Abstract
Aim
To describe and analyse the clinical and genetic characteristics of digenic familial exudative vitreoretinopathy (FEVR).
Methods
The study cohort consisted of patients with FEVR (n = 13) to identify patients with two mutations in two different genes. A genetic analysis of the LRP5, FZD4, TSPAN12, and ZNF408 genes was performed with next-generation sequencing (NGS). The genotype data obtained from the patients with FEVR were analysed and correlated with their clinical manifestations. They were then further evaluated in conjunction with other data that were available for these genes. The probands and parents/relatives underwent comprehensive age-appropriate ophthalmic examinations.
Results
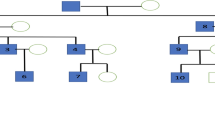
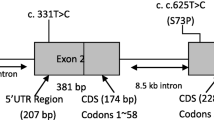
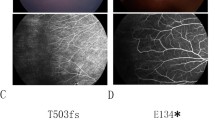
The medical history and genetic reports of 487 patients with FEVR were reviewed. In all, we identified 13 probands (2.67%, 13/487) with simultaneous mutations in two disease-causing genes. A total of 25 of mutations were found, including10 in FZD4, 8 in LRP5, 3 in ZNF408, 2 in NDP, and 2 in TSPAN12. The most frequent mutations were those in FZD4 and LRP5. We identified 8 mutations that had previously been identified and 17 novel variants. Among 26 eyes, 65.38% exhibited a phenotype, and 10 (38.46%) were stage 4, while 7 (26.92%) were stage 5.
Conclusions
This is the first study to report a group of patients with digenic FEVR. In most affected eyes, the stage was more severe than stage 3. We speculate that the phenotype of FEVR is more severe in patients with digenic rather than monogenic variants of FEVR-related genes.


Similar content being viewed by others
References
Criswick VG, Schepens CL (1969) Familial exudative vitreoretinopathy. Am J Ophthalmol 68:578–594
Boonstra FN, van Nouhuys CE, Schuil J, de Wijs IJ, van der Donk KP, Nikopoulos K, Mukhopadhyay A, Scheffer H, Tilanus MA, Cremers FP, Hoefsloot LH (2009) Clinical and molecular evaluation of probands and family members with familial exudative vitreoretinopathy. Invest Ophthalmol Vis Sci 50:4379–4385. https://doi.org/10.1167/iovs.08-3320
Pendergast SD, Trese MT (1998) Familial exudative vitreoretinopathy: results of surgical management. Ophthalmology 105:1015–1023. https://doi.org/10.1016/S0161-6420(98)96002-X
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR (2010) A method and server for predicting damaging missense mutations. Nat Methods 7:248–249. https://doi.org/10.1038/nmeth0410-248
Kumar P, Henikoff S, Ng PC (2009) Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 4:1073–1081. https://doi.org/10.1038/nprot.2009.86
Schwarz JM, Cooper DN, Schuelke M, Seelow D (2014) MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods 11:361–362. https://doi.org/10.1038/nmeth.2890
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, Committee ALQA (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17:405–424. https://doi.org/10.1038/gim.2015.30
Toomes C, Downey L (1993) Familial exudative vitreoretinopathy, autosomal dominant. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, LJH B, Stephens K, Amemiya A (eds) GeneReviews((R)). University of Washington, Seattle
Muller M, Kusserow C, Orth U, Klar-Dissars U, Laqua H, Gal A (2008) Mutations of the frizzled-4 gene. Their impact on medical care of patients with autosomal dominant exudative vitreoretinopathy. Der Ophthalmol 105:262–268. https://doi.org/10.1007/s00347-007-1617-7
Toomes C, Bottomley HM, Scott S, Mackey DA, Craig JE, Appukuttan B, Stout JT, Flaxel CJ, Zhang K, Black GC, Fryer A, Downey LM, Inglehearn CF (2004) Spectrum and frequency of FZD4 mutations in familial exudative vitreoretinopathy. Invest Ophthalmol Vis Sci 45:2083–2090
Gilmour DF (2015) Familial exudative vitreoretinopathy and related retinopathies. Eye (Lond) 29:1–14. https://doi.org/10.1038/eye.2014.70
Qin M, Hayashi H, Oshima K, Tahira T, Hayashi K, Kondo H (2005) Complexity of the genotype-phenotype correlation in familial exudative vitreoretinopathy with mutations in the LRP5 and/or FZD4 genes. Hum Mutat 26:104–112. https://doi.org/10.1002/humu.20191
Stiegel E, Say EA, Carter BC, Thomas MJ, Shields CL (2013) Simultaneous fzd4 and lrp5 mutation in autosomal dominant familial exudative vitreoretinopathy. Retin Cases Brief Rep 7:26–28. https://doi.org/10.1097/ICB.0b013e31827537eb
Schatz P, Khan AO (2017) Variable familial exudative vitreoretinopathy in a family harbouring variants in both FZD4 and TSPAN12. Acta Ophthalmol 95:705–709. https://doi.org/10.1111/aos.13411
Salvo J, Lyubasyuk V, Xu M, Wang H, Wang F, Nguyen D, Wang K, Luo H, Wen C, Shi C, Lin D, Zhang K, Chen R (2015) Next-generation sequencing and novel variant determination in a cohort of 92 familial exudative vitreoretinopathy patients. Invest Ophthalmol Vis Sci 56:1937–1946. https://doi.org/10.1167/iovs.14-16065
Kramer GD, Say EA, Shields CL (2016) Simultaneous novel mutations of LRP5 and TSPAN12 in a case of familial exudative vitreoretinopathy. J Pediatr Ophthalmol Strabismus 53(online):e1–e5. https://doi.org/10.3928/01913913-20151215-01
Fei P, Zhang Q, Huang L, Xu Y, Zhu X, Tai Z, Gong B, Ma S, Yao Q, Li J, Zhao P, Yang Z (2014) Identification of two novel LRP5 mutations in families with familial exudative vitreoretinopathy. Mol Vis 20:395–409
Nikopoulos K, Venselaar H, Collin RW, Riveiro-Alvarez R, Boonstra FN, Hooymans JM, Mukhopadhyay A, Shears D, van Bers M, de Wijs IJ, van Essen AJ, Sijmons RH, Tilanus MA, van Nouhuys CE, Ayuso C, Hoefsloot LH, Cremers FP (2010) Overview of the mutation spectrum in familial exudative vitreoretinopathy and Norrie disease with identification of 21 novel variants in FZD4, LRP5, and NDP. Hum Mutat 31:656–666. https://doi.org/10.1002/humu.21250
Hiraoka M, Takahashi H, Orimo H, Hiraoka M, Ogata T, Azuma N (2010) Genetic screening of Wnt signaling factors in advanced retinopathy of prematurity. Mol Vis 16:2572–2577
Kondo H, Kusaka S, Yoshinaga A, Uchio E, Tawara A, Hayashi K, Tahira T (2011) Mutations in the TSPAN12 gene in Japanese patients with familial exudative vitreoretinopathy. Am J Ophthalmol 151(1095–1100):e1091. https://doi.org/10.1016/j.ajo.2010.11.026
Collin RW, Nikopoulos K, Dona M, Gilissen C, Hoischen A, Boonstra FN, Poulter JA, Kondo H, Berger W, Toomes C, Tahira T, Mohn LR, Blokland EA, Hetterschijt L, Ali M, Groothuismink JM, Duijkers L, Inglehearn CF, Sollfrank L, Strom TM, Uchio E, van Nouhuys CE, Kremer H, Veltman JA, van Wijk E, Cremers FP (2013) ZNF408 is mutated in familial exudative vitreoretinopathy and is crucial for the development of zebrafish retinal vasculature. Proc Natl Acad Sci U S A 110:9856–9861. https://doi.org/10.1073/pnas.1220864110
Iarossi G, Bertelli M, Maltese PE, Gusson E, Marchini G, Bruson A, Benedetti S, Volpetti S, Catena G, Buzzonetti L, Ziccardi L (2017) Genotype-phenotype characterization of novel variants in six Italian patients with familial exudative vitreoretinopathy. J Ophthalmol 2017:3080245. https://doi.org/10.1155/2017/3080245
Funding
This research was supported by the National Natural Science Foundation Project of China (81770964, 15XD1502800).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Rights and permissions
About this article
Cite this article
Li, Y., Peng, J., Li, J. et al. The characteristics of digenic familial exudative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol 256, 2149–2156 (2018). https://doi.org/10.1007/s00417-018-4076-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-018-4076-8