Abstract
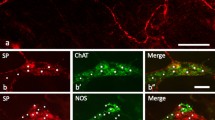
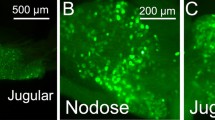
The calcitonin-gene-related peptide (CGRP) receptor is a heterodimer of calcitonin-receptor-like receptor (CLR) and receptor-activity-modifying protein 1 (RAMP1). Despite the importance of CGRP in regulating gastrointestinal functions, nothing is known about the distribution and function of CLR/RAMP1 in the esophagus, where up to 90 % of spinal afferent neurons contain CGRP. We detected CLR/RAMP1 in the mouse esophagus using immunofluorescence and confocal laser scanning microscopy and examined their relationship with neuronal elements of the myenteric plexus. Immunoreactivity for CLR and RAMP1 colocalized with VGLUT2-positive intraganglionic laminar endings (IGLEs), which were contacted by CGRP-positive varicose axons presumably of spinal afferent origin, typically at sites of CRL/RAMP1 immunoreactivity. This provides an anatomical basis for interaction between spinal afferent fibers and IGLEs. Immunoreactive CLR and RAMP1 also colocalized in myenteric neurons. Thus, CGRP-containing spinal afferents may interact with both vagal IGLEs and myenteric neurons in the mouse esophagus, possibly modulating motility reflexes and inflammatory hypersensitivity.










Similar content being viewed by others
References
Adams J (1992) Biotin amplification of biotin and horseradish peroxidase signals in histochemical stains. J Histochem Cytochem 40(10):1457–1463
Altschuler SM, Bao XM, Bieger D, Hopkins DA, Miselis RR (1989) Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol 283(2):248–268. doi:10.1002/cne.902830207
Ammons WS, Blair RW, Foreman RD (1983) Vagal afferent inhibition of primate thoracic spinothalamic neurons. J Neurophysiol 50(4):926–940
Barrett RT, Bao X, Miselis RR, Altschuler SM (1994) Brain stem localization of rodent esophageal premotor neurons revealed by transneuronal passage of pseudorabies virus. Gastroenterology 107(3):728–737
Berthoud HR, Powley TL (1992) Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol 319(2):261–276
Berthoud HR, Patterson LM, Neumann F, Neuhuber WL (1997a) Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol (Berl) 195(2):183–191
Berthoud HR, Patterson LM, Willing AE, Mueller K, Neuhuber WL (1997b) Capsaicin-resistant vagal afferent fibers in the rat gastrointestinal tract: anatomical identification and functional integrity. Brain Res 746(1–2):195–206
Bieger D, Hopkins DA (1987) Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Comp Neurol 262(4):546–562. doi:10.1002/cne.902620408
Bomberger JM, Parameswaran N, Hall CS, Aiyar N, Spielman WS (2005) Novel function for receptor activity-modifying proteins (RAMPs) in post-endocytic receptor trafficking. J Biol Chem 280(10):9297–9307. doi:10.1074/jbc.M413786200
Boudaka A, Wörl J, Shiina T, Saito S, Atoji Y, Kobayashi H, Shimizu Y, Takewaki T (2007) Key role of mucosal primary afferents in mediating the inhibitory influence of capsaicin on vagally mediated contractions in the mouse esophagus. J Vet Med Sci 69(4):365–372
Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I (1985) Calcitonin gene-related peptide is a potent vasodilator. Nature 313(5997):54–56
Carlton SM, Westlund KN, Zhang DX, Sorkin LS, Willis WD (1990) Calcitonin gene-related peptide containing primary afferent fibers synapse on primate spinothalamic tract cells. Neurosci Lett 109(1–2):76–81
Chandler MJ, Hobbs SF, Bolser DC, Foreman RD (1991) Effects of vagal afferent stimulation on cervical spinothalamic tract neurons in monkeys. Pain 44(1):81–87
Clerc N, Mazzia C (1994) Morphological relationships of choleragenoid horseradish peroxidase-labeled spinal primary afferents with myenteric ganglia and mucosal associated lymphoid tissue in the cat esophagogastric junction. J Comp Neurol 347(2):171–186. doi:10.1002/cne.903470203
Conner AC, Simms J, Barwell J, Wheatley M, Poyner DR (2007) Ligand binding and activation of the CGRP receptor. Biochem Soc Trans 35(Pt 4):729–732. doi:10.1042/BST0350729
Cottrell GS, Roosterman D, Marvizon JC, Song B, Wick E, Pikios S, Wong H, Berthelier C, Tang Y, Sternini C, Bunnett NW, Grady EF (2005) Localization of calcitonin receptor-like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. J Comp Neurol 490(3):239–255. doi:10.1002/cne.20669
Cottrell GS, Alemi F, Kirkland JG, Grady EF, Corvera CU, Bhargava A (2012) Localization of calcitonin receptor-like receptor (CLR) and receptor activity-modifying protein 1 (RAMP1) in human gastrointestinal tract. Peptides 35(2):202–211. doi:10.1016/j.peptides.2012.03.020
Cox HM, Ferrar JA, Cuthbert AW (1989) Effects of alpha- and beta-calcitonin gene-related peptides upon ion transport in rat descending colon. Br J Pharmacol 97(4):996–998
Cunningham ET Jr, Sawchenko PE (1989) A circumscribed projection from the nucleus of the solitary tract to the nucleus ambiguus in the rat: anatomical evidence for somatostatin-28-immunoreactive interneurons subserving reflex control of esophageal motility. J Neurosci 9(5):1668–1682
De Jonge F, De Laet A, Van Nassauw L, Brown JK, Miller HRP, van Bogaert P-P, Timmermans J-P, Kroese ABA (2004) In vitro activation of murine DRG neurons by CGRP-mediated mucosal mast cell degranulation. Am J Physiol Gastrointest Liver Physiol 287(1):G178–G191. doi:10.1152/ajpgi.00528.2003
Demir I, Schäfer K-H, Tieftrunk E, Friess H, Ceyhan G (2013) Neural plasticity in the gastrointestinal tract: chronic inflammation, neurotrophic signals, and hypersensitivity. Acta Neuropathol 125(4):491–509. doi:10.1007/s00401-013-1099-4
Dennis T, Fournier A, Stpierre S, Quirion R (1989) Structure-activity profile of calcitonin gene-related peptide in peripheral and brain-tissues—evidence for receptor multiplicity. J Pharmacol Exp Ther 251(2):718–725
Dütsch M, Eichhorn U, Wörl J, Wank M, Berthoud HR, Neuhuber WL (1998) Vagal and spinal afferent innervation of the rat esophagus: a combined retrograde tracing and immunocytochemical study with special emphasis on calcium-binding proteins. J Comp Neurol 398(2):289–307
Esfandyari T, Macnaughton WK, Quirion R, St Pierre S, Junien JL, Sharkey KA (2000) A novel receptor for calcitonin gene-related peptide (CGRP) mediates secretion in the rat colon: implications for secretory function in colitis. FASEB J Off Publ Fed Am Soc Exp Biol 14(10):1439–1446
Evans BN, Rosenblatt MI, Mnayer LO, Oliver KR, Dickerson IM (2000) CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J Biol Chem 275(40):31438–31443. doi:10.1074/jbc.M005604200
Ewald P, Neuhuber WL, Raab M (2006) Vesicular glutamate transporter 1 immunoreactivity in extrinsic and intrinsic innervation of the rat esophagus. Histochem Cell Biol 125(4):377–395
Foreman JC (1987) Peptides and neurogenic inflammation. Br Med Bull 43(2):386–400
Furness JB (2006) The enteric nervous system. Blackwell, Carlton
Harrington AM, Brierley SM, Isaacs NJ, Young RL, Ashley Blackshaw L (2013) Identifying spinal sensory pathways activated by noxious esophageal acid. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. doi:10.1111/nmo.12180
Hay DL, Poyner DR, Quirion R (2008) International Union of Pharmacology. LXIX. Status of the calcitonin gene-related peptide subtype 2 receptor. Pharmacol Rev 60(2):143–145. doi:10.1124/pr.108.00372
Hermann GE, Travagli RA, Rogers RC (2006) Esophageal-gastric relaxation reflex in rat: dual control of peripheral nitrergic and cholinergic transmission. Am J Physiol Regul Integr Comp Physiol 290(6):R1570–R1576. doi:10.1152/ajpregu.00717.2005
Holzer P (1988) Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience 24(3):739–768
Holzer P (2002) Sensory neurone responses to mucosal noxae in the upper gut: relevance to mucosal integrity and gastrointestinal pain. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc 14(5):459–475
Hong Y, Hay DL, Quirion R, Poyner DR (2012) The pharmacology of adrenomedullin 2/intermedin. Br J Pharmacol 166(1):110–120. doi:10.1111/j.1476-5381.2011.01530.x
Horling L, Neuhuber WL, Raab M (2012) Pitfalls using tyramide signal amplification (TSA) in the mouse gastrointestinal tract: endogenous streptavidin-binding sites lead to false positive staining. J Neurosci Methods 204(1):124–132. doi:10.1016/j.jneumeth.2011.11.009
Husmann K, Born W, Fischer JA, Muff R (2003) Three receptor-activity-modifying proteins define calcitonin gene-related peptide or adrenomedullin selectivity of the mouse calcitonin-like receptor in COS-7 cells. Biochem Pharmacol 66(11):2107–2115
Izumi N, Matsuyama H, Ko M, Shimizu Y, Takewaki T (2003) Role of intrinsic nitrergic neurones on vagally mediated striated muscle contractions in the hamster oesophagus. J Physiol 551(Pt 1):287–294. doi:10.1113/jphysiol.2003.044669
Jansson G (1969) Vago-vagal reflex relaxation of the stomach in the cat. Acta Physiol Scand 75(1):245–252
Kestler C, Neuhuber WL, Raab M (2009) Distribution of P2X(3) receptor immunoreactivity in myenteric ganglia of the mouse esophagus. Histochem Cell Biol 131(1):13–27. doi:10.1007/s00418-008-0498-4
Kraus T, Neuhuber WL, Raab M (2007) Distribution of vesicular glutamate transporter 1 (VGLUT1) in the mouse esophagus. Cell Tissue Res 329(2):205–219
Kressel M, Radespiel-Tröger M (1999) Anterograde tracing and immunohistochemical characterization of potentially mechanosensitive vagal afferents in the esophagus. J Comp Neurol 412(1):161–172
Kuramoto H, Oomori Y, Murabayashi H, Kadowaki M, Karaki S, Kuwahara A (2004) Localization of neurokinin 1 receptor (NK1R) immunoreactivity in rat esophagus. J Comp Neurol 478(1):11–21
Kuwasako K (2013) The RAMP-interacting Family B G protein-coupled receptors and their specific bioactive peptides. Curr Protein Pept Sci 14(4):243–245
Kuwasako K, Shimekake Y, Masuda M, Nakahara K, Yoshida T, Kitaura M, Kitamura K, Eto T, Sakata T (2000) Visualization of the calcitonin receptor-like receptor and its receptor activity-modifying proteins during internalization and recycling. J Biol Chem 275(38):29602–29609. doi:10.1074/jbc.M004534200
Kuwasako K, Hay DL, Nagata S, Murakami M, Kitamura K, Kato J (2013) Functions of third extracellular loop and Helix 8 of Family B GPCRs complexed with RAMPs and characteristics of their receptor trafficking. Curr Protein Pept Sci 14(5):416–428
Lennerz JK, Ruhle V, Ceppa EP, Neuhuber WL, Bunnett NW, Grady EF, Messlinger K (2008) Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J Comp Neurol 507(3):1277–1299. doi:10.1002/cne.21607
Mallee JJ, Salvatore CA, LeBourdelles B, Oliver KR, Longmore J, Koblan KS, Kane SA (2002) Receptor activity-modifying protein 1 determines the species selectivity of non-peptide CGRP receptor antagonists. J Biol Chem 277(16):14294–14298. doi:10.1074/jbc.M109661200
Marvizón JCG, Pérez OA, Song B, Chen W, Bunnett NW, Grady EF, Todd AJ (2007) Calcitonin receptor-like receptor and receptor activity modifying protein 1 in the rat dorsal horn: localization in glutamatergic presynaptic terminals containing opioids and adrenergic α2C receptors. Neuroscience 148(1):250–265. doi:10.1016/j.neuroscience.2007.05.036
Mazzia C, Clerc N (1997) Ultrastructural relationships of spinal primary afferent fibres with neuronal and non-neuronal cells in the myenteric plexus of the cat oesophago-gastric junction. Neuroscience 80(3):925–937. doi:10.1016/s0306-4522(97)00058-4
McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM (1998) RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393(6683):333–339. doi:10.1038/30666
McNeill DL, Chandler MJ, Fu QG, Foreman RD (1991) Projection of nodose ganglion cells to the upper cervical spinal cord in the rat. Brain Res Bull 27(2):151–155
Medda BK, Sengupta JN, Lang IM, Shaker R (2005) Response properties of the brainstem neurons of the cat following intra-esophageal acid–pepsin infusion. Neuroscience 135(4):1285–1294. doi:10.1016/j.neuroscience.2005.07.016
Miolan JP, Roman C (1984) The role of oesophageal and intestinal receptors in the control of gastric motility. J Auton Nerv Syst 10(3–4):235–241
Neuhuber WL (1987) Sensory vagal innervation of the rat esophagus and cardia: a light and electron microscopic anterograde tracing study. J Auton Nerv Syst 20(3):243–255
Neuhuber WL, Clerc N (1990) Afferent innervation of the esophagus in cat and rat. In: Zenker W, Neuhuber WL (eds) The primary afferent neuron. Plenum Press, New York, pp 93–107
Neuhuber WL, Raab M, Berthoud HR, Wörl J (2006) Innervation of the mammalian esophagus. Adv Anat Embryol Cell Biol 185:1–84
Parsons AM, Seybold VS (1997) Calcitonin gene-related peptide induces the formation of second messengers in primary cultures of neonatal rat spinal cord. Synapse 26(3):235–242. doi:10.1002/(SICI)1098-2396(199707)26:3<235:AID-SYN5>3.0.CO;2-8
Partosoedarso ER, Blackshaw LA (1997) Vagal efferent fibre responses to gastric and oesophageal mechanical and chemical stimuli in the ferret. J Auton Nerv Syst 66(3):169–178
Patterson LM, Zheng H, Ward SM, Berthoud HR (2003) Vanilloid receptor (VR1) expression in vagal afferent neurons innervating the gastrointestinal tract. Cell Tissue Res 311(3):277–287
Peghini PL, Johnston BT, Leite LP, Castell DO (1996) Mucosal acid exposure sensitizes a subset of normal subjects to intra-oesophageal balloon distension. Eur J Gastroenterol Hepatol 8(10):979–983
Peles S, Medda BK, Zhang Z, Banerjee B, Lehmann A, Shaker R, Sengupta JN (2009) Differential effects of transient receptor vanilloid one (TRPV1) antagonists in acid-induced excitation of esophageal vagal afferent fibers of rats. Neuroscience 161(2):515–525. doi:10.1016/j.neuroscience.2009.03.040
Phillips RJ, Powley TL (2000) Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev 34(1–2):1–26
Phillips RJ, Powley TL (2007) Innervation of the gastrointestinal tract: patterns of aging. Auton Neurosci Basic Clin 136(1–2):1–19
Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, Muff R, Fischer JA, Foord SM (2002) International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev 54(2):233–246
Raab M, Neuhuber WL (2003) Vesicular glutamate transporter 2 immunoreactivity in putative vagal mechanosensor terminals of mouse and rat esophagus: indication of a local effector function? Cell Tissue Res 312(2):141–148
Raab M, Neuhuber WL (2004) Intraganglionic laminar endings and their relationships with neuronal and glial structures of myenteric ganglia in the esophagus of rat and mouse. Histochem Cell Biol 122(5):445–459
Raab M, Neuhuber WL (2005) Number and distribution of intraganglionic laminar endings in the mouse esophagus as demonstrated with two different immunohistochemical markers. J Histochem Cytochem 53(8):1023–1031
Randich A, Gebhart GF (1992) Vagal afferent modulation of nociception. Brain Res Brain Res Rev 17(2):77–99
Ren K, Randich A, Gebhart GF (1990) Electrical stimulation of cervical vagal afferents. I. Central relays for modulation of spinal nociceptive transmission. J Neurophysiol 64(4):1098–1114
Rodrigo J, Hernandez J, Vidal MA, Pedrosa JA (1975) Vegetative innervation of the esophagus. II. Intraganglionic laminar endings. Acta Anat (Basel) 92(1):79–100
Rodrigo J, de Felipe J, Robles-Chillida EM, Perez Anton JA, Mayo I, Gomez A (1982) Sensory vagal nature and anatomical access paths to esophagus laminar nerve endings in myenteric ganglia. Determination by surgical degeneration methods. Acta Anat (Basel) 112(1):47–57
Rodrigo J, Polak JM, Fernandez L, Ghatei MA, Mulderry P, Bloom SR (1985) Calcitonin gene-related peptide immunoreactive sensory and motor nerves of the rat, cat, and monkey esophagus. Gastroenterology 88(2):444–451
Rosenfeld MG, Mermod JJ, Amara SG, Swanson LW, Sawchenko PE, Rivier J, Vale WW, Evans RM (1983) Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature 304(5922):129–135
Ryu PD, Gerber G, Murase K, Randic M (1988) Calcitonin gene-related peptide enhances calcium current of rat dorsal root ganglion neurons and spinal excitatory synaptic transmission. Neurosci Lett 89(3):305–312
Segond von Banchet G, Pastor A, Biskup C, Schlegel C, Benndorf K, Schaible HG (2002) Localization of functional calcitonin gene-related peptide binding sites in a subpopulation of cultured dorsal root ganglion neurons. Neuroscience 110(1):131–145
Shapiro RE, Miselis RR (1985) The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol 238(4):473–488. doi:10.1002/cne.902380411
Shindler K, Roth K (1996) Double immunofluorescent staining using two unconjugated primary antisera raised in the same species. J Histochem Cytochem 44(11):1331–1335
Sternini C (1992) Enteric and visceral afferent CGRP neurons. Targets of innervation and differential expression patterns. Ann N Y Acad Sci 657:170–186
Travagli RA, Hermann GE, Browning KN, Rogers RC (2003) Musings on the wanderer: what’s new in our understanding of vago-vagal reflexes? III. Activity-dependent plasticity in vago-vagal reflexes controlling the stomach. Am J Physiol Gastrointest Liver Physiol 284(2):G180–G187. doi:10.1152/ajpgi.00413.2002
Wang ZJ, Neuhuber WL (2003) Intraganglionic laminar endings in the rat esophagus contain purinergic P2X2 and P2X3 receptor immunoreactivity. Anat Embryol (Berl) 207(4–5):363–371. doi:10.1007/s00429-003-0351-4
Wang FB, Powley TL (2000) Topographic inventories of vagal afferents in gastrointestinal muscle. J Comp Neurol 421(3):302–324
Wank M, Neuhuber WL (2001) Local differences in vagal afferent innervation of the rat esophagus are reflected by neurochemical differences at the level of the sensory ganglia and by different brainstem projections. J Comp Neurol 435(1):41–59
Wimalawansa SJ (1996) Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr Rev 17(5):533–585
Wimalawansa SJ (1997) Amylin, calcitonin gene-related peptide, calcitonin, and adrenomedullin: a peptide superfamily. Crit Rev Neurobiol 11(2–3):167–239
Zagorodnyuk VP, Brookes SJ (2000) Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J Neurosci 20(16):6249–6255
Zagorodnyuk VP, Chen BN, Brookes SJ (2001) Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol 534(Pt 1):255–268
Zagorodnyuk VP, Chen BN, Costa M, Brookes SJ (2003) Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea-pig oesophagus. J Physiol 553(Pt 2):575–587
Acknowledgments
The skillful technical assistance of Karin Löschner, Hedwig Symowski, Stefanie Link, Anita Hecht and Andrea Hilpert is gratefully acknowledged. This study was supported by Johannes und Frieda Marohn-Stiftung, Erlangen, and by the National Health and Medical Research Council (NWB), Australia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Horling, L., Bunnett, N.W., Messlinger, K. et al. Localization of receptors for calcitonin-gene-related peptide to intraganglionic laminar endings of the mouse esophagus: peripheral interaction between vagal and spinal afferents?. Histochem Cell Biol 141, 321–335 (2014). https://doi.org/10.1007/s00418-013-1162-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00418-013-1162-1