Abstract
Background
Culture of retinal pigment epithelium (RPE) cells might be a future option in the therapy of various degenerative retinal diseases. However, the molecular changes which occur during in vitro expansion of RPE cells during culture are not fully elucidated. The aim of this study was to evaluate molecular changes in the RPE cell line ARPE-19 after stimulation with different growth factors.
Methods
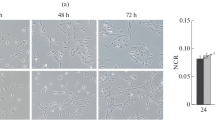
Cultured ARPE-19 cells were stimulated for 72 hours with rh-EGF, rh-IGF-1, rh-VEGF or rh-bFGF, and transcriptional changes of the differentiation markers cytokeratin 18 and RPE65 and of the key molecules of the wnt pathway, β-catenin, and glycogen synthase kinase-3 (GSK-3) were evaluated by real time RT-PCR.
Results
We found a significant decrease of cytokeratin 18 and RPE65 transcription after stimulation with rh-EGF (0.47 ± 0.42 and 0.32 ± 0.57-fold, respectively; p < 0.05). A significant reduction of β-catenin and GSK-3 mRNA was found in ARPE-19 cells stimulated with rh-IGF-1 (0.61 ± 0.25 and 0.52 ± 0.02-fold, respectively) or rh-EGF (0.55 ± 0.19 and 0.76 ± 0.26-fold, respectively). No changes of β-catenin mRNA were observed after stimulation with rh-VEGF or bFGF.
Conclusion
Our data suggest an inhibition of the β-catenin-pathway in ARPE-19 cells by IGF-1 and EGF, suggesting that ARPE-19 cell proliferation is, at least in part, driven by the β-catenin pathway. Furthermore, induction of proliferation by EGF results in a loss of differentiation markers in these cells. Maintaining the RPE phenotype is still one of the main problems for RPE- transplantation.




Similar content being viewed by others
References
Lamb TD, Pugh JEN (2004) Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res 23:307
Bok D (1993) The retinal pigment epithelium: aversatile partner in vision. J Cell Sci Suppl 17:189–195
Binder S, Stolba U, Krebs I et al (2002) Transplantation of autologous retinal pigment epithelium in eyes with foveal neovascularisation resulting from age-related macular degeneration: a pilot study. Am J Ophthalmol 133:215–225
Gouras P, Algvere P (1996) Retinal cell transplantation in the macula: new techniques. Vision Res 36:4121–4125
Binder S, Krebs I, Hilgers RD, Abri A, Stolba U, Assadoulina A, Kellner L, Stanzel BV, Jahn C, Feichtinger H (2004) Outcome of transplantation of autologous retinal pigment epithelium in age-related macular degeneration: a prospective trial. Invest Ophthalmol Vis Sci 45:4151–4160
Kim KS, Tezel TH, Del Priore LV (1998) Minimum number of adult human retinal pigment epithelial cells required to establish a confluent monolayer in vitro. Curr Eye Res 17:962–969
Ishida M, Lui GM, Yamani A, Sugino IK, Zarbin MA (1998) Culture of human retinal pigment epithelial cells from peripheral scleral flap biopsies. Curr Eye Res 17:392–402
Zhu M, Provis JM, Penfold PL (1998) Isolation, culture and characteristics of human foetal and adult retinal pigment epithelium. Aust N Z J Ophthalmol 26(Suppl 1):S50–S52
Hecquet C, Lefevre G, Valtink M, Engelmann K, Mascarelli F (2002) Activation and role of MAP kinase-dependent pathways in retinal pigment epithelial cells: ERK and RPE cell proliferation. Invest Ophthalmol Vis Sci 43:3091–3098
Defoe DM, Grindstaff RD (2004) Epithermal growth factor stimulation of RPE cell survival: contribution of phosphatidylinositol 3-linase and mitogen- activated protein kinase pathways. Exp Eye Res 79:51–59
Liou GI, Matragoon S, Samuel S, Behzadian MA, Tsai N, Gu X, Roon P, Hunt DM, Hunt RC, Caldwell RB, Marcus DM (2002) MAP kinase and β-catenin signalling in HGF induced RPE cell proliferation. Mol Vis 8:483–493
Jin M, Barron E, He S, Ryan S, Hinton DR (2002) Regulation of RPE intercellular junction integrity and function by hepatocyte growth factor. Invest Ophthalmol Vis Sci 43:2782–2790
Dale TC (1998) Signal transduction by the wnt family ligands. Biochem J 329:209–223
Doble BW, Woodgett JR (2003) GSK-3: tricks of the trade for a multitasking kinase. J Cell Sci 116:1175–1186
Cross DAE, Alessi DR, Vandenheede JR, McDowell H, Hundal HS, Cohen P (1994) The inhibition of glycogen synthase kinase-3 by insulin-like growth factor 1 in the rat skeletal muscle cell line L6 is blocked by wortmannin but not by rapamycin: evidence that wortmannin blocks activation of the mitogen-activated protein kinase pathway in L6 cells between Ras and Raf. Biochem J 303:21–26
Eldar-Finkelman H, Seger R, Vandenheede JR, Krebs EG (1995) Inactivation of glycogen synthase kinase-3 by epidermal growth factor is mediated by mitogen-activated protein kinase/p90 ribosomal protein S6 kinase signaling pathway in NIH/3T3 cells. J Biol Chem 270:987–990
Bachelder RE, Yoon S, Franci C, Garcia de Herreros A, Mercurio AM (2005) Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. J Cell Biol 168:29–33
Mariner DJ, Davis MA, Reynolds AB (2003) EGFR signaling to p120-catenin through phosphorylation at Y228. J Cell Sci 117:1339–1350
Hoschuetzky H, Aberle H, Kemler R (1994) β-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol 127:1375–1380
PE Applied Biosystems (2001) ABI prism 7700 sequence detection system: relative quantitation of gene expression. Applied Biosystems, Bulletin 2, pp 1–36
Sharma RK, Orr WE, Schmitt AD, Johnson DA (2005) A functional profile of gene expression in ARPE-19 cells. BMC Ophthalmology 5:25
Kanuga N, Winton HL, Beauchene L, Koman A, Zerbib A, Halford S, Couraud P, Keegan D, Coffey P, Lund RD, Adamson P, Greenwood J (2002) Characterization of genetically modified human retinal pigment epithelial cells developed for in vitro and transplantation studies. Invest Ophthalmol Vis Sci 43:546–555
Wang Q, Zhou Y, Wang X, Evers BM (2006) Glycogen synthase kinase-3 is a negative regulator of extracellular signal-regulated kinase. Oncogene 25:43–50
Reya T, Clevers H (2005) Wnt signaling in stem cells and cancer. Nature 434:843–850
Casaroli-Marano RP, Pagan R, Vilaro S (1999) Epithelial-mesenchymal transition in proliferative vitreoretinopathy: intermediate filament protein expression in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 40:2062–2072
Thiery JP (2002) Epithelial-mesenchymal transitions in tumor progression. Nat Rev Cancer 2:442–454
Saika S, Kono-Saika S, Tanaka T, Yamanaka O, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Yoo J, Flanders KC, Roberts AB (2004) Smad3 is required for dedifferentiation of retinal pigment epithelium following retinal detachment in mice. Lab Invest 84:1245–1258
Shi Y, Massague J (2003) Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113:685–700
Ten Dijke P, Goumans MJ, Itoh F, Itoh S (2002) Regulation of cell proliferation by Smad proteins. J Cell Physiol 191:1–16
Fujimoto K, Sheng H, Shao J, Beauchamp RD (2001) Transforming growth factor-beta1 promotes invasiveness after cellular transformation with activated Ras in intestinal epithelial cells. Exp Cell Res 266:239–249
Grände M, Franzen A, Karlsson JO, Ericson LE, Heldin NE, Nilsson M (2002) Transforming growth factor-β and epidermal growth factor synergistically stimulate epithelial to mesenchymal transition (EMT) through a MEK-dependent mechanism in primary cultured pig thymocytes. J Cell Sci 115:4227–4236
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krugluger, W., Seidel, S., Steindl, K. et al. Epidermal growth factor inhibits glycogen synthase kinase-3 (GSK-3) and β-catenin transcription in cultured ARPE-19 cells. Graefes Arch Clin Exp Ophthalmol 245, 1543–1548 (2007). https://doi.org/10.1007/s00417-007-0635-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-007-0635-0