Abstract
Purpose
To investigate the influence of complement component C5a inhibition on laser-induced choroidal neovascularization (CNV) in mice using a C5a specific l-aptamer.
Methods
In C57BL/6 J mice CNV was induced by argon-laser, C5a-inhibitor (NOX-D20) was intravitreally injected in three concentrations: 0.3, 3.0, and 30 mg/ml. The unPEGylated derivate (NOX-D20001) was applied at 3.0 mg/ml; the vehicle (5 % glucose) was injected in controls. Vascular leakage was evaluated using fluorescence angiography, CNV area was examined immunohistochemically. Activated immune cells surrounding the CNV lesion and potential cytotoxicity were analyzed.
Results
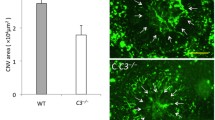
Compared to controls, CNV areas were significantly reduced after NOX-D20 injection at a concentration of 0.3 and 3.0 mg/ml (p = 0.042; p = 0.016). NOX-D20001 significantly decreased CNV leakage but not the area (p = 0.007; p = 0.276). At a concentration of 30 mg/ml, NOX-D20 did not reveal significant effects on vascular leakage or CNV area (p = 0.624; p = 0.121). The amount of CD11b positive cells was significantly reduced after treatment with 0.3 and 3.0 mg/ml NOX-D20 (p = 0.027; p = 0.002). No adverse glial cell proliferation or increased apoptosis were observed at effective dosages.
Conclusions
Our findings demonstrate that the targeted inhibition of complement component C5a reduces vascular leakage and neovascular area in laser-induced CNV in mice. NOX-D20 was proven to be an effective and safe agent that might be considered as a therapeutic candidate for CNV treatment. The deficiency of activated immune cells highlights promising new aspects in the pathology of choroidal neovascularization, and warrants further investigations.








Similar content being viewed by others
References
Pascolini D, Mariotti SP, Pokharel GP, Pararajasegaram R, Etya’ale D, Négrel AD, Resnikoff S (2004) 2002 global update of available data on visual impairment: a compilation of population-based prevalence studies. Ophthalmic Epidemiol 11:67–115
Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE, Hofman A, Jensen S, Wang JJ, de Jong PT (2001) Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology 108:697–704
Rosenfeld P, Brown D, Heier J, Boyer D, Kaiser P, Chung C, Kim R (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355:1419–1431
Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ, Group CR (2011) Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 364:1897–1908. doi:10.1056/NEJMoa1102673
Browning DJ, Kaiser PK, Rosenfeld PJ, Stewart MW (2012) Aflibercept for age-related macular degeneration: a game-changer or quiet addition? Am J Ophthalmol 154:222–226. doi:10.1016/j.ajo.2012.04.020
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U, Groups VVS (2012) Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 119:2537–2548. doi:10.1016/j.ophtha.2012.09.006
Sivaprasad S, Chong NV (2006) The complement system and age-related macular degeneration. Eye (Lond) 20:867–872. doi:10.1038/sj.eye.6702176
Ricklin D, Hajishengallis G, Yang K, Lambris JD (2010) Complement: a key system for immune surveillance and homeostasis. Nat Immunol 11:785–797. doi:10.1038/ni.1923
Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM (2009) Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci 50:5818–5827. doi:10.1167/iovs.09-3928
Penfold PL, Liew SC, Madigan MC, Provis JM (1997) Modulation of major histocompatibility complex class II expression in retinas with age-related macular degeneration. Invest Ophthalmol Vis Sci 38:2125–2133
Umeda S, Suzuki MT, Okamoto H, Ono F, Mizota A, Terao K, Yoshikawa Y, Tanaka Y, Iwata T (2005) Molecular composition of drusen and possible involvement of anti-retinal autoimmunity in two different forms of macular degeneration in cynomolgus monkey (macaca fascicularis). FASEB J 19:1683–1685. doi:10.1096/fj.04-3525fje
Köhl J (2001) Anaphylatoxins and infectious and non-infectious inflammatory diseases. Mol Immunol 38:175–187
Björk J, Hugli TE, Smedegård G (1985) Microvascular effects of anaphylatoxins C3a and C5a. J Immunol 134:1115–1119
Joussen AM, Poulaki V, Qin W, Kirchhof B, Mitsiades N, Wiegand SJ, Rudge J, Yancopoulos GD, Adamis AP (2002) Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol 160:501–509. doi:10.1016/S0002-9440(10)64869-9
Cortright DN, Meade R, Waters SM, Chenard BL, Krause JE (2009) C5a, but not C3a, increases VEGF secretion in ARPE-19 human retinal pigment epithelial cells. Curr Eye Res 34:57–61. doi:10.1080/02713680802546658
Nozaki M, Raisler BJ, Sakurai E, Sarma JV, Barnum SR, Lambris JD, Chen Y, Zhang K, Ambati BK, Baffi JZ, Ambati J (2006) Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A 103:2328–2333. doi:10.1073/pnas.0408835103
Hoehlig K, Maasch C, Shushakova N, Buchner K, Huber-Lang M, Purschke WG, Vater A, Klussmann S (2013) A novel C5a-neutralizing mirror-image (l-)aptamer prevents organ failure and improves survival in experimental sepsis. Mol Ther 21(12):2236–2246. doi:10.1038/mt.2013.178
Klussmann S, Nolte A, Bald R, Erdmann VA, Fürste JP (1996) Mirror-image RNA that binds D-adenosine. Nat Biotechnol 14:1112–1115. doi:10.1038/nbt0996-1112
Vater A, Sahlmann J, Kröger N, Zöllner S, Lioznov M, Maasch C, Buchner K, Vossmeyer D, Schwoebel F, Purschke WG, Vonhoff S, Kruschinski A, Hübel K, Humphrey M, Klussmann S, Fliegert F (2013) Hematopoietic stem and progenitor cell mobilization in mice and humans by a first-in-class mirror-image oligonucleotide inhibitor of CXCL12. Clin Pharmacol Ther 94(1):150–157. doi:10.1038/clpt.2013.58
Semkova I, Peters S, Welsandt G, Janicki H, Jordan J, Schraermeyer U (2003) Investigation of laser-induced choroidal neovascularization in the rat. Invest Ophthalmol Vis Sci 44:5349–5354
Xia X, Wen R, Chou TH, Li Y, Wang Z, Porciatti V (2014) Protection of pattern electroretinogram and retinal ganglion cells by oncostatin m after optic nerve injury. PLoS One 9, e108524. doi:10.1371/journal.pone.0108524
Greenberg KP, Geller SF, Schaffer DV, Flannery JG (2007) Targeted transgene expression in muller glia of normal and diseased retinas using lentiviral vectors. Invest Ophthalmol Vis Sci 48:1844–1852. doi:10.1167/iovs.05-1570
Jasielska M, Semkova I, Shi X, Schmidt K, Karagiannis D, Kokkinou D, Mackiewicz J, Kociok N, Joussen AM (2010) Differential role of tumor necrosis factor (TNF)-alpha receptors in the development of choroidal neovascularization. Invest Ophthalmol Vis Sci 51:3874–3883. doi:10.1167/iovs.09-5003
Sheets KG, Zhou Y, Ertel MK, Knott EJ, Regan CE, Elison JR, Gordon WC, Gjorstrup P, Bazan NG (2010) Neuroprotectin D1 attenuates laser-induced choroidal neovascularization in mouse. Mol Vis 16:320–329
Müther PS, Semkova I, Schmidt K, Abari E, Kuebbeler M, Beyer M, Abken H, Meyer KL, Kociok N, Joussen AM (2010) Conditions of retinal glial and inflammatory cell activation after irradiation in a GFP-chimeric mouse model. Invest Ophthalmol Vis Sci 51:4831–4839. doi:10.1167/iovs.09-4923
Shi X, Semkova I, Müther PS, Dell S, Kociok N, Joussen AM (2006) Inhibition of TNF-alpha reduces laser-induced choroidal neovascularization. Exp Eye Res 83:1325–1334. doi:10.1016/j.exer.2006.07.007
Kociok N, Krohne TU, Poulaki V, Joussen AM (2007) Geldanamycin treatment reduces neovascularization in a mouse model of retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 245:258–266. doi:10.1007/s00417-006-0355-x
Kociok N, Radetzky S, Krohne TU, Gavranic C, Liang Y, Semkova I, Joussen AM (2009) ICAM-1 depletion does not alter retinal vascular development in a model of oxygen-mediated neovascularization. Exp Eye Res 89:503–510. doi:10.1016/j.exer.2009.05.005
Michalewski J, Nawrocki J, Izdebski B, Michalewska Z (2014) Morphological changes in spectral domain optical coherence tomography guided bevacizumab injections in wet age-related macular degeneration, 12 months results. Indian J Ophthalmol 62:554–560. doi:10.4103/0301-4738.133485
Falkenstein IA, Cheng L, Wong-Staal F, Tammewar AM, Barron EC, Silva GA, Li QX, Yu D, Hysell M, Liu G, Ke N, Macdonald JE, Freeman WR (2008) Toxicity and intraocular properties of a novel long-acting anti-proliferative and anti-angiogenic compound IMS2186. Curr Eye Res 33:599–609. doi:10.1080/02713680802226582
Zhuang P, Shen Y, Lin BQ, Zhang WY, Chiou GC (2011) Effect of quercetin on formation of choroidal neovascularization (CNV) in age-related macular degeneration(AMD). Yan Ke Xue Bao 26:23–29. doi:10.3969/j.issn.1000-4432.2011.01.006
Semkova I, Fauser S, Lappas A, Smyth N, Kociok N, Kirchhof B, Paulsson M, Poulaki V, Joussen AM (2006) Overexpression of FasL in retinal pigment epithelial cells reduces choroidal neovascularization. FASEB J 20:1689–1691. doi:10.1096/fj.05-5653fje
Kemper C, Atkinson JP (2007) T-cell regulation: with complements from innate immunity. Nat Rev Immunol 7:9–18. doi:10.1038/nri1994
Rohrer B, Long Q, Coughlin B, Wilson RB, Huang Y, Qiao F, Tang PH, Kunchithapautham K, Gilkeson GS, Tomlinson S (2009) A targeted inhibitor of the alternative complement pathway reduces angiogenesis in a mouse model of age-related macular degeneration. Invest Ophthalmol Vis Sci 50:3056–3064. doi:10.1167/iovs.08-2222
Rohrer B, Coughlin B, Bandyopadhyay M, Holers VM (2012) Systemic human CR2-targeted complement alternative pathway inhibitor ameliorates mouse laser-induced choroidal neovascularization. J Ocul Pharmacol Ther 28:402–409. doi:10.1089/jop.2011.0212
Fukuoka Y, Strainic M, Medof ME (2003) Differential cytokine expression of human retinal pigment epithelial cells in response to stimulation by C5a. Clin Exp Immunol 131:248–253
Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, Rollins BJ, Ambati BK (2003) An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med 9:1390–1397. doi:10.1038/nm950
Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G, Hauser MA, Rickman CB, Bok D, Hageman GS, Johnson LV (2010) The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res 29:95–112. doi:10.1016/j.preteyeres.2009.11.003
Bora PS, Sohn JH, Cruz JM, Jha P, Nishihori H, Wang Y, Kaliappan S, Kaplan HJ, Bora NS (2005) Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J Immunol 174:491–497
Yu M, Zou W, Peachey NS, McIntyre TM, Liu J (2012) A novel role of complement in retinal degeneration. Invest Ophthalmol Vis Sci 53:7684–7692. doi:10.1167/iovs.12-10069
Zarbin MA, Rosenfeld PJ (2010) Pathway-based therapies for age-related macular degeneration: an integrated survey of emerging treatment alternatives. Retina 30:1350–1367. doi:10.1097/IAE.0b013e3181f57e30
Khan MA, Maasch C, Vater A, Klussmann S, Morser J, Leung LL, Atkinson C, Tomlinson S, Heeger PS, Nicolls MR (2013) Targeting complement component 5a promotes vascular integrity and limits airway remodeling. Proc Natl Acad Sci U S A 110:6061–6066. doi:10.1073/pnas.1217991110
Remtulla S, Hallett PE (1985) A schematic eye for the mouse, and comparisons with the rat. Vis Res 25:21–31
Yu DY, Cringle SJ (2006) Oxygen distribution in the mouse retina. Invest Ophthalmol Vis Sci 47:1109–1112. doi:10.1167/iovs.05-1118
Tobe T, Ortega S, Luna JD, Ozaki H, Okamoto N, Derevjanik NL, Vinores SA, Basilico C, Campochiaro PA (1998) Targeted disruption of the FGF2 gene does not prevent choroidal neovascularization in a murine model. Am J Pathol 153:1641–1646. doi:10.1016/S0002-9440(10)65753-7
Solovjov DA, Pluskota E, Plow EF (2005) Distinct roles for the alpha and beta subunits in the functions of integrin alphaMbeta2. J Biol Chem 280:1336–1345. doi:10.1074/jbc.M406968200
Zhang C, Lei B, Lam TT, Yang F, Sinha D, Tso MO (2004) Neuroprotection of photoreceptors by minocycline in light-induced retinal degeneration. Invest Ophthalmol Vis Sci 45:2753–2759. doi:10.1167/iovs.03-1344
Morimura T, Neuchrist C, Kitz K, Budka H, Scheiner O, Kraft D, Lassmann H (1990) Monocyte subpopulations in human gliomas: expression of Fc and complement receptors and correlation with tumor proliferation. Acta Neuropathol 80:287–294
Graeber MB, Streit WJ, Kreutzberg GW (1988) Axotomy of the rat facial nerve leads to increased CR3 complement receptor expression by activated microglial cells. J Neurosci Res 21:18–24. doi:10.1002/jnr.490210104
Arnaout MA (1990) Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood 75:1037–1050
Larson RS, Springer TA (1990) Structure and function of leukocyte integrins. Immunol Rev 114:181–217
Todd RF (1996) The continuing saga of complement receptor type 3 (CR3). J Clin Invest 98:1–2. doi:10.1172/JCI118752
Thurman JM, Kulik L, Orth H, Wong M, Renner B, Sargsyan SA, Mitchell LM, Hourcade DE, Hannan JP, Kovacs JM, Coughlin B, Woodell AS, Pickering MC, Rohrer B, Holers VM (2013) Detection of complement activation using monoclonal antibodies against C3d. J Clin Invest 123:2218–2230. doi:10.1172/JCI65861
Thurman JM, Rohrer B (2013) Noninvasive detection of complement activation through radiologic imaging. Adv Exp Med Biol 735:271–282
Framme C, Wolf S, Wolf-Schnurrbusch U (2010) Small dense particles in the retina observable by spectral-domain optical coherence tomography in age-related macular degeneration. Invest Ophthalmol Vis Sci 51:5965–5969. doi:10.1167/iovs.10-5779
Acknowledgments
Karin Oberlaender and Gabriele Fels are sincerely acknowledged for their excellent technical assistance. In equal measure, we want to thank Lucas Bethge and Tino Struck for synthesis and formulation of NOX-D20 and NOX-D20001, as well as Kai Hoehlig and Christian Maasch for verifying the drug activity in the chosen vehicle for ocular administration.
Conflicts of interest statement
Financial support was received from Noxxon Pharma AG. Axel Vater and Sven Klussmann are employees of Noxxon Pharma AG. All other authors certify that they have no affiliation with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. ESM 1
VEGF-A secretion by ARPE-19 cells: VEGF secretion was measured as VEGF concentration in the cell culture medium after 24 h incubation with C5a. The baseline secretion rate was doubled using 50 nM C5a stimulation over 24 h; *p < 0.05 (GIF 21 kb)
Fig. ESM 2
Effect of Spiegelmer NOX-D20 on VEGF secretion by ARPE-19 cells: VEGF secretion was measured as VEGF concentration in the culture medium after a 24 h-long stimulation of 50 nM C5a in the presence of different NOX-D20 concentrations; *p < 0.05. Please note that NOX-D20 binds C5a in a 1:1 fashion. Therefore, at 25 nM NOX-D20, 50 % of C5a can be neutralized even though its affinity is < 1 nM for human C5a (GIF 22 kb)
Rights and permissions
About this article
Cite this article
Brockmann, C., Brockmann, T., Dege, S. et al. Intravitreal inhibition of complement C5a reduces choroidal neovascularization in mice. Graefes Arch Clin Exp Ophthalmol 253, 1695–1704 (2015). https://doi.org/10.1007/s00417-015-3041-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-015-3041-z