine. Adv Enzymol Relat Areas Mol Biol. 1982;53:201–37.
Newsholme EA, Crabtree B, Ardawi MS. Glutamine metabolism in lymphocytes: its biochemical, physiological and clinical importance. Q J Exp Physiol. 1985;70(4):473–89.
Ardawi MS, Newsholme EA. Metabolism in lymphocytes and its importance in the immune response. Essays Biochem. 1985;21:1–44.
Lacey JM, Wilmore DW. Is glutamine a conditionally essential amino acid? Nutr Rev. 1990;48(8):297–309.
Wirén M, Magnusson KE, Larsson J. The role of glutamine, serum and energy factors in growth of enterocyte-like cell lines. Int J Biochem Cell Biol. 1998;30(12):1331–6.
Curi TC, De Melo MP, De Azevedo RB, Zorn TM, Curi R. Glutamine utilization by rat neutrophils: presence of phosphate-dependent glutaminase. Am J Phys. 1997;273(4 Pt 1):C1124–9.
Ardawi MS, Newsholme EA. Fuel utilization in colonocytes of the rat. Biochem J. 1985;231(3):713–9.
Bode BP. Recent molecular advances in mammalian glutamine transport. J Nutr. 2001;131(9 Suppl):2475S–85S discussion 2486S–2477S.
Mackenzie B, Erickson JD. Sodium-coupled neutral amino acid (system N/A) transporters of the SLC38 gene family. Pflugers Arch. 2004;447(5):784–95.
Scalise M, Pochini L, Galluccio M, Indiveri C. Glutamine transport. From energy supply to sensing and beyond. Biochim Biophys Acta. 2016;1857(8):1147–57.
Brosnan JT. Amino acids, then and now--a reflection on Sir Hans Krebs’ contribution to nitrogen metabolism. IUBMB Life. 2001;52(6):265–70.
Kuhn KS, Schuhmann K, Stehle P, Darmaun D, Fürst P. Determination of glutamine in muscle protein facilitates accurate assessment of proteolysis and de novo synthesis-derived endogenous glutamine production. Am J Clin Nutr. 1999;70(4):484–9.
Chang TW, Goldberg AL. The metabolic fates of amino acids and the formation of glutamine in skeletal muscle. J Biol Chem. 1978;253(10):3685–93.
Stumvoll M, Perriello G, Meyer C, Gerich J. Role of glutamine in human carbohydrate metabolism in kidney and other tissues. Kidney Int. 1999;55(3):778–92.
Curi R, Lagranha CJ, Doi SQ, et al. Molecular mechanisms of glutamine action. J Cell Physiol. 2005;204(2):392–401.
Newsholme P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J Nutr. 2001;131(9 Suppl):2515S–22S discussion 2523S–2514S.
Newsholme P, Lima MM, Procopio J, et al. Glutamine and glutamate as vital metabolites. Braz J Med Biol Res. 2003;36(2):153–63.
Roth E, Oehler R, Manhart N, et al. Regulative potential of glutamine--relation to glutathione metabolism. Nutrition. 2002;18(3):217–21.
Roth E. Nonnutritive effects of glutamine. J Nutr. 2008;138(10):2025S–31S.
Wernerman J, Hammarqvist F. Modulation of endogenous glutathione availability. Curr Opin Clin Nutr Metab Care. 1999;2(6):487–92.
Matés JM, Pérez-Gómez C, Núñez de Castro I, Asenjo M, Márquez J. Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int J Biochem Cell Biol. 2002;34(5):439–58.
Wang X, Pierre JF, Heneghan AF, Busch RA, Kudsk KA. Glutamine improves innate immunity and prevents bacterial enteroinvasion during parenteral nutrition. JPEN J Parenter Enteral Nutr. 2015;39(6):688–97.
Wang B, Wu Z, Ji Y, Sun K, Dai Z, Wu G. L-glutamine enhances tight junction integrity by activating CaMK kinase 2-AMP-activated protein kinase signaling in intestinal porcine epithelial cells. J Nutr. 2016;146(3):501–8.
Dai ZL, Li XL, Xi PB, Zhang J, Wu G, Zhu WY. L-glutamine regulates amino acid utilization by intestinal bacteria. Amino Acids. 2013;45(3):501–12.
Senf SM, Howard TM, Ahn B, Ferreira LF, Judge AR. Loss of the inducible Hsp70 delays the inflammatory response to skeletal muscle injury and severely impairs muscle regeneration. PLoS One. 2013;8(4):e62687.
Senf SM. Skeletal muscle heat shock protein 70: diverse functions and therapeutic potential for wasting disorders. Front Physiol. 2013;4:330.
Rose WC. The nutritive significance of the amino acids and certain related compounds. Science. 1937;86(2231):298–300.
Bermon S, Castell LM, Calder PC, et al. Consensus statement immunonutrition and exercise. Exerc Immunol Rev. 2017;23:8–50.
Castell L. Glutamine supplementation in vitro and in vivo, in exercise and in immunodepression. Sports Med. 2003;33(5):323–45.
Fläring UB, Rooyackers OE, Wernerman J, Hammarqvist F. Glutamine attenuates post-traumatic glutathione depletion in human muscle. Clin Sci (Lond). 2003;104(3):275–82.
Rohde T, MacLean DA, Hartkopp A, Pedersen BK. The immune system and serum glutamine during a triathlon. Eur J Appl Physiol Occup Physiol. 1996;74(5):428–34.
Parry-Billings M, Evans J, Calder PC, Newsholme EA. Does glutamine contribute to immunosuppression after major burns? Lancet. 1990;336(8714):523–5.
Lambertucci AC, Lambertucci RH, Hirabara SM, et al. Glutamine supplementation stimulates protein-synthetic and inhibits protein-degradative signaling pathways in skeletal muscle of diabetic rats. PLoS One. 2012;7(12):e50390.
Wagenmakers AJ. Muscle amino acid metabolism at rest and during exercise: role in human physiology and metabolism. Exerc Sport Sci Rev. 1998;26:287–314.
Antonio J, Street C. Glutamine: a potentially useful supplement for athletes. Can J Appl Physiol. 1999;24(1):1–14.
Morris CR, Hamilton-Reeves J, Martindale RG, Sarav M, Ochoa Gautier JB. Acquired amino acid deficiencies: a focus on arginine and glutamine. Nutr Clin Pract. 2017;32(1_suppl):30S–47S.
Ogle CK, Ogle JD, Mao JX, et al. Effect of glutamine on phagocytosis and bacterial killing by normal and pediatric burn patient neutrophils. JPEN J Parenter Enteral Nutr. 1994;18(2):128–33.
Petry ER, Cruzat VF, Heck TG, Leite JS, Homem de Bittencourt PI, Tirapegui J. Alanyl-glutamine and glutamine plus alanine supplements improve skeletal redox status in trained rats: involvement of heat shock protein pathways. Life Sci. 2014;94(2):130–6.
Petry É, Cruzat VF, Heck TG, Homem de Bittencourt PI, Tirapegui J. L-glutamine supplementations enhance liver glutamine-glutathione axis and heat shock factor-1 expression in endurance-exercise trained rats. Int J Sport Nutr Exerc Metab. 2015;25(2):188–97.
Rogero MM, Tirapegui J, Pedrosa RG, Castro IA, Pires IS. Effect of alanyl-glutamine supplementation on plasma and tissue glutamine concentrations in rats submitted to exhaustive exercise. Nutrition. 2006;22(5):564–71.
Cruzat VF, Rogero MM, Tirapegui J. Effects of supplementation with free glutamine and the dipeptide alanyl-glutamine on parameters of muscle damage and inflammation in rats submitted to prolonged exercise. Cell Biochem Funct. 2010;28(1):24–30.
Brooks K, Carter J. Overtraining, exercise, and adrenal insufficiency. J Nov Physiother. 2013;3(125).
Cooper DM, Radom-Aizik S, Schwindt C, Zaldivar F. Dangerous exercise: lessons learned from dysregulated inflammatory responses to physical activity. J Appl Physiol (1985). 2007;103(2):700–9.
Morton JP, Kayani AC, McArdle A, Drust B. The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med. 2009;39(8):643–62.
Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000;80(3):1055–81.
Baltusnikas J, Venckunas T, Kilikevicius A, Fokin A, Ratkevicius A. Efflux of creatine kinase from isolated soleus muscle depends on age, sex and type of exercise in mice. J Sports Sci Med. 2015;14(2):379–85.
Finsterer J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet Disord. 2012;13:218.
Peake JM, Della Gatta P, Suzuki K, Nieman DC. Cytokine expression and secretion by skeletal muscle cells: regulatory mechanisms and exercise effects. Exerc Immunol Rev. 2015;21:8–25.
Peake JM, Suzuki K, Hordern M, Wilson G, Nosaka K, Coombes JS. Plasma cytokine changes in relation to exercise intensity and muscle damage. Eur J Appl Physiol. 2005;95(5–6):514–21.
Côte CH, Tremblay MH, Duchesne E, Lapoite BM. Inflammation-induced leukocyte accumulation in injured skeletal muscle: role of mast cells. Muscle Nerve. 2008;37(6):754–63.
Burzyn D, Kuswanto W, Kolodin D, et al. A special population of regulatory T cells potentiates muscle repair. Cell. 2013;155(6):1282–95.
Heredia JE, Mukundan L, Chen FM, et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153(2):376–88.
Zhang J, Xiao Z, Qu C, Cui W, Wang X, Du J. CD8 T cells are involved in skeletal muscle regeneration through facilitating MCP-1 secretion and Gr1(high) macrophage infiltration. J Immunol. 2014;193(10):5149–60.
Brigitte M, Schilte C, Plonquet A, et al. Muscle resident macrophages control the immune cell reaction in a mouse model of notexin-induced myoinjury. Arthritis Rheum. 2010;62(1):268–79.
Nieman DC, Mitmesser SH. Potential impact of nutrition on immune system recovery from heavy exertion: a metabolomics perspective. Nutrients. 2017;9(5).
Della Gatta PA, Cameron-Smith D, Peake JM. Acute resistance exercise increases the expression of chemotactic factors within skeletal muscle. Eur J Appl Physiol. 2014;114(10):2157–67.
Lu H, Huang D, Saederup N, Charo IF, Ransohoff RM, Zhou L. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J. 2011;25(1):358–69.
Peterson JM, Pizza FX. Cytokines derived from cultured skeletal muscle cells after mechanical strain promote neutrophil chemotaxis in vitro. J Appl Physiol (1985). 2009;106(1):130–7.
Peterson JM, Feeback KD, Baas JH, Pizza FX. Tumor necrosis factor-alpha promotes the accumulation of neutrophils and macrophages in skeletal muscle. J Appl Physiol (1985). 2006;101(5):1394–9.
Ihalainen J, Walker S, Paulsen G, et al. Acute leukocyte, cytokine and adipocytokine responses to maximal and hypertrophic resistance exercise bouts. Eur J Appl Physiol. 2014;114(12):2607–16.
Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81(11 Suppl):S52–69.
Legault Z, Bagnall N, Kimmerly DS. The influence of oral L-glutamine supplementation on muscle strength recovery and soreness following unilateral knee extension eccentric exercise. Int J Sport Nutr Exerc Metab. 2015;25(5):417–26.
Walsh NP, Gleeson M, Shephard RJ, et al. Position statement. Part one: immune function and exercise. Exerc Immunol Rev. 2011;17:6–63.
Walsh NP, Gleeson M, Pyne DB, et al. Position statement. Part two: maintaining immune health. Exerc Immunol Rev. 2011;17:64–103.
Gleeson M, Pyne DB. Respiratory inflammation and infections in high-performance athletes. Immunol Cell Biol. 2016;94(2):124–31.
Soares S, Ferreira-Junior JB, Pereira MC, et al. Dissociated time course of muscle damage recovery between single and multi-joint exercises in highly resistance trained men. J Strength Cond Res. 2015;9:2594–9.
Freidenreich DJ, Volek JS. Immune responses to resistance exercise. Exerc Immunol Rev. 2012;18:8–41.
Urso ML. Anti-inflammatory interventions and skeletal muscle injury: benefit or detriment? J Appl Physiol (1985). 2013;115(6):920–8.
McGlory C, Devries MC, Phillips SM. Skeletal muscle and resistance exercise training; the role of protein synthesis in recovery and remodeling. J Appl Physiol (1985). 2017;122(3):541–8.
Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985). 2005;98(4):1154–62.
Paulsen G, Mikkelsen UR, Raastad T, Peake JM. Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc Immunol Rev. 2012;18:42–97.
Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18(49):6853–66.
Vella L, Caldow MK, Larsen AE, et al. Resistance exercise increases NF-κB activity in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2012;302(6):R667–73.
Ji LL, Gomez-Cabrera MC, Steinhafel N, Vina J. Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. FASEB J. 2004;18(13):1499–506.
Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88(4):1379–406.
Hirose L, Nosaka K, Newton M, et al. Changes in inflammatory mediators following eccentric exercise of the elbow flexors. Exerc Immunol Rev. 2004;10:75–90.
de Nadal E, Ammerer G, Posas F. Controlling gene expression in response to stress. Nat Rev Genet. 2011;12(12):833–45.
Wischmeyer PE, Kahana M, Wolfson R, Ren H, Musch MM, Chang EB. Glutamine induces heat shock protein and protects against endotoxin shock in the rat. J Appl Physiol (1985). 2001;90(6):2403–10.
Wischmeyer PE, Musch MW, Madonna MB, Thisted R, Chang EB. Glutamine protects intestinal epithelial cells: role of inducible HSP70. Am J Phys. 1997;272(4 Pt 1):G879–84.
Yoo CG, Lee S, Lee CT, Kim YW, Han SK, Shim YS. Anti-inflammatory effect of heat shock protein induction is related to stabilization of I kappa B alpha through preventing I kappa B kinase activation in respiratory epithelial cells. J Immunol. 2000;164(10):5416–23.
Kiang JG, Tsokos GC. Heat shock protein 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998;80(2):183–201.
Simar D, Malatesta D, Badiou S, Dupuy AM, Caillaud C. Physical activity modulates heat shock protein-72 expression and limits oxidative damage accumulation in a healthy elderly population aged 60 90 years. J Gerontol A Biol Sci Med Sci. 2007;62(12):1413–9.
Paulsen G, Hanssen KE, Rønnestad BR, et al. Strength training elevates HSP27, HSP70 and αB-crystallin levels in musculi vastus lateralis and trapezius. Eur J Appl Physiol. 2012;112(5):1773–82.
Paulsen G, Vissing K, Kalhovde JM, et al. Maximal eccentric exercise induces a rapid accumulation of small heat shock proteins on myofibrils and a delayed HSP70 response in humans. Am J Physiol Regul Integr Comp Physiol. 2007;293(2):R844–53.
Archer AE, Von Schulze AT, Geiger PC. Exercise, heat shock proteins and insulin resistance. Philos Trans R Soc Lond Ser B Biol Sci. 2018;373(1738).
Krause M, Heck TG, Bittencourt A, et al. The chaperone balance hypothesis: the importance of the extracellular to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediat Inflamm. 2015;2015:249205.
Cahill CM, Waterman WR, Xie Y, Auron PE, Calderwood SK. Transcriptional repression of the prointerleukin 1beta gene by heat shock factor 1. J Biol Chem. 1996;271(40):24874–9.
Singleton KD, Wischmeyer PE. Glutamine induces heat shock protein expression via O-glycosylation and phosphorylation of HSF-1 and Sp1. JPEN J Parenter Enteral Nutr. 2008;32(4):371–6.
Wu C. Activating protein factor binds in vitro to upstream control sequences in heat shock gene chromatin. Nature. 1984;311(5981):81–4.
Voellmy R. Transduction of the stress signal and mechanisms of transcriptional regulation of heat shock/stress protein gene expression in higher eukaryotes. Crit Rev Eukaryot Gene Expr. 1994;4(4):357–401.
Xiao X, Zuo X, Davis AA, et al. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999;18(21):5943–52.
Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5(22):2592–601.
Oishi Y, Taniguchi K, Matsumoto H, Ishihara A, Ohira Y, Roy RR. Muscle type-specific response of HSP60, HSP72, and HSC73 during recovery after elevation of muscle temperature. J Appl Physiol (1985). 2002;92(3):1097–103.
Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17(2):162–84.
Phillips BE, Williams JP, Gustafsson T, et al. Molecular networks of human muscle adaptation to exercise and age. PLoS Genet. 2013;9(3):e1003389.
Takayama S, Reed JC, Homma S. Heat-shock proteins as regulators of apoptosis. Oncogene. 2003;22(56):9041–7.
Thompson HS, Scordilis SP, Clarkson PM, Lohrer WA. A single bout of eccentric exercise increases HSP27 and HSC/HSP70 in human skeletal muscle. Acta Physiol Scand. 2001;171(2):187–93.
Mikami T, Sumida S, Ishibashi Y, Ohta S. Endurance exercise training inhibits activity of plasma GOT and liver caspase-3 of mice [correction of rats] exposed to stress by induction of heat shock protein 70. J Appl Physiol (1985). 2004;96(5):1776–81.
Shi Y, Tu Z, Tang D, et al. The inhibition of LPS-induced production of inflammatory cytokines by HSP70 involves inactivation of the NF-kappaB pathway but not the MAPK pathways. Shock. 2006;26(3):277–84.
Chu EK, Ribeiro SP, Slutsky AS. Heat stress increases survival rates in lipopolysaccharide-stimulated rats. Crit Care Med. 1997;25(10):1727–32.
Schmidt JA, Abdulla E. Down-regulation of IL-1 beta biosynthesis by inducers of the heat-shock response. J Immunol. 1988;141(6):2027–34.
Benjamin IJ, Christians E. Exercise, estrogen, and ischemic cardioprotection by heat shock protein 70. Circ Res. 2002;90(8):833–5.
Whitham M, Halson SL, Lancaster GI, Gleeson M, Jeukendrup AE, Blannin AK. Leukocyte heat shock protein expression before and after intensified training. Int J Sports Med. 2004;25(7):522–7.
Wischmeyer PE. Glutamine and heat shock protein expression. Nutrition. 2002;18(3):225–8.
Kim M, Wischmeyer PE. Glutamine. World Rev Nutr Diet. 2013;105:90–6.
Wischmeyer PE, Riehm J, Singleton KD, et al. Glutamine attenuates tumor necrosis factor-alpha release and enhances heat shock protein 72 in human peripheral blood mononuclear cells. Nutrition. 2003;19(1):1–6.
Xue H, Slavov D, Wischmeyer PE. Glutamine-mediated dual regulation of heat shock transcription factor-1 activation and expression. J Biol Chem. 2012;287(48):40400–13.
Hamiel CR, Pinto S, Hau A, Wischmeyer PE. Glutamine enhances heat shock protein 70 expression via increased hexosamine biosynthetic pathway activity. Am J Physiol Cell Physiol. 2009;297(6):C1509–19.
Bevilacqua A, Fiorenza MT, Mangia F. A developmentally regulated GAGA box-binding factor and Sp1 are required for transcription of the hsp70.1 gene at the onset of mouse zygotic genome activation. Development. 2000;127(7):1541–51.
Wischmeyer PE. Clinical applications of L-glutamine: past, present, and future. Nutr Clin Pract. 2003;18(5):377–85.
Ren X, Zou L, Zhang X, et al. Redox signaling mediated by thioredoxin and glutathione systems in the central nervous system. Antioxid Redox Signal. 2017;27(13):989–1010.
Alves WF, Aguiar EE, Guimarães SB, et al. L-alanyl-glutamine preoperative infusion in patients with critical limb ischemia subjected to distal revascularization reduces tissue damage and protects from oxidative stress. Ann Vasc Surg. 2010;24(4):461–7.
Liu S, Yang Y, Song YQ, Geng J, Chen QL. Protective effects of N(2)-L-alanyl-L-glutamine mediated by the JAK2/STAT3 signaling pathway on myocardial ischemia reperfusion. Mol Med Rep. 2018;17(4):5102–8.
Chen G, Shi J, Qi M, Yin H, Hang C. Glutamine decreases intestinal nuclear factor kappa B activity and pro-inflammatory cytokine expression after traumatic brain injury in rats. Inflamm Res. 2008;57(2):57–64.
Lesueur C, Bôle-Feysot C, Bekri S, Husson A, Lavoinne A, Brasse-Lagnel C. Glutamine induces nuclear degradation of the NF-κB p65 subunit in Caco-2/TC7 cells. Biochimie. 2012;94(3):806–15.
Kozakowska M, Pietraszek-Gremplewicz K, Jozkowicz A, Dulak J. The role of oxidative stress in skeletal muscle injury and regeneration: focus on antioxidant enzymes. J Muscle Res Cell Motil. 2015;36(6):377–93.
McRae MP. Therapeutic benefits of glutamine: an umbrella review of meta-analyses. Biomed Rep. 2017;6(5):576–84.
Ortega E, Giraldo E, Hinchado MD, et al. Role of Hsp72 and norepinephrine in the moderate exercise-induced stimulation of neutrophils’ microbicide capacity. Eur J Appl Physiol. 2006;98(3):250–5.
Castell L, Vance C, Abbott R, Marquez J, Eggleton P. Granule localization of glutaminase in human neutrophils and the consequence of glutamine utilization for neutrophil activity. J Biol Chem. 2004;279(14):13305–10.
Gleeson M. Dosing and efficacy of glutamine supplementation in human exercise and sport training. J Nutr. 2008;138(10):2045S–9S.
Castell LM, Newsholme EA. The effects of oral glutamine supplementation on athletes after prolonged, exhaustive exercise. Nutrition. 1997;13(7–8):738–42.
Jepson MM, Bates PC, Broadbent P, Pell JM, Millward DJ. Relationship between glutamine concentration and protein synthesis in rat skeletal muscle. Am J Phys. 1988;255(2 Pt 1):E166–72.
MacLennan PA, Brown RA, Rennie MJ. A positive relationship between protein synthetic rate and intracellular glutamine concentration in perfused rat skeletal muscle. FEBS Lett. 1987;215(1):187–91.
Boza JJ, Turini M, Moënnoz D, et al. Effect of glutamine supplementation of the diet on tissue protein synthesis rate of glucocorticoid-treated rats. Nutrition. 2001;17(1):35–40.
Salehian B, Mahabadi V, Bilas J, Taylor WE, Ma K. The effect of glutamine on prevention of glucocorticoid-induced skeletal muscle atrophy is associated with myostatin suppression. Metabolism. 2006;55(9):1239–47.
Bowtell JL, Gelly K, Jackman ML, Patel A, Simeoni M, Rennie MJ. Effect of oral glutamine on whole body carbohydrate storage during recovery from exhaustive exercise. J Appl Physiol (1985). 1999;86(6):1770–7.
Blomstrand E, Essén-Gustavsson B. Changes in amino acid concentration in plasma and type I and type II fibres during resistance exercise and recovery in human subjects. Amino Acids. 2009;37(4):629–36.
Miles MP, Naukam RJ, Hackney AC, Clarkson PM. Blood leukocyte and glutamine fluctuations after eccentric exercise. Int J Sports Med. 1999;20(5):322–7.
Kreider RB. Dietary supplements and the promotion of muscle growth with resistance exercise. Sports Med. 1999;27(2):97–110.
Phillips GC. Glutamine: the nonessential amino acid for performance enhancement. Curr Sports Med Rep. 2007;6(4):265–8.
Wilkinson SB, Kim PL, Armstrong D, Phillips SM. Addition of glutamine to essential amino acids and carbohydrate does not enhance anabolism in young human males following exercise. Appl Physiol Nutr Metab. 2006;31(5):518–29.
Candow DG, Chilibeck PD, Burke DG, Davison KS, Smith-Palmer T. Effect of glutamine supplementation combined with resistance training in young adults. Eur J Appl Physiol. 2001;86(2):142–9.
Wu G. Intestinal mucosal amino acid catabolism. J Nutr. 1998;128(8):1249–52.
Fürst P. New developments in glutamine delivery. J Nutr. 2001;131(9 Suppl):2562S–8S.
Lima AA, Carvalho GH, Figueiredo AA, et al. Effects of an alanyl-glutamine-based oral rehydration and nutrition therapy solution on electrolyte and water absorption in a rat model of secretory diarrhea induced by cholera toxin. Nutrition. 2002;18(6):458–62.
Favano A, Santos-Silva PR, Nakano EY, Pedrinelli A, Hernandez AJ, Greve JM. Peptide glutamine supplementation for tolerance of intermittent exercise in soccer players. Clinics (Sao Paulo). 2008;63(1):27–32.
Rogero MM, Tirapegui J, Pedrosa RdG, Pires ISdO, Alves IdC. Plasma and tissue glutamine response to acute and chronic supplementation with L-glutamine and L-alanyl-L-glutamine in rats. In. Nutrition Research. 2004;24(4):261–270.
Lochs H, Roth E, Gasic S, Hübl W, Morse EL, Adibi SA. Splanchnic, renal, and muscle clearance of alanylglutamine in man and organ fluxes of alanine and glutamine when infused in free and peptide forms. Metabolism. 1990;39(8):833–6.
McCormack WP, Hoffman JR, Pruna GJ, et al. Effects of L-alanyl-L-glutamine ingestion on one-hour run performance. J Am Coll Nutr. 2015;34(6):488–96.
Wang W, Choi RH, Solares GJ, et al. L-alanylglutamine inhibits signaling proteins that activate protein degradation, but does not affect proteins that activate protein synthesis after an acute resistance exercise. Amino Acids. 2015;47(7):1389–98.
Pruna GJ, Hoffman JR, McCormack WP, et al. Effect of acute L-alanyl-L-glutamine and electrolyte ingestion on cognitive function and reaction time following endurance exercise. Eur J Sport Sci. 2016;16(1):72–9.
de Souza HM, Borba-Murad GR, Ceddia RB, Curi R, Vardanega-Peicher M, Bazotte RB. Rat liver responsiveness to gluconeogenic substrates during insulin-induced hypoglycemia. Braz J Med Biol Res. 2001;34(6):771–7.
Hoffman JR, Ratamess NA, Kang J, et al. Examination of the efficacy of acute L-alanyl-L-glutamine ingestion during hydration stress in endurance exercise. J Int Soc Sports Nutr. 2010;7:8.
Hoffman JR, Williams DR, Emerson NS, et al. L-alanyl-L-glutamine ingestion maintains performance during a competitive basketball game. J Int Soc Sports Nutr. 2012;9(1):4.
Leite JS, Raizel R, Hypólito TM, Rosa TD, Cruzat VF, Tirapegui J. L-glutamine and L-alanine supplementation increase glutamine-glutathione axis and muscle HSP-27 in rats trained using a progressive high-intensity resistance exercise. Appl Physiol Nutr Metab. 2016;41(8):842–9.
Raizel R, Leite JS, Hypólito TM, et al. Determination of the anti-inflammatory and cytoprotective effects of L-glutamine and L-alanine, or dipeptide, supplementation in rats submitted to resistance exercise. Br J Nutr. 2016;116(3):470–9.
Ardawi MS, Newsholme EA. Glutamine metabolism in lymphocytes of the rat. Biochem J. 1983;212(3):835–42.
Coqueiro AY, Raizel R, Bonvini A, et al. Effects of glutamine and alanine supplementation on central fatigue markers in rats submitted to resistance training. Nutrients. 2018;10(2). https://doi.org/10.3390/nu10020119.
Acknowledgements
Not applicable
Funding
Not applicable
Availability of data and materials
Not applicable
Author information
Authors and Affiliations
Contributions
RR and JT were responsible for all the steps of the review. The figure was drawn by RR. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Raizel, R., Tirapegui, J. Role of glutamine, as free or dipeptide form, on muscle recovery from resistance training: a review study. Nutrire 43, 28 (2018). https://doi.org/10.1186/s41110-018-0087-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41110-018-0087-9