Abstract
Purpose
To compare chorioretinal atrophy (CRA) progression in myopic choroidal neovascularization (mCNV) between intravitreal injections of ranibizumab (IVR) and aflibercept (IVA) in the eyes with mCNV.
Methods
Thirty eyes (28 patients) with treatment-naïve mCNV were included in this study. IVR or IVA was administered for up to 1 year. The best-corrected visual acuity (BCVA) was measured, and fundus photographs and fundus autofluorescence were obtained before and 1, 3, 6, and 12 months after the initial treatment. The clinical characteristics including the macular choroidal thickness in various areas and CRA progression were compared between the drugs. The clinical characteristics and macular choroidal thicknesses were compared between eyes with and without CRA progression.
Results
The BCVA improved significantly (p < 0.05 for all comparisons) from 0.44 to 0.26, 0.19, 0.20, and 0.17 after 1, 3, 6, and 12 months, respectively. CRA progressed in 12 (40%) eyes over 1 year. The CRA progression did not differ significantly between aflibercept and ranibizumab. The foveal choroid was significantly (p = 0.0043) thinner in aflibercept-treated eyes compared with ranibizumab-treated eyes at 1 year. Subfoveal CNV tended to cause CRA progression more frequently at 1 year, although this did not reach significance.
Conclusions
IVA to treat mCNV caused more severe thinning of the foveal choroid than ranibizumab; however, no significant difference was seen in CRA progression between the drugs and the choroidal thickness should not be associated with CRA progression. The CNV location may predict CRA progression after anti-vascular endothelial growth factor therapy for mCNV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myopic choroidal neovascularization (mCNV), a severe complication of pathologic myopia, occurs in up to 10% of myopic patients and up to 40% in highly myopic patients [1,2,3]. The long-term visual prognosis is poor without treatment [4]. Although various treatments, including laser photocoagulation, macular translocation, surgical CNV removal, photodynamic therapy (PDT), and anti-vascular endothelial growth factor (anti-VEGF) therapy, have been performed [5], the long-term visual outcomes are extremely poor primarily due to the development of chorioretinal atrophy (CRA) around the regressed CNV [6,7,8,9,10,11,12]. To improve the long-term visual outcomes of mCNV, treatments are needed that also prevent development of CRA. The mechanism of the development of CRA around the CNV is unknown, although Ohno-Matsui et al. observed holes in Bruch’s membrane at the myopic choroidal atrophy including CRA around the CNV using swept-source optical coherence tomography (OCT) [13]. Several risk factors for CRA, including age, CNV size and location, and choroidal thickness, have been reported [12, 14, 15], in contrast, Oishi et al. reported that no patient characteristics significantly affected the CRA [9].
In the eyes with age-related macular degeneration (AMD), macular atrophy develops despite anti-VEGF therapy and affects the long-term visual outcomes in some cases [16]. Macular atrophy develops in about 10% of patients with AMD and 37% of those with retinal angiomatous proliferation (RAP) [17, 18]. The mechanism of the development of macular atrophy is unknown; however, several investigators have reported that macular atrophy was associated significantly with the baseline macular choroidal thickness and the number of anti-VEGF injections administered [17, 19].
The first-line treatment for mCNV recently has become anti-VEGF therapy. Two large, multi-center, double-masked, randomized, controlled clinical trials, i.e., the RADIANCE (Ranibizumab And PDT evAluation iN myopic Choroidal nEovascularization) study and the MYRROR (Intravitreal Aflibercept Injection in Patients with Myopic Choroidal Neovascularization) study, have reported beneficial effects of treatment [5, 20, 21]. However, it is unclear if anti-VEGF therapy also prevents later development of CRA in high myopia. In addition, although a recent study reported that the incidence of macular atrophy was higher in the eyes with RAP treated with intravitreal injections of aflibercept (IVA) (Eylea, Regeneron Pharmaceuticals, Tarrytown, NY, Bayer AG, Leverkusen, Germany) compared with intravitreal injections of ranibizumab (IVR) (Lucentis, Genentech, South San Francisco, CA) [18, 22], it is unclear if the prevalence rates of CRA development after administration of anti-VEGF therapy for mCNV differ between IVA and IVR. The current study compared the progression of CRA between IVA and IVR and investigated the difference between those with and without CRA enlargement.
Methods
We retrospectively reviewed the records of patients who were treated with IVA or IVR for mCNV. The diagnosis of mCNV was based on the characteristic findings on fluorescein angiography (FA), indocyanine green angiography (ICGA) images, and fundus photographs. The inclusion criteria were a refractive error of − 6.0 diopters (D) or higher or axial length of 26.5 mm or higher, the presence of subfoveal or juxtafoveal CNV, and treatment with IVA or IVR at Osaka University Hospital. The exclusion criteria included any treatment for mCNV other than anti-VEGF therapy before or during the observation period, a follow-up period of less than 12 months, intraocular surgery other than cataract surgery, or development of other ocular diseases during the follow-up.
The best-corrected visual acuity (BCVA) was measured and a dilated fundus examination that included indirect ophthalmoscopy, color fundus photography, FA, OCT, and fundus autofluorescence (FAF) was performed. FA and OCT were used to determine the location of the CNV, which was defined as subfoveal if the CNV was present in the foveal center, juxtafoveal if the CNV edge was within 200 μm of the foveal center, and extrafoveal if the CNV edge was more than 200 μm from the foveal center. For the purposes of analysis, the location of the CNV was classified as subfoveal or not subfoveal. When it was difficult to determine the location of the CNV using only FA images, OCT images were used to determine if the CNV was beneath the foveal center. OCT findings including intraretinal and subretinal fluid and CNV with a fuzzy border also were observed. The BCVA was converted to the logarithm of the minimum angle of resolution for analysis.
After topical anesthesia was applied, aflibercept (2.0 mg/0.05 ml) or ranibizumab (0.5 mg/0.05 ml) was injected 3.5 to 4.0 mm posterior to the corneal limbus into the vitreous cavity using a 30-gauge needle. Prophylactic topical antibiotics were applied for 3 days after the injection. Follow-up evaluations were conducted at 1, 3, 6, and 12 months. Additional follow-up visits were planned for each patient at the clinician’s discretion. After the initial treatment, additional treatment was administered as needed. The criteria for retreatment were determined based on objective/subjective visual declines, exudative changes seen on the OCT images, and/or dye leakage on FA.
The research adhered to the tenets of the Declaration of Helsinki. The institutional review board of Osaka University Hospital approved this retrospective study.
FA, ICGA, and FAF examinations
All examinations were performed using the Spectralis instrument (Heidelberg Retina Angiograph+OCT, Heidelberg, Germany) and a fundus camera (TRC-50DX, Topcon Corporation, Tokyo, Japan).
Choroidal and retinal thickness measurements
Swept-source OCT DRI OCT-1 Atlantis (Topcon Corporation) was used to obtain the measurements. The choroidal thicknesses were manually segmented and defined as the distance from the retinal pigment epithelial (RPE) line to the hyperreflective line behind the large vessel layers of the choroid, presumed to be the choroidal-scleral interface.
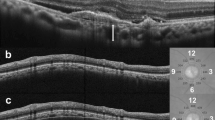
A five-line cross scan centered on the fovea was performed. After the B-scan scale was adjusted to 1:1 and the size of the image, the observer measured the choroidal thickness using the caliper function, which is a built-in linear measuring tool (Fig. 1). These measurements were performed manually and obtained at the fovea and 3.0 mm superior, inferior, nasal, and temporal to the fovea. The choroidal thickness at 12 months and % of foveal choroidal thickness in proportion to the baseline choroidal thickness at 3.0 mm nasal to the fovea were excluded from the analysis because the choroidal thickness at 3.0 mm nasally was too thin to be measured due to peripapillary atrophy. The retinal thickness was defined as the distance between the outer surface of the internal limiting membrane to the inner surface of the RPE line.
The measurement of the choroidal thickness on optical coherence tomography. Top: A scanning laser ophthalmoscopy fundus image. Bottom: The green lines indicate choroidal thickness measurements taken from the outer edge of the hyperreflective retinal pigment epithelium to the inner sclera at the fovea and 3 mm superior and inferior to the fovea
Development or enlargement of CRA
Two authors (K.S. and S.U.) determined if the CRA had progressed based on fundus photographs and FAF images. When the two authors disagreed, a third author (Y.I.) arbitrated the decision. CRA progression was defined as enlargement of patchy and diffuse atrophy adjacent to the CNV. Subtle color changes in tessellation and CRAs nonadjacent to the CNV were not counted (Figs. 3 and 4).
Statistical analysis
Statistical analyses were performed using JMP software version 10.0 (SAS Inc., Cary, NC). The comparisons were performed to identify significant differences in the BCVA among the examinations (before and 1, 3, 6, and 12 months after treatment) using Tukey’s HSD test. The parameters evaluated using the Student’s t test were the differences in age, axial length, BCVA, number of injections, and choroidal thicknesses in various areas between those treated with IVA and IVR, and with and without CRA progression. The chi-square test assessed the differences in the OCT findings between IVR and IVA, and gender, location of the CNV (subfoveal/not subfoveal), and the OCT findings between those with and without CRA progression. p < 0.05 was considered significant.
Results
Thirty eyes of 28 patients (7 men, 21 women; mean patient age, 65.8 ± 8.6 years) were eligible for the study. The mean axial length was 29.4 ± 1.6 mm. The refractive error was − 11.9 ± 3.5 D (range, −6 to −20) after excluding the 16 eyes that underwent cataract or refractive surgery. Fifteen eyes each received IVR or IVA. The mean number of anti-VEGF injections was 2.2 ± 1.2 (range, 1 to 5; IVR, 2.2 ± 1.4, range 1 to 5; IVR, 2.3 ± 1.1, range 1 to 4) over 12 months.
The occurrence rate of CRA progression was 40% at 12 months after IVR and IVA treatment.
Table 1 shows the characteristics of the patients treated with IVR and IVA. Only % of foveal choroidal thickness in proportion to the baseline at 1 year from baseline was significantly (p = 0.0018) thinner in the eyes treated with aflibercept compared with ranibizumab (Fig. 2). Table 2 shows the differences in the OCT findings between IVR and IVA. There were no significant differences in any OCT findings between both before treatment and 12 months after treatment. Tables 3 and 4, respectively, show the characteristics of the patients with and without CRA progression at baseline and 12 months after the initial treatment. No parameters were affected significantly; however, the subfoveal CNV tended to cause CRA progression more frequently at 12 months, and visual improvement was better in those without CRA progression than those with CRA progression, although the data did not reach significance. The choroidal thickness and % of the choroidal thickness in proportion to the baseline at the fovea and in various areas were not associated with CRA progression.
Percent of foveal choroidal thickness (CT) in proportion to the baseline after treatment in eyes with myopic choroidal neovascularization. The red line indicates percent of foveal CT in proportion to the baseline in all eyes; the blue line indicates percent of foveal CT in proportion to the baseline in the intravitreal ranibizumab (IVR)-treated eyes; the yellow line indicates percent of foveal CT in proportion to the baseline in the intravitreal aflibercept (IVA)-treated eyes. In all eyes and the IVA-treated eyes, the SCT decreased significantly from baseline 1 month after treatment and remained decreased until 1 month later. In the IVR-treated eyes, the SCT decreased compared with baseline; however, the difference did not reach significance. Comparison of the SCTs between the IVR- and IVA-treated eyes shows that percent of foveal CT in proportion to the baseline are significantly less in the IVR-treated eyes during the follow-up period. M = months; yr = years
Overall, the BCVA improved significantly (p < 0.05 for all comparisons) from 0.44 to 0.26, 0.19, 0.20, and 0.17 after 1, 3, 6, and 12 months, respectively.
Figures 3 and 4 show representative cases.
Left: Color fundus photographs; middle: fundus autofluorescence images; right: optical coherence tomography images at upper: baseline and bottom: 12 months after treatment. Upper right: A fluorescein angiography image at baseline obtained from a 60-year-old woman. The axial length of the right eye is 32.92 mm. The patient has subfoveal choroidal neovascularization (CNV) and was treated with one intravitreal ranibizumab injection. Comparison of the baseline color fundus photograph and fundus autofluorescence image and those obtained 12 months after treatment shows the obvious macular atrophy around the CNV (arrowheads) and the patchy atrophy close to the enlarged CNV (arrows). Her visual acuity, calculated in logarithm of the minimum angle of resolution units, improved from 0.82 to 0.40 at 1 year. The foveal choroidal thickness was 26 μm before the treatment and 27 μm at 1 year
Left: Color fundus photographs; middle: fundus autofluorescence images; right: optical coherence tomography images at upper: baseline and bottom: 12 months after treatment. Upper right: A fluorescein angiography image at baseline obtained from a 61-year-old man. The axial length of the right eye is 29.55 mm. The patient has subfoveal choroidal neovascularization (CNV) and was treated with two aflibercept intravitreal injections. Comparison of the baseline color fundus photograph and fundus autofluorescence images and those obtained 12 months after the treatment shows that macular atrophy developed around the CNV (arrowheads). His visual acuity, calculated in logarithm of the minimum angle of resolution units, improved from 1.52 to 1.10 at 1 year. The foveal choroidal thickness was 113 μm before the treatment and 103 μm at 1 year
Discussion
The current study compared CRA progression associated with IVA and IVR, and investigated the difference between those with and without CRA progression in the eyes with treatment-naïve mCNV. The overall rate of progression of CRA with IVA or IVR was 40% over the course of the study. No significant difference in CRA progression was seen between IVA and IVR, although the eyes with mCNV treated with IVA had significantly more severe thinning of the foveal choroid compared to IVR. The choroidal thickness and % of the choroidal thickness in proportion to the baseline were not associated with CRA progression.
Anti-VEGF therapy is the first-line therapy for mCNV, because the effect of PDT, which is an effective treatment for AMD, is limited to maintaining vision due to the high rate of CRA progression [5, 23, 24]. However, several investigators have reported that the VA improved significantly from baseline just after treatment, was maintained for 3 or 4 years, then declined slightly thereafter mainly due to CRA progression [6,7,8,9,10,11,12]. Oishi et al. evaluated the long-term outcomes in 22 eyes with mCNV after intravitreal injection of bevacizumab (IVB) (Avastin, Genentech) for at least 4 years [9]. The VA improved significantly from baseline and was maintained for 3 years, but the improvement became marginally nonsignificant 4 years after treatment presumably due to CRA, as 73% of eyes were found to have CRA progression at the last visit. In that study, CRA progression was seen in 40.9% in the first year, 63.6% in the second year, and 72.7% in the third and fourth years. Lee et al., who studied 50 eyes with mCNV that were treated with IVB or IVR for at least 2 years, estimated the occurrence rates of CRA progression as 10% in the first year, 19.1% in the second year, 23.6% in the third and fourth years, and 35.4% in the fifth year [12]. In the current study, the occurrence rate of CRA progression 12 months after treatment corresponded to the results reported by Oishi et al. [9], but was higher than that of Lee et al. [12]. While the reason for this discrepancy is unknown, one hypothesis might be differences in the diagnosis and definition of CRA progression.
In the eyes with AMD, macular atrophy after anti-VEGF therapy is considered problematic. After anti-VEGF therapy is administered to the eyes with AMD, the long-term progress is poor due to macular atrophy, which is CRA at the macular area [16]. The incidence of macular atrophy in AMD after anti-VEGF therapy is about 10% in the first year. Based on disease type, 12 months after treatment, the occurrence rates were reported to be 36.6% in the eyes with RAP, 3.8% in the eyes with AMD other than RAP treated with IVR, and 10.6% treated with IVA [17, 18, 25]. In another report, the incidence rates of macular atrophy in the eyes with RAP were 19% in the IVR-treated group and 42.9% in the IVA-treated group 12 months after treatment, a difference that reached significance [22]. Several factors such as the frequency of injections, baseline choroidal thickness, and AMD subtype have been reported as risk factors for macular atrophy, but no definitive conclusions have been reached [17, 19]. In addition, it is unknown why the IVA treatment group has a greater occurrence rate of macular atrophy in the eyes with AMD compared to the IVR treatment group. In the current study, the overall occurrence rate of CRA progression was 40% and there was no difference between the two treatment groups. The current occurrence rate of CRA progression is comparable to the incidence of macular atrophy in the eyes with RAP. Certainly, the choroid is thin in the eyes with RAP and high myopia [26, 27], and the choroid becomes thinner after treatment with IVA than with IVR [28]. Lee et al. reported that the choroidal thicknesses at the fovea and in various areas were not associated significantly with CRA progression, and only the ratio of the subfoveal choroid to the inferior choroid at 3 mm was associated strongly with CRA progression [12]. The current study did not analyze the association between the ratio of the subfoveal choroid to the choroid in various areas and CRA progression; however, considering that the baseline and 12-month choroidal thicknesses and the choroid thickness changes were not associated with CRA in the current report, we speculated that the choroidal circulation is related somehow to the atrophy of the macular region and IVA might have more of a negative impact on the choroidal circulation compared to IVR. However, the choroidal circulation might not necessarily be evaluated by the choroidal thickness. Future studies that include use of other methods to evaluate the choroidal circulation, such as laser speckle flowgraphy and OCT angiography, are needed to elucidate the pathology of CRA progression [29, 30] as are longer studies with more eyes, which might help differentiate the effect on the choroidal morphology between treatment with IVA and IVR in the eyes with mCNV.
Another interesting result in the current study was the location of the CNV. The subfoveal CNV tended to cause CRA progression more frequently at 12 months after treatment than the non-subfoveal CNV did, although this difference did not reach significance. Previous studies have reported that subfoveal CNV, older age, and large CNV size are risk factors for CRA progression [12, 14, 15]. Several investigators have reported that eyes with juxtafoveal CNV have a better prognosis than those with subfoveal CNV that was untreated or after anti-VEGF therapy [31,32,33]. Moreover, CRA progression caused by PDT occurred more often in the eyes with subfoveal CNV than with juxtafoveal CNV [34]. It is unclear why subfoveal CNV results in more frequent CRA progression. When CNV regresses, a retraction force is generated in the surrounding RPE and Bruch’s membrane, which then leads to atrophy or a hole in Bruch’s membrane, resulting in CRA [13]. We hypothesized that an intrinsic difference in the choroidal and scleral morphology might affect CNV regression after treatment resulting in CRA progression.
The current study had several limitations, including its retrospective design and the small sample size. Since the occurrence of CRA increases over time, the long-term results may differ from the current results. Future prospective longitudinal studies with longer follow-up periods are needed to confirm the current results. Choroidal thickness measurements of extremely thin choroids should be performed very cautiously. Thus, a direct comparison should be undertaken in a study with a large number of cases to determine the effect of drugs on the choroid thickness, although the results of several previous studies of the eyes with AMD support the current results [35,36,37].
In summary, we found that in the eyes with mCNV, the choice of IVR or IVR should not be considered as a risk factor contributing to CRA progression, although IVA can cause more severe choroidal thinning compared to IVR. The location of the CNV may predict the frequency of CRA progression after anti-VEGF therapy for mCNV.
References
Ohno-Matsui K (2016) Pathologic myopia. Asia Pac J Ophthalmol 5:415–423
Curtin BJ (1963) The pathogenesis of congenital myopia. A study of 66 cases. Arch Ophthalmol 69:166–173
Hotchkiss ML, Fine SL (1981) Pathologic myopia and choroidal neovascularization. Am J Ophthalmol 91:177–183
Yoshida T, Ohno-Matsui K, Yasuzumi K et al (2003) Myopic choroidal neovascularization: a 10-year follow-up. Ophthalmology 110:1297–1305
Ohno-Matsui K, Ikuno Y, Lai TYY, Gemmy Cheung CM (2018) Diagnosis and treatment guideline for myopic choroidal neovascularization due to pathologic myopia. Prog Retin Eye Res 63:92–106
Ikuno Y, Sayanagi K, Soga K et al (2009) Intravitreal bevacizumab for choroidal neovascularization attributable to pathological myopia: one-year results. Am J Ophthalmol 147:94–100
Lalloum F, Souied EH, Bastuji-Garin S et al (2010) Intravitreal ranibizumab for choroidal neovascularization complicating pathologic myopia. Retina 30:399–406
Kasahara K, Moriyama M, Morohoshi K et al (2017) Six-year outcomes of intravitreal bevacizumab for choroidal neovascularization in patients with pathologic myopia. Retina 37:1055–1064
Oishi A, Yamashiro K, Tsujikawa A et al (2013) Long-term effect of intravitreal injection of anti-VEGF agent for visual acuity and chorioretinal atrophy progression in myopic choroidal neovascularization. Graefes Arch Clin Ophthalmol 251:1–7
Ruiz-Moreno JM, Montero JA, Araiz J et al (2015) Intravitreal anti-vascular endothelial growth factor therapy for choroidal neovascularization secondary to pathologic myopia: six years outcome. Retina 35:2450–2456
Sarao V, Veritti D, Macor S, Lanzetta P (2016) Intravitreal bevacizumab for choroidal neovascularization due to pathologic myopia: long-term outcomes. Graefes Arch Clin Exp Ophthalmol 254:445–454
Lee JH, Lee SC, Kim SH et al (2017) Choroidal thickness and chorioretinal atrophy in myopic choroidal neovascularization with anti-vascular endothelial growth factor therapy. Retina 37:1516–1522
Ohno-Matsui K, Jonas JB, Spaide RF (2016) Macular Bruch membrane holes in choroidal neovascularization-related myopic macular atrophy by swept-source optical coherence tomography. Am J Ophthalmol 162:133–139
Kojima A, Ohno-Matsui K, Teramukai S et al (2004) Factors associated with the development of chorioretinal atrophy around choroidal neovascularization in pathologic myopia. Graefes Arch Clin Exp Ophthalmol 242(2):114–119
Hayashi K, Shimada N, Moriyama M et al (2012) Two-year outcomes of intravitreal bevacizumab for choroidal neovascularization in Japanese patients with pathologic myopia. Retina 32:687–695
Bhisitkul RB, Desai SJ, Boyer DS, Sadda SR, Zhang K (2016) Fellow eye comparisons for 7-year outcomes in ranibizumab-treated AMD subjects from ANCHOR, MARINA, and HORIZON (SEVEN-UP Study). Ophthalmology 123:1269–1277
Kuroda Y, Yamashiro K, Ooto S et al (2018) Macular atrophy and macular morphology in aflibercept-treated neovascular age-related macular degeneration. Retina 38:1743–1750
Hata M, Yamashiro K, Oishi A et al (2017) Retinal pigment epithelial atrophy after anti-vascular endothelial growth factor injections for retinal angiomatous proliferation. Retina 37:2069–2077
Lois N, McBain V, Abdelkader E, Scott NW, Kumari R (2013) Retinal pigment epithelial atrophy in patients with exudative age-related macular degeneration undergoing anti-vascular endothelial growth factor therapy. Retina 33:13–22
Wolf S, Balciuniene VJ, Laganovska G et al (2014) RADIANCE: a randomized controlled study of ranibizumab in patients with choroidal neovascularization secondary to pathologic myopia. Ophthalmology 121:682–692.e2
Ikuno Y, Ohno-Matsui K, Wong TY et al (2015) Intravitreal aflibercept injection in patients with myopic choroidal neovascularization: the MYRROR study. Ophthalmology 122:1220–1227
Cho HJ, Hwang HJ, Kim HS et al (2018) Intravitreal aflibercept and ranibizumab injections for type 3 neovascularization. Retina. https://doi.org/10.1097/IAE.0000000000001862
Ruiz-Moreno JM, Amat P, Montero JA, Lugo F (2008) Photodynamic therapy to treat choroidal neovascularisation in highly myopic patients: 4 years’ outcome. Br J Ophthalmol 92:792–794
Ikuno Y, Nagai Y, Matsuda S et al (2010) Two-year visual results for older Asian women treated with photodynamic therapy or bevacizumab for myopic choroidal neovascularization. Am J Ophthalmol 149:140–146
Kuroda Y, Yamashiro K, Tsujikawa A et al (2016) Retinal pigment epithelial atrophy in neovascular age-related macular degeneration after ranibizumab treatment. Am J Ophthalmol 161:94–103.e1
Kim JH, Kim JR, Kang SW, Kim SJ, Ha HS (2013) Thinner choroid and greater drusen extent in retinal angiomatous proliferation than in typical exudative age-related macular degeneration. Am J Ophthalmol 155:743–749
Ikuno Y, Fujimoto S, Jo Y, Asai T, Nishida K (2013) Choroidal thinning in high myopia measured by optical coherence tomography. Clin Ophthalmol 7:889–893
Gharbiya M, Giustolisi R, Marchiori J et al (2018) Comparison of short-term choroidal thickness and retinal morphological changes after intravitreal anti-VEGF therapy with ranibizumab or aflibercept in treatment-naive eyes. Curr Eye Res 43:391–396
Araie M (1999) In vivo measurement of ocular circulation with the laser speckle method—development of apparatus and application in ophthalmological research. Nippon Ganka Gakkai Zasshi 103:871–909
Maruko I, Spaide RF, Koizumi H et al (2017) Choroidal blood flow visualization in high myopia using a projection artifact method in optical coherence tomography angiography. Retina 37:460–465
Calvo-Gonzalez C, Reche-Frutos J, Donate J, Fernandez-Perez C, Garcia-Feijoo J (2011) Intravitreal ranibizumab for myopic choroidal neovascularization: factors predictive of visual outcome and need for retreatment. Am J Ophthalmol 151:529–534
Hayashi K, Ohno-Matsui K, Yoshida T et al (2005) Characteristics of patients with a favorable natural course of myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 243:13–19
Nakanishi H, Tsujikawa A, Yodoi Y et al (2011) Prognostic factors for visual outcomes 2-years after intravitreal bevacizumab for myopic choroidal neovascularization. Eye (Lond) 25:375–381
Hayashi K, Ohno-Matsui K, Shimada N et al (2011) Long-term results of photodynamic therapy for choroidal neovascularization in Japanese patients with pathologic myopia. Am J Ophthalmol 151:137–147
Koizumi H, Kano M, Yamamoto A, Saito M et al (2015) Short-term changes in choroidal thickness after aflibercept therapy for neovascular age-related macular degeneration. Am J Ophthalmol 159:627–633
Yamazaki T, Koizumi H, Yamagishi T, Kinoshita S (2012) Subfoveal choroidal thickness after ranibizumab therapy for neovascular age-related macular degeneration: 12-month results. Ophthalmology 119:1621–1627
Ellabban AA, Tsujikawa A, Ogino K et al (2012) Choroidal thickness after intravitreal ranibizumab injections for choroidal neovascularization. Clin Ophthalmol 6:837–844
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sayanagi, K., Uematsu, S., Hara, C. et al. Effect of intravitreal injection of aflibercept or ranibizumab on chorioretinal atrophy in myopic choroidal neovascularization. Graefes Arch Clin Exp Ophthalmol 257, 749–757 (2019). https://doi.org/10.1007/s00417-018-04214-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-018-04214-w