Abstract
Purpose
The model of oxygen-induced retinopathy (OIR) is widely used to analyze pathomechanisms in retinal neovascularization. Previous studies have shown that macrophages (MP) play a key role in vessel formation in OIR, the influence of microglia (MG) having been discussed. The aim of our study was to analyze the spatial and temporal distribution and activation of MP/MG expressing CD115 and CD11b during the process of neovascularization in OIR.
Methods
We used MacGreen mice expressing the green fluorescence protein (GFP) under the promoter for CD115. CD115+ cells were investigated in vivo by scanning laser ophthalmoscopy at postnatal days (P) 17 and 21 in MacGreen mice with OIR (75% oxygen from P7 to P12), and were compared to MacGreen room-air controls. In addition MP/MG were examined ex vivo using immunohistochemistry for CD11b+ detection on retinal flatmounts at P14, P17, and P21 of wild type mice with OIR.
Results
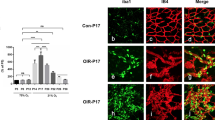
In-vivo imaging revealed the highest density of activated MP/MG in tuft areas at P17 of MacGreen mice with OIR. Tufts and regions with a high density of CD115+ cells were detected close to veins, rather to arteries. In peripheral, fully vascularized areas, the distribution of CD115+ cells in MacGreen mice with OIR was similar to MacGreen room-air controls. Correspondingly, immunohistochemical analyses of retinal flatmounts from wild type mice with OIR induction revealed that the number of CD11b+ cells significantly varies between vascular, avascular, and tuft areas as well as between the retinal layers. Activated CD11b+ cells were almost exclusively found in avascular areas and tufts of wild type mice with OIR induction; here, the proportion of activated cells related to the total number of CD11b+ cells remained stable over the course of time.
Conclusions
Using two different approaches to monitor MP/MG cells, our findings demonstrated that MP/MG concentrate within pathologically vascularized areas during OIR. We were able to clarify that reactive changes of CD11b+ cell distribution to OIR primarily occur in the deep retinal layers. Furthermore, we found the highest proportion of activated CD11b+ cells in regions with pathologic neovascularization processes. Our findings support previous reports about activated MP/MG guiding revascularization in avascular areas and playing a key role in the formation and regression of neovascular tufts.








Similar content being viewed by others
References
Smith LE, Wesolowski E, McLellan A, Kostyk SK, D’Amato R, Sullivan R, D’Amore PA (1994) Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 35:101–111
Stahl A, Connor KM, Sapieha P, Chen J, Dennison RJ, Krah NM, Seaward MR, Willett KL, Aderman CM, Guerin KI, Hua J, Löfqvist C, Hellström A, Smith LE (2010) The mouse retina as an angiogenesis model. Invest Ophthalmol Vis Sci 51:2813–2826. https://doi.org/10.1167/iovs.10-5176
Hartnett ME, Penn JS (2012) Mechanisms and management of retinopathy of prematurity. N Engl J Med 367:2515–2526. https://doi.org/10.1056/NEJMra1208129
Caprara C, Grimm C (2012) From oxygen to erythropoietin: relevance of hypoxia for retinal development, health and disease. Prog Retin Eye Res 31:89–119. https://doi.org/10.1016/j.preteyeres.2011.11.003
Uemura A, Kusuhara S, Katsuta H, Nishikawa S (2006) Angiogenesis in the mouse retina: a model system for experimental manipulation. Exp Cell Res 312:676–683. https://doi.org/10.1016/j.yexcr.2005.10.030
Naug HL, Browning J, Gole GA, Gobé G (2000) Vitreal macrophages express vascular endothelial growth factor in oxygen-induced retinopathy. Clin Experiment Ophthalmol 28:48–52
Kataoka K, Nishiguchi KM, Kaneko H, van Rooijen N, Kachi S, Terasaki H (2011) The roles of vitreal macrophages and circulating leukocytes in retinal neovascularization. Invest Ophthalmol Vis Sci 52:1431–1438. https://doi.org/10.1167/iovs.10-5798
Yang Y, Liu F, Tang M, Yuan M, Hu A, Zhan Z, Li Z, Li J, Ding X, Lu L (2016) Macrophage polarization in experimental and clinical choroidal neovascularization. Sci Rep 6:30933. https://doi.org/10.1038/srep30933
He L, Marneros AG (2014) Doxycycline inhibits polarization of macrophages to the proangiogenic M2-type and subsequent neovascularization. J Biol Chem 289:8019–8028. https://doi.org/10.1074/jbc.M113.535765
Fischer F, Martin G, Agostini HT (2011) Activation of retinal microglia rather than microglial cell density correlates with retinal neovascularization in the mouse model of oxygen-induced retinopathy. J Neuroinflammation 8:120. https://doi.org/10.1186/1742-2094-8-120
Dorrell MI, Aguilar E, Jacobson R, Trauger SA, Friedlander J, Siuzdak G, Friedlander M (2010) Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy. Glia 58:43–54. https://doi.org/10.1002/glia.20900
Ritter MR, Banin E, Moreno SK, Aguilar E, Dorrell MI, Friedlander M (2006) Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J Clin Invest 116:3266–3276. https://doi.org/10.1172/JCI29683
Vessey KA, Wilkinson-Berka JL, Fletcher EL (2011) Characterization of retinal function and glial cell response in a mouse model of oxygen-induced retinopathy. J Comp Neurol 519:506–527. https://doi.org/10.1002/cne.22530
Checchin D, Sennlaub F, Levavasseur E, Leduc M, Chemtob S (2006) Potential role of microglia in retinal blood vessel formation. Invest Ophthalmol Vis Sci 47:3595–3602. https://doi.org/10.1167/iovs.05-1522
Chen L, Yang P, Kijlstra A (2002) Distribution, markers, and functions of retinal microglia. Ocul Immunol Inflamm 10:27–39
Egensperger R, Maslim J, Bisti S, Holländer H, Stone J (1996) Fate of DNA from retinal cells dying during development: uptake by microglia and macroglia (Müller cells). Brain Res Dev Brain Res 97:1–8
Yang P, de Vos AF, Kijlstra A (1996) Macrophages in the retina of normal Lewis rats and their dynamics after injection of lipopolysaccharide. Invest Ophthalmol Vis Sci 37:77–85
Alt C, Runnels JM, Mortensen LJ, Zaher W, Lin CP (2014) In vivo imaging of microglia turnover in the mouse retina after ionizing radiation and dexamethasone treatment. Invest Ophthalmol Vis Sci 55:5314–5319. https://doi.org/10.1167/iovs.14-14254
Jolivel V, Bicker F, Binamé F, Ploen R, Keller S, Gollan R, Jurek B, Birkenstock J, Poisa-Beiro L, Bruttger J, Opitz V, Thal SC, Waisman A, Bäuerle T, Schäfer MK, Zipp F, Schmidt MH (2015) Perivascular microglia promote blood vessel disintegration in the ischemic penumbra. Acta Neuropathol 129:279–295. https://doi.org/10.1007/s00401-014-1372-1
Crespo-Garcia S, Reichhart N, Hernandez-Matas C, Zabulis X, Kociok N, Brockmann C, Joussen AM, Strauß O (2015) In vivo analysis of the time and spatial activation pattern of microglia in the retina following laser-induced choroidal neovascularization. Exp Eye Res 139:13–21. https://doi.org/10.1016/j.exer.2015.07.012
Sherr CJ, Roussel MF, Rettenmier CW (1988) Colony-stimulating factor-1 receptor (c-fms). J Cell Biochem 38:179–187. https://doi.org/10.1002/jcb.240380305
Mitrasinovic OM, Grattan A, Robinson CC, Lapustea NB, Poon C, Ryan H, Phong C, Murphy GM (2005) Microglia overexpressing the macrophage colony-stimulating factor receptor are neuroprotective in a microglial–hippocampal organotypic coculture system. J Neurosci 25:4442–4451. https://doi.org/10.1523/JNEUROSCI.0514-05.2005
Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, Ostrowski MC, Himes SR, Hume DA (2003) A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 101:1155–1163. https://doi.org/10.1182/blood-2002-02-0569
Sedgwick JD, Schwender S, Imrich H, Dörries R, Butcher GW, ter Meulen V (1991) Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci U S A 88:7438–7442
Sedgwick JD, Ford AL, Foulcher E, Airriess R (1998) Central nervous system microglial cell activation and proliferation follows direct interaction with tissue-infiltrating T cell blasts. J Immunol 160:5320–5330
Campanella M, Sciorati C, Tarozzo G, Beltramo M (2002) Flow cytometric analysis of inflammatory cells in ischemic rat brain. Stroke 33:586–592
Mattapallil MJ, Wawrousek EF, Chan CC, Zhao H, Roychoudhury J, Ferguson TA, Caspi RR (2012) The Rd8 mutation of the Crb1 gene is present in vendor lines of C57BL/6N mice and embryonic stem cells, and confounds ocular induced mutant phenotypes. Invest Ophthalmol Vis Sci 53:2921–2927. https://doi.org/10.1167/iovs.12-9662
Brockmann C, Brockmann T, Dege S, Busch C, Kociok N, Vater A, Klussmann S, Strauß O, Joussen AM (2015) Intravitreal inhibition of complement C5a reduces choroidal neovascularization in mice. Graefes Arch Clin Exp Ophthalmol 253(10):1695–1704. https://doi.org/10.1007/s00417-015-3041-z
Müther PS, Semkova I, Schmidt K, Abari E, Kuebbeler M, Beyer M, Abken H, Meyer KL, Kociok N, Joussen AM (2010) Conditions of retinal glial and inflammatory cell activation after irradiation in a GFP-chimeric mouse model. Invest Ophthalmol Vis Sci 51:4831–4839. https://doi.org/10.1167/iovs.09-4923
Kociok N, Radetzky S, Krohne TU, Gavranic C, Joussen AM (2006) Pathological but not physiological retinal neovascularization is altered in TNF-Rp55-receptor-deficient mice. Invest Ophthalmol Vis Sci 47:5057–5065. https://doi.org/10.1167/iovs.06-0407
Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LE (2009) Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc 4:1565–1573. https://doi.org/10.1038/nprot.2009.187
Kettenmann H (2007) Neuroscience: the brain’s garbage men. Nature 446:987–989. https://doi.org/10.1038/nature05713
Stence N, Waite M, Dailey ME (2001) Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia 33:256–266
Davies MH, Eubanks JP, Powers MR (2006) Microglia and macrophages are increased in response to ischemia-induced retinopathy in the mouse retina. Mol Vis 12:467–477
Jonas RA, Yuan TF, Liang YX, Jonas JB, Tay DK, Ellis-Behnke RG (2012) The spider effect: morphological and orienting classification of microglia in response to stimuli in vivo. PLoS ONE 7:e30763. https://doi.org/10.1371/journal.pone.0030763
Shen J, Xie B, Dong A, Swaim M, Hackett SF, Campochiaro PA (2007) In vivo immunostaining demonstrates macrophages associate with growing and regressing vessels. Invest Ophthalmol Vis Sci 48:4335–4341. https://doi.org/10.1167/iovs.07-0113
Fruttiger M (2002) Development of the mouse retinal vasculature: angiogenesis versus vasculogenesis. Invest Ophthalmol Vis Sci 43:522–527
Ganesan P, He S, Xu H (2010) Analysis of retinal circulation using an image-based network model of retinal vasculature. Microvasc Res 80:99–109. https://doi.org/10.1016/j.mvr.2010.02.005
Lang RA, Bishop JM (1993) Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell 74:453–462
Stephan AH, Barres BA, Stevens B (2012) The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci 35:369–389. https://doi.org/10.1146/annurev-neuro-061010-113810
Diez-Roux G, Lang RA (1997) Macrophages induce apoptosis in normal cells in vivo. Development 124:3633–3638
Acknowledgements
Karin Oberländer is sincerely acknowledged for her excellent technical assistance.
Funding
This work was funded by Ernst und Berta Grimmke-Stiftung (Lf.Nr. 8/13) and Deutsche Forschungsgemeinschaft (DFG, Jo 324/10-2). Claudia Brockmann and Tobias Brockmann are participants in the BIH-Charité Clinical Scientist Program funded by the Charité -Universitätsmedizin Berlin and the Berlin Institute of Health. Sabrina Dege received a scholarship from the Sonnenfeld-Stiftung. Sergio Crespo-Garcia received a Marie Curie grant from the European Commission in the framework of the REVAMMAD ITN (Initial Training Research network).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Financial disclosure
None of the authors has any financial interests in the present work.
Electronic supplementary material
Fig. S1
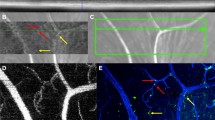
Representative images showing the method of quantification of auto-fluorescent CD115+ cells in MacGreen mice. a Fluorescence angiography in an eye with oxygen-induced retinopathy at postnatal day 17 representing avascular areas and tufts. b Same image with marked regions of interest (ROIs); yellow: avascular areas; red: tufts. c Corresponding auto-fluorescence image of the same eye showing auto-fluorescent CD115+ cells. d Same image with applied ROIs. (GIF 78 kb)
Fig. S2
Representative images showing the method of quantification of auto-fluorescent CD115+ cells in MacGreen mice. a Fluorescence angiography in an eye of room-air controls at postnatal day 21 representing physiological retinal vascularization. b Same image with marked regions of interest (ROIs); green: vascular area. c Corresponding auto-fluorescence image of the same eye showing auto-fluorescent CD115+ cells. d Same image with applied ROIs. (GIF 83 kb)
Rights and permissions
About this article
Cite this article
Brockmann, C., Dege, S., Crespo-Garcia, S. et al. Spatial distribution of CD115+ and CD11b+ cells and their temporal activation during oxygen-induced retinopathy in mice. Graefes Arch Clin Exp Ophthalmol 256, 313–323 (2018). https://doi.org/10.1007/s00417-017-3845-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-017-3845-0