Abstract
Background
Familial Exudative Vitreoretinopathy (FEVR) is a hereditary disorder characterized by peripheral avascular retina with neovascularization. Although FEVR has been thoroughly described in multiple literature publications from different countries, there are currently limited articles describing the phenotypes of FEVR among South-East Asian Descendent. This paper describes the clinical phenotype of the FZD4 gene with c.1501_1502 deletion in a 4-generation case series of a South East Asian family.
Methods
We reviewed a 4-generation case series of a South-East Asian descendent family consisting of 27 family members with 10 members diagnosed with FEVR. We observed the clinical phenotype of these series of patients, including some of the family members who underwent whole-exome sequencing, PCR amplification and DNA sequencing techniques to identify the mutated gene.
Results
Frameshift mutation (c.1501_1502del) were found in FZD4 gene in this series of patients with the age ranging from 1 month old to 69 years old. There was a 100% (4/4) of our paediatric patients being diagnosed within 21 days of life. It was also found that 75% of patients (6/8) less than 40 years old exhibited disease asymmetry of 2 stages or more and 80% (8/10) had a history of vitreoretinal surgery or diode laser photocoagulation, with a further 50% of the adult patients identified as legally blind; the mean age of blindness was 18-years-old.
Conclusions
Phenotypic manifestation of FZD4 gene with c.1501_1502del mutation can be identified within the neonatal period. They have relatively greater clinical asymmetry of 2 stages or more compared to other mutations. Without treatment, most of them will have bilateral severe visual impairment around the adolescent age group.
Similar content being viewed by others
Background
Familial exudative vitreoretinopathy (FEVR) was first described by Criswick and Schepens in 1969 as congenital, bilateral vitreoretinopathy with no history of premature birth and oxygen therapy [1]. FEVR is characterized by the peripheral avascular retina and subsequently lead to complication due to retina ischemia [2]. These includes peripheral neovascularization, vitreous haemorrhage, retinal traction with temporal dragging, macular dragging, falciform retinal fold and retinal detachment [2, 3].
FEVR can be inherited in different modes which include autosomal dominant, autosomal recessive and X-linked recessive [4,5,6]. Autosomal dominant FEVR is the most common, it involves a mutation in frizzled class receptor 4 (FZD4), low-density lipoprotein receptor protein 5 (LRP5) or tetraspanin 12 (TSPAN12) [7,8,9].
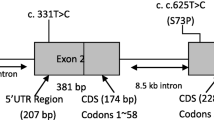
FZD4 genes are located in chromosome 11q14.2 and encode a 7-transmembrane protein of 537 amino acids [10]. FZD4 genes encode Wnt receptor which plays an important role in retinal angiogenesis [9]. To date, there are more than 50 mutations in FZD4 genes linked with FEVR reported [11]. Deletion of c.1501_1502 is one of the reported mutations in FZD4 genes. In regards to FZD4 genes related FEVR disease, most of the published articles originate from Caucasian, South Asian (Indian) East Asian (Chinese and Japanese) [9, 11,12,13]. To our best knowledge, there is currently no published literature describing the phenotypic manifestations of FZD4 c.1501_1502 deletion among South-East Asian descents.
Methods
Written informed consent was taken from the patients or their guardians before clinical data and blood samples were collected. This research adhered to the tenets of the Declaration of Helsinki and was registered under the Malaysian National Medical Research Registry (NMRR ID-21-02310-O5A). This study was conducted in Hospital Kuala Lumpur from April 2021 until December 2021.
The diagnosis of FEVR was based on the presence of typical clinical features: peripheral retinal avascular areas or proliferative changes, dragged disc or macula, retinal detachment with exudation or falciform retinal folds and no history of prematurity or oxygen supplementation. We identified one Malaysian patient as a proband with autosomal dominant FEVR and investigated the molecular basis of the disorder with Whole Exome Sequencing (WES). Once the pathogenic gene was identified, some family members who have the phenotype of FEVR went for polymerase chain reaction (PCR) and DNA sequencing techniques for the determination of the targeted mutation. We conducted clinical examination on the other family members and all the clinical data were documented and analysed. Some of the family members were previously treated in our centre, whereby retrospective data extraction was done from the clinical notes.
Whole exome sequencing (WES)
Whole exome sequencing was performed on genomic DNA using Agilent v6CREv2 targeted sequence capture method to enrich the exome. Direct sequencing of the amplified captured regions was performed using 2 × 100 bp reads on Illumina next-generation sequencing (NGS) systems. Alignments to the human reference genome (hg 19) are performed and annotated variants are identified in the targeted region. Primary data analysis was performed using Illumina DRAGON Bio-IT Platform v.2.03. Secondary and tertiary data analysis was performed using PerkinElmer’s internal ODIN v.1.01 software for single nucleotide variants and Biodiscovery’s NxClinical v.4.3 or Illumina DRAGEN Bio-IT Platform v.2.03 for copy number variation and absence of heterozygosity.
Results
We identified 10 FEVR patients (20 eyes) within 1 family of South-East Asian descendent. Data were retrospectively extracted and analysed. Of the 10 patients, 7 (70%) were male, their age ranged from 1-month-old to 69 years old. The family pedigree is displayed in Fig. 1.
Genotype: Frameshift mutation (c.1501_1502del) in FZD4 gene
Proband is Patient No. 10, WES was done to identify the genetic mutation. Results revealed deletion of two nucleotides at position c.1501_1502 of the FZD4 gene causing a frameshift in the protein reading frame. PCR amplification and DNA sequencing techniques were performed on Patient No. 9 to detect the c.1501_1502delCT mutation in the FZD4 gene. Results were positive.
Phenotype: extremely asymmetric clinical severity
The clinical severity of FEVR was graded using Revised FEVR Clinical Staging System 2014 [14]. The grading ranged from stage 1 to 5B (Table 1). Among 10 patients, only 4 (40%) have similar staging in both eyes. Clinical asymmetry become more apparent among younger patients, 6 out of 8 patients (75%) that are younger than 40-years-old have asymmetry FEVR clinical grade. All of the patients (100%) that have clinical asymmetry, have differences of at least 2 stages between their eyes (Fig. 2).
Visual acuity ranged from logMAR 0.2 to no perception of light. We could not take the visual acuity for the 1-month-old patient. Only 3 out of 9 patients (33.3%) have visual acuity of logMAR 1.0 or better in either eye. All 3 of them had laser therapy or vitreoretinal surgery performed on their better eye.
Among paediatric patients, all 4 patients (No. 5, 6, 7 & 10) were diagnosed in our centre before 21 days of life (Table 2). None of them was born prematurely. All of them had asymmetric retinal folds during our first examination (Fig. 3). The better eye ranged from stage 1 to 4A, the worse eye ranged from 3B to 5B. Diode laser was given to all 4 of them and monitored closely to look for any progression. Despite laser photocoagulation, patient no. 7 still progressed to Stage 4A with retinal fold touching posterior capsule of the crystalline lens. To date, patients 5, 6 & 10 have been clinically stable, but they are too young to conclude that the disease is static.
Fundus photo of patient no. 6 shows asymmetric clinical severity. A Right eye shows extramacular full-thickness retina fold with haemorrhage. No obvious exudation was seen. Stage 3A FEVR. B Left eye posterior pole appears flat, arrow shows the border of vascularized retinal with minimal exudates, no laser marks over avascular retina area. Stage 1B FEVR
The mean age for adult family members is 43.1 years old. (Table 3) 50% (No. 1, 2, 8) of the adult family members are legally blind. All 3 of them are the eldest among all 10 patients. They gave a history of bilateral eye blindness when they were teenagers (ages ranged from 15 to 21 years old). The mean age of their blindness was 18 years old.
All 6 adult patients complained of unilateral blurring of vision at a very young age (ranged 3 to 10 years old). Subsequently, the better eye will deteriorate and 3 (50%) of them underwent vitreoretinal surgery on their better eye. 1 patient (16.6%) underwent lens extraction over the better eye and suffered from secondary glaucoma, his FEVR remained stable despite absent of vitreoretinal surgery. The remaining 2 adult patients (33.3%) did not seek any medical treatment. The disease remained static after adolescence.
Discussion
Familial exudative vitreoretinopathy (FEVR, OMIM: 133,780) caused by FZD4 gene with c.1501_1502 deletion was only briefly described by Toomes et al. [7]. This mutation has been previously reported in individuals with FEVR (PMID: 12172548). There is a paucity of the detailed phenotypic description on c.1501_1502 deletion. To our best knowledge, to date, there has been no article describing the genotypic-phenotypic correlation of this mutation among South-East Asian descents.
Early detection
FZD4 gene with c.1501_1502 deletion showed signs of FEVR as early as 10 days of life. Our study managed to diagnose all the 4th generation children during their neonatal period (mean age of diagnosis is 14 days of life) due to positive FEVR family history, compared to a large study that has a mean age of diagnosis of 6 years old.[15] This shows that FEVR caused by FZD4 gene c.1501_1502delCT mutation can be diagnosed at a very young age within the neonatal period. Screening should be done as early as 10 days old for neonates who have a family history of FEVR, especially for those who had a definitive genetic diagnosis of c.1501_1502 deletion.
Disease progression and age group
Disease progression was expected to occur before age of 20 years old [16]. Among the 10 patients in our series, 2 of them (No. 1 & 7) exhibited disease progression despite treatment. Progression occurred at the age of 3-years-old (No. 7) and 17-years-old (No. 1). Disease severity remained static for the other 6 patients who received treatment. Only 2 patients did not receive any form of treatment (No. 2 & 8), and both of them translated the natural history of progression in FEVR and became legally blind around adolescent age.
Some studies described FEVR as a lifelong disease where progression can occur at any age after varying periods of apparent quiescent [13, 16]. However, these studies lacked genotypic information. Patients with deletion of c.1501_1502 in FZD4 gene might have a relatively lesser risk of progression after the adolescent period.
Disease asymmetry
Interocular clinical asymmetry among FEVR has been largely reported by multiple articles [3, 9, 13, 17]. Generally, 57% of FEVR patients have asymmetry staging, in which 71% presented with the 2 eyes within 1 stage of each other [15]. FEVR with FDZ4 gene mutation shows 47.4% of asymmetric disease severity which is higher than FEVR patients with TSPAN12, KIF11 or NDP mutation [17].
Our results showed greater asymmetry in younger patients with 75% of patients (6/8) who aged younger than 40-years-old exhibited disease asymmetry. All 6 of them (100%) have a difference of 2 stages or more. Ranchod et al. reported 71% of FEVR patients have 2 eyes within 1 stage of each other [3]. We hypothesize that the FZD4 gene c.1501_1502 deletion can produce greater clinical asymmetry compared to other genotype mutations.
Genotype-phenotype association will be the future of diagnostic medicine. FEVR can be caused by varies genotype mutations, and detailed description of the phenotype manifestation, correlating with the genotype mutations for each mutation will help both physicians and patients to understand and manage the disease better, with a more realistic visual prognostic expectations, instead of generalising the disease into a broad spectrum of phenotypic manifestations.
Conclusions
To our best knowledge, this is the first case series describing the phenotypic expression of FZD4 gene with c.1501_1502 deletion in South-East Asian descents, spanning a 4-generation case series. Our study highlights that FEVR signs due to c.1501_1502del can appear very early in life (neonatal period) and thus fundus screening is very important among newborns with a family history of FEVR. A deletion in c.1501_1502 could manifest greater clinical asymmetry of 2 stages or more compared to other mutation. Further studies are needed for detail phenotype description in other FEVR genetic mutations.
Availability of data and materials
All data and materials gathered during this study are included in this study.
Abbreviations
- FEVR:
-
Familial Exudative Vitreoretinopathy
- FZD4:
-
Frizzled Class Receptor 4
- PCR:
-
Polymerase chain reaction
- DNA:
-
deoxyribonucleic acid
- LRP5:
-
Low-density lipoprotein receptor protein 5
- TSPAN12:
-
Tetraspanin 12
- WES:
-
Whole exome sequencing
- VA:
-
Visual acuity
- RE:
-
Right eye
- LE:
-
Left eye
- BE:
-
Both eye
- PL:
-
Perception of light
- NPL:
-
Non perception of light
- VR op:
-
Vitreo-retinal operation
- IOL:
-
Intraocular lens
- KIF11:
-
Kinesin Family Member 11
- NDP:
-
Norrin Cystine Knot Growth Factor
References
Criswick VG, Schepens CL. Familial exudative vitreoretinopathy. Am J Ophthalmol. 1969;68(4):578–94.
Gow J, Oliver GL. Familial exudative vitreoretinopathy. An expanded view. Arch Ophthalmol (Chicago, Ill 1960). 1971;86(2):150–5.
Ranchod TM, Ho LY, Drenser KA, Capone AJ, Trese MT. Clinical presentation of familial exudative vitreoretinopathy. Ophthalmology. 2011;118(10):2070–5.
Feldman EL, Norris JL, Cleasby GW. Autosomal dominant exudative vitreoretinopathy. Arch Ophthalmol. 1983;101(10):1532–5. https://doi.org/10.1001/archopht.1983.01040020534004.
de Crecchio G, Simonelli F, Nunziata G, Mazzeo S, Greco GM, Rinaldi E, et al. Autosomal recessive familial exudative vitreoretinopathy: evidence for genetic heterogeneity. Clin Genet. 1998;54(4):315–20.
Plager DA, Orgel IK, Ellis FD, Hartzer M, Trese MT, Shastry BS. X-Linked recessive familial exudative vitreoretinopathy. Am J Ophthalmol. 1992;114(2):145–8.
Toomes C, Bottomley HM, Jackson RM, Towns KV, Scott S, Mackey DA, et al. Mutations in LRP5 or FZD4 underlie the common familial exudative vitreoretinopathy locus on chromosome 11q. Am J Hum Genet. 2004;74(4):721–30.
Nikopoulos K, Gilissen C, Hoischen A, van Nouhuys CE, Boonstra FN, Blokland EAW, et al. Next-generation sequencing of a 40 Mb linkage interval reveals TSPAN12 mutations in patients with familial exudative vitreoretinopathy. Am J Hum Genet. 2010;86(2):240–7.
Kaykas A, Yang-Snyder J, Héroux M, Shah KV, Bouvier M, Moon RT. Mutant Frizzled 4 associated with vitreoretinopathy traps wild-type Frizzled in the endoplasmic reticulum by oligomerization. Nat Cell Biol. 2004;6(1):52–8.
Warden SM, Andreoli CM, Mukai S. The Wnt signaling pathway in familial exudative vitreoretinopathy and Norrie disease. Semin Ophthalmol. 2007;22(4):211–7.
Tang M, Ding X, Li J, Hu A, Yuan M, Yang Y, et al. Novel mutations in FZD4 and phenotype–genotype correlation in chinese patients with familial exudative vitreoretinopathy. Mol Vis. 2016;22:917–32.
Omoto S, Hayashi T, Kitahara K, Takeuchi T, Ueoka Y. Autosomal dominant familial exudative vitreoretinopathy in two Japanese families with FZD4 mutations (H69Y and C181R). Ophthalmic Genet. 2004;25(2):81–90.
Shukla D, Singh J, Sudheer G, Soman M, John RK, Ramasamy K, et al. Familial exudative vitreoretinopathy (FEVR). Clinical profile and management. Indian J Ophthalmol. 2003;51(4):323–8.
Kashani AH, Learned D, Nudleman E, Drenser KA, Capone A, Trese MT. High prevalence of peripheral retinal vascular anomalies in family members of patients with familial exudative vitreoretinopathy. Ophthalmology. 2014;121(1):262–8.
Ranchod TM, Ho LY, Drenser KA, Capone A, Trese MT. Clinical presentation of familial exudative vitreoretinopathy. Ophthalmology. 2011;118(10):2070–5.
Benson WE. Familial exudative vitreoretinopathy. Trans Am Ophthalmol Soc. 1995;93:473–521.
Wang Z, Chen C, Sun L, Zhang A, Liu C, Huang L, et al. Symmetry of folds in FEVR: a genotype-phenotype correlation study. Exp Eye Res. 2019;186:107720.
Acknowledgements
The authors would like to thank the Director-General of Ministry of Health Malaysia for his kind permission to publish this article.
Funding
There is no financial support received for this study.
Author information
Authors and Affiliations
Contributions
YZW gathered the necessary information about the patient. YZW and YYC wrote the manuscript under supervision of NH and JR. LTL did a thorough revision of the work. NH and JR are the main consultants that managed the patients. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This case series is registered under Malaysian National Medical Research Registry (NMRR ID-21-02310-O5A) which follows the tenets of the Declaration of Helsinki. Written consent was taken from the patients for participation .
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wai, Y.Z., Chong, Y.Y., Lim, L.T. et al. Familial exudative vitreoretinopathy in a 4 generations family of South-East Asian Descendent with FZD4 mutation (c.1501_1502del). Int J Retin Vitr 8, 30 (2022). https://doi.org/10.1186/s40942-022-00384-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40942-022-00384-2