Abstract
The contribution of microglia to ischemic cortical stroke is of particular therapeutic interest because of the impact on the survival of brain tissue in the ischemic penumbra, a region that is potentially salvable upon a brain infarct. Whether or not tissue in the penumbra survives critically depends on blood flow and vessel perfusion. To study the role of microglia in cortical stroke and blood vessel stability, CX3CR1+/GFP mice were subjected to transient middle cerebral artery occlusion and then microglia were investigated using time-lapse two-photon microscopy in vivo. Soon after reperfusion, microglia became activated in the stroke penumbra and started to expand cellular protrusions towards adjacent blood vessels. All microglia in the penumbra were found associated with blood vessels within 24 h post reperfusion and partially fully engulfed them. In the same time frame blood vessels became permissive for blood serum components. Migration assays in vitro showed that blood serum proteins leaking into the tissue provided molecular cues leading to the recruitment of microglia to blood vessels and to their activation. Subsequently, these perivascular microglia started to eat up endothelial cells by phagocytosis, which caused an activation of the local endothelium and contributed to the disintegration of blood vessels with an eventual break down of the blood brain barrier. Loss-of-microglia-function studies using CX3CR1GFP/GFP mice displayed a decrease in stroke size and a reduction in the extravasation of contrast agent into the brain penumbra as measured by MRI. Potentially, medication directed at inhibiting microglia activation within the first day after stroke could stabilize blood vessels in the penumbra, increase blood flow, and serve as a valuable treatment for patients suffering from ischemic stroke.








Similar content being viewed by others
References
Abdullah N, Voronovitch L, Taylor S, Lippmann S (2003) Olanzapine and quick-response hyperglycemia. Psychosomatics 44:175–176
Adams RA, Bauer J, Flick MJ et al (2007) The fibrin-derived gamma377-395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med 204:571–582
Arimura K, Ago T, Kamouchi M et al (2012) PDGF receptor beta signaling in pericytes following ischemic brain injury. Curr Neurovasc Res 9:1–9
Asahi M, Wang X, Mori T et al (2001) Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci 21:7724–7732
Astrup J, Symon L, Branston NM, Lassen NA (1977) Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke 8:51–57
Belayev L, Busto R, Zhao W, Ginsberg MD (1996) Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res 739:88–96
Carmeliet P, De Smet F, Loges S, Mazzone M (2009) Branching morphogenesis and antiangiogenesis candidates: tip cells lead the way. Nat Rev Clin Oncol 6:315–326
Cunningham CL, Martinez-Cerdeno V, Noctor SC (2013) Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci 33:4216–4233
Davalos D, Grutzendler J, Yang G et al (2005) ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8:752–758
Davalos D, Ryu JK, Merlini M et al (2012) Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun 3:1227
del Zoppo GJ, Milner R, Mabuchi T et al (2007) Microglial activation and matrix protease generation during focal cerebral ischemia. Stroke 38:646–651
Denes A, Ferenczi S, Halasz J, Kornyei Z, Kovacs KJ (2008) Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J Cereb Blood Flow Metab 28:1707–1721
Denes A, Ferenczi S, Halasz J, Kornyei Z, Kovacs KJ (2008) Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J Cereb Blood Flow Metab 28:1707–1721
Enzmann G, Mysiorek C, Gorina R et al (2013) The neurovascular unit as a selective barrier to polymorphonuclear granulocyte (PMN) infiltration into the brain after ischemic injury. Acta Neuropathol 125:395–412
Ferrara N, Gerber HP, LeCouter J (2003) The biology of VEGF and its receptors. Nat Med 9:669–676
Floris S, Blezer EL, Schreibelt G et al (2004) Blood-brain barrier permeability and monocyte infiltration in experimental allergic encephalomyelitis: a quantitative MRI study. Brain 127:616–627
Furlan M, Marchal G, Viader F, Derlon JM, Baron JC (1996) Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol 40:216–226
Gelderblom M, Leypoldt F, Steinbach K et al (2009) Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke 40:1849–1857
Girard S, Brough D, Lopez-Castejon G, Giles J, Rothwell NJ, Allan SM (2013) Microglia and macrophages differentially modulate cell death after brain injury caused by oxygen-glucose deprivation in organotypic brain slices. Glia 61:813–824
Heiss WD (2000) Ischemic penumbra: evidence from functional imaging in man. J Cereb Blood Flow Metab 20:1276–1293
Heiss WD (2012) The ischemic penumbra: how does tissue injury evolve? Ann N Y Acad Sci 1268:26–34
Iadecola C, Anrather J (2011) The immunology of stroke: from mechanisms to translation. Nat Med 17:796–808
Jin R, Yang G, Li G (2010) Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 87:779–789
Justicia C, Panes J, Sole S et al (2003) Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J Cereb Blood Flow Metab 23:1430–1440
Kalimo H, del Zoppo GJ, Paetau A, Lindsberg PJ (2013) Polymorphonuclear neutrophil infiltration into ischemic infarctions: myth or truth? Acta Neuropathol 125:313–316
Karperien A, Ahammer H, Jelinek HF (2013) Quantitating the subtleties of microglial morphology with fractal analysis. Front Cell Neurosci 7:3
Katsura K, Kristian T, Siesjo BK (1994) Energy metabolism, ion homeostasis, and cell damage in the brain. Biochem Soc Trans 22:991–996
Kitamura Y, Takata K, Inden M et al (2004) Intracerebroventricular injection of microglia protects against focal brain ischemia. J Pharmacol Sci 94:203–206
Kitamura Y, Yanagisawa D, Inden M et al (2005) Recovery of focal brain ischemia-induced behavioral dysfunction by intracerebroventricular injection of microglia. J Pharmacol Sci 97:289–293
Knowland D, Arac A, Sekiguchi KJ et al (2014) Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron 82:603–617
Kopatz J, Beutner C, Welle K et al (2013) Siglec-h on activated microglia for recognition and engulfment of glioma cells. Glia 61:1122–1133
Kuntz M, Mysiorek C, Petrault O et al (2014) Stroke-induced brain parenchymal injury drives blood-brain barrier early leakage kinetics: a combined in vivo/in vitro study. J Cereb Blood Flow Metab 34:95–107
Kuroiwa T, Ting P, Martinez H, Klatzo I (1985) The biphasic opening of the blood-brain barrier to proteins following temporary middle cerebral artery occlusion. Acta Neuropathol 68:122–129
Lee CZ, Xu B, Hashimoto T, McCulloch CE, Yang G-Y, Young WL (2004) Doxycycline suppresses cerebral matrix metalloproteinase-9 and angiogenesis induced by focal hyperstimulation of vascular endothelial growth factor in a mouse model. Stroke 35:1715–1719
Li T, Pang S, Yu Y, Wu X, Guo J, Zhang S (2013) Proliferation of parenchymal microglia is the main source of microgliosis after ischaemic stroke. Brain 136:3578–3588
Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD (2004) Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol 187:94–104
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Lloyd-Jones D, Adams R, Carnethon M et al (2009) Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 119:480–486
Lo EH (2008) A new penumbra: transitioning from injury into repair after stroke. Nat Med 14:497–500
Lo EH, Dalkara T, Moskowitz MA (2003) Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 4:399–415
Madinier A, Bertrand N, Mossiat C et al (2009) Microglial involvement in neuroplastic changes following focal brain ischemia in rats. PLoS One 4:e8101
Marti HJ, Bernaudin M, Bellail A et al (2000) Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol 156:965–976
Martin RL, Lloyd HG, Cowan AI (1994) The early events of oxygen and glucose deprivation: setting the scene for neuronal death? Trends Neurosci 17:251–257
Masuda T, Croom D, Hida H, Kirov SA (2011) Capillary blood flow around microglial somata determines dynamics of microglial processes in ischemic conditions. Glia 59:1744–1753
Morancho A, Hernández-Guillamon M, Boada C et al (2013) Cerebral ischaemia and matrix metalloproteinase-9 modulate the angiogenic function of early and late outgrowth endothelial progenitor cells. J Cell Mol Med 17:1543–1553
Moskowitz MA, Lo EH, Iadecola C (2010) The science of stroke: mechanisms in search of treatments. Neuron 67:181–198
Nag S, Kapadia A, Stewart DJ (2011) Review: molecular pathogenesis of blood-brain barrier breakdown in acute brain injury. Neuropathol Appl Neurobiol 37:3–23
Neumann H, Kotter MR, Franklin RJ (2009) Debris clearance by microglia: an essential link between degeneration and regeneration. Brain 132:288–295
Nikolic I, Plate KH, Schmidt MHH (2010) EGFL7 meets miRNA-126: an angiogenesis alliance. J Angiogenes Res 2:9
Nikolic I, Stankovic ND, Bicker F et al (2013) EGFL7 ligates alphavbeta3 integrin to enhance vessel formation. Blood 121:3041–3050
Nimmerjahn A, Kirchhoff F, Helmchen F (2005) Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308:1314–1318
Pillai DR, Shanbhag NC, Dittmar MS, Bogdahn U, Schlachetzki F (2013) Neurovascular protection by targeting early blood-brain barrier disruption with neurotrophic factors after ischemia-reperfusion in rats. J Cereb Blood Flow Metab 33:557–566
Plate KH, Beck H, Danner S, Allegrini PR, Wiessner C (1999) Cell type specific upregulation of vascular endothelial growth factor in an MCA-occlusion model of cerebral infarct. J Neuropathol Exp Neurol 58:654–666
Pugh CW, Ratcliffe PJ (2003) Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 9:677–684
Richardson DL, Pepper DS, Kay AB (1976) Chemotaxis for human monocytes by fibrinogen-derived peptides. Br J Haematol 32:507–513
Rosell A, Ortega-Aznar A, Alvarez-Sabín J et al (2006) Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke 37:1399–1406
Saleh A, Schroeter M, Ringelstein A et al (2007) Iron oxide particle-enhanced MRI suggests variability of brain inflammation at early stages after ischemic stroke. Stroke 38:2733–2737
Sandoval KE, Witt KA (2008) Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis 32:200–219
Schilling M, Besselmann M, Muller M, Strecker JK, Ringelstein EB, Kiefer R (2005) Predominant phagocytic activity of resident microglia over hematogenous macrophages following transient focal cerebral ischemia: an investigation using green fluorescent protein transgenic bone marrow chimeric mice. Exp Neurol 196:290–297
Schmidt MHH, Bicker F, Nikolic I et al (2009) Epidermal growth factor-like domain 7 (EGFL7) modulates Notch signalling and affects neural stem cell renewal. Nat Cell Biol 11:873–880
Sierra A, Abiega O, Shahraz A, Neumann H (2013) Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci 7:6
Sierra A, Encinas JM, Deudero JJ et al (2010) Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7:483–495
Stence N, Waite M, Dailey ME (2001) Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia 33:256–266
Strasser GA, Kaminker JS, Tessier-Lavigne M (2010) Microarray analysis of retinal endothelial tip cells identifies CXCR4 as a mediator of tip cell morphology and branching. Blood 115:5102–5110
Ueyama T, Lennartz MR, Noda Y et al (2004) Superoxide production at phagosomal cup/phagosome through beta I protein kinase C during Fc gamma R-mediated phagocytosis in microglia. J Immunol 173:4582–4589
Yang JP, Liu HJ, Liu XF (2010) VEGF promotes angiogenesis and functional recovery in stroke rats. J Invest Surg 23:149–155
Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG (2006) Microglia potentiate damage to blood-brain barrier constituents: improvement by minocycline in vivo and in vitro. Stroke 37:1087–1093
Zhang J, Defelice AF, Hanig JP, Colatsky T (2010) Biomarkers of endothelial cell activation serve as potential surrogate markers for drug-induced vascular injury. Toxicol Pathol 38:856–871
Zhang ZG, Zhang L, Jiang Q et al (2000) VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest 106:829–838
Zhao TZ, Xia YZ, Li L et al (2009) Bovine serum albumin promotes IL-1beta and TNF-alpha secretion by N9 microglial cells. Neurol Sci 30:379–383
Acknowledgments
We thank Heike Ehrengard, Christin Liefländer, Christine Oswald, and Andreas Zymny for their excellent technical assistance and Darragh O’Neill for proofreading the manuscript. This work was supported by the Foundation Rhineland-Palatinate to FZ, by the German Research Foundation (DFG) via the collaborative research center 1080, projects A3 (MHHS) and B6 (MKS+FZ), DFG FOR1336 (AW) as well as the DFG Grant SCHM 2159/2-1 to MHHS.
Conflict of interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
V. Jolivel and F. Bicker contributed equally as first but M. K. Schäfer, F. Zipp and M. H. H. Schmidt contributed equally as last authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
401_2014_1372_MOESM1_ESM.tif
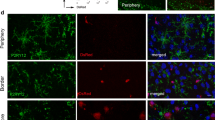
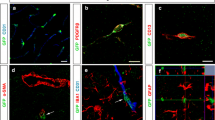
Suppl. Fig. 1 At 24 h post reperfusion brain sections of MCAO-treated mice were immunohistochemically stained for Iba-1 (microglia) and CD31 (ECs) in combination with either the smooth muscle cell marker SMA (a–d) or the pericyte marker NG2 (e–h) Iba-1-positive cells are neither smooth muscle cells nor pericytes. Furthermore, perivascular microglia did not phagocytize these cell types. Cell nuclei were visualized with DAPI. Bars represent 25 µm. i–l Double staining of Iba-1 (microglia) and a combination of the neuronal marker MAP2 and the neurofilament marker NF160 revealed phagocytosis of endothelial (yellow particles, arrowheads) but not neuronal structures (magenta, absent) by perivascular microglia. Cell nuclei were visualized with DAPI. Bars represent 10 µm (TIFF 7741 kb)
401_2014_1372_MOESM2_ESM.tif
Suppl. Fig. 2 At 24 h post-reperfusion brain sections of MCAO-treated mice were immunohistochemically stained for the vessel markers CD31 and a–c Glut-1, d–f caveolin-1, g–i claudin-5, and j–l podocalyxin. All markers overlap to a large extent with CD31 in the brain endothelium. Cell nuclei were visualized with DAPI. Bars represent 10 µm (TIFF 5047 kb)
401_2014_1372_MOESM3_ESM.tif
Suppl. Fig. 3 Immunohistochemical double staining of brain sections of MCAO-treated mice 24 h post reperfusion using the EC markers a–c Glut1, d–f caveolin1, g–i claudin-5, or j–l podocalyxin and Iba-1 (microglia) reveals phagocytosis of blood vessel components by perivascular microglia as indicated by arrowheads. Cell nuclei were visualized with DAPI. Bars represent 10 µm (TIFF 6970 kb)
401_2014_1372_MOESM4_ESM.tif
Suppl. Fig. 4 At 24 h post-reperfusion brain sections of MCAO-treated mice were immunohistochemically stained for EGFL7 and the EC activation markers a–c VEGF, d–f PDGF-B, g–i vWF, and j–l ICAM-1. Blood vessels in the ischemic penumbra stained positive for EGFL7 and either EC activation marker. Cell nuclei were visualized with DAPI. Bar represents 10 µm (TIFF 8902 kb)
401_2014_1372_MOESM5_ESM.avi
Suppl. Movie 1 At 4 h after reperfusion microglia extend cellular processes towards blood vessels in the ischemic penumbra. In this 38-min-long time-lapse movie, the dynamic extension and retraction of GFP-positive microglia processes (green) towards rhodamine–dextran-labeled blood vessels (red) is studied using intravital two-photon microscopy in the ischemic penumbra. At 4 h post reperfusion, activation of microglia was detectable by microglial processes that switched from random palpation to a targeted extension towards blood vessels (arrow). Each frame in this movie represents a z-projection of a 70-µm-thick stack acquired at 1-min intervals in vivo (AVI 1758 kb)
Suppl. Movie 2 At 8 h after reperfusion perivascular microglia assume a spherical shape in the ischemic penumbra. In this 4.5-h-long time-lapse movie starting 4 h post reperfusion, activation of microglia was measured by intravital two-photon microscopy. At 8 h post reperfusion, microglia cell bodies changed from an elongated to a spherical shape (arrows). GFP-positive microglia are indicated in green, rhodamine–dextran-labeled blood vessels in red. Each frame in this movie represents a z-projection of a 70-µm-thick stack acquired at 1-min intervals in vivo (AVI 13793 kb)
Suppl. Movie 3 At 8 h after reperfusion microglia migrate towards blood vessels. In this 55-min-long time-lapse movie starting 8 h post reperfusion, intravital two-photon microscopy revealed the migration of GFP-positive microglia (green) towards rhodamine–dextran-labeled blood vessels (red) 8 h post reperfusion (arrows). Each frame in this movie represents a z-projection of a 70-µm-thick stack acquired at 1-min intervals in vivo (AVI 2064 kb)
Suppl. Movie 4 At 8 h after reperfusion a microglia cell migrates towards a blood vessel. This movie shows a magnification of a cell derived from supplementary movie 3 migrating towards a blood vessel 8 h post reperfusion. Each frame in this movie represents a z-projection of a 70-µm-thick stack acquired at 1-min intervals in vivo (AVI 300 kb)
Suppl. Movie 5 At 24 h post reperfusion microglia phagocytize blood vessel components. This 70-min-long intravital two-photon microscopy time-lapse movie shows GFP-positive microglia (green) aligning with rhodamine–dextran-labeled blood vessels (red, small arrow). At 24 h post reperfusion these cells started to phagocytize rhodamine–dextran-positive blood vessel components (yellow, big arrow). Each frame in this movie represents a z-projection of a 70-µm-thick stack acquired at 1-min intervals in vivo (AVI 4281 kb)
Suppl. Movie 6 At 24 h post reperfusion microglia disintegrate blood vessels. This 88-min-long intravital two-photon microscopy time-lapse movie shows GFP-positive activated microglia (green) that are mainly associated with blood vessels 24 h post reperfusion. Two of the microglia cells actively migrate towards blood vessels (big arrows) and scan them with their processes. The disintegration of the blood vasculature has already started, as demonstrated by patchy extravasation of rhodamine–dextran in the lower part of the video (small arrows). Each frame in this movie represents a z-projection of a 70-µm-thick stack acquired at 1-min intervals in vivo (AVI 7722 kb)
Rights and permissions
About this article
Cite this article
Jolivel, V., Bicker, F., Binamé, F. et al. Perivascular microglia promote blood vessel disintegration in the ischemic penumbra. Acta Neuropathol 129, 279–295 (2015). https://doi.org/10.1007/s00401-014-1372-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-014-1372-1