Abstract
Background and objective
Transcranial brain parenchyma sonography (TCS) has been recommended as a tool for the early and differential diagnosis of Parkinson’s disease (PD) in German and European clinical guidelines. Still, the brain structures to be examined for the diagnostic questions and the requirements for being a qualified investigator were not specified in detail. These issues have now been addressed in the 2023 update of the clinical guideline on PD by the German Society of Neurology (DGN).
Methods
The recommendations were based on a systematic literature review following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Results
Three diagnostic questions were defined: (1) What is the accuracy of TCS in the differential diagnosis of PD versus atypical and secondary Parkinsonian syndromes? (2) What is the accuracy of TCS in the differential diagnosis of PD versus essential tremor? (3) What is the accuracy of TCS in the diagnosis of PD in persons with typical early symptoms, compared with the diagnosis established by clinical follow-up? The brain structures to be assessed and the level of recommendation were formulated for these questions. The training requirements for being regarded as qualified TCS investigator were stipulated by the responsible medical societies (German Society of Ultrasound in Medicine, DEGUM; German Society for Clinical Neurophysiology and Functional Imaging, DGKN). Finally, the recommendations for these diagnostic questions reached strong consensus (each ≥ 97%) of the guideline committee. Here, the details of review and recommendations are presented.
Conclusion
The updated guideline clarifies the diagnostic uses and limitations of TCS in PD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the first description of the characteristic transcranial sonography (TCS) finding of enlarged echogenic appearance (“hyperechogenicity”) of the substantia nigra in Parkinson’s disease (PD) [1], numerous studies have underpinned the diagnostic value of TCS in PD [2,3,4,5,6,7,8,9,10,11,12,13,14]. Specific advantages of TCS compared to other brain imaging methods are its non-invasiveness and low interference with patients head movements. Applying high-end ultrasound systems with standardized settings [9,10,11,12,13,14], high image resolution of echogenic deep brain structures of up to 0.7 × 1.1 mm is achieved [15]. For planimetric measurement of substantia nigra echogenicity by an experienced investigator high intra-rater (ICC 0.97 and 0.93, respectively, for both hemispheres) and inter-rater reliability (ICC 0.84 and 0.89) have been demonstrated [16]. For the diagnostic work-up of Parkinsonian syndromes, two standardized transtemporal axial scanning planes are used: the mesencephalic plane in which the substantia nigra is assessed, and the third ventricular/thalamic plane in which the ventricular system and the basal ganglia are assessed (Fig. 1). The diagnostic evaluation of these structures by TCS has been described earlier in detail [9,10,11,12,13,14]. Meanwhile, TCS of substantia nigra, basal ganglia and ventricles is well established as a supportive diagnostic tool in PD. TCS by an experienced investigator has been included in the European clinical guidelines as an optionally recommended method for:
-
(i)
the differential diagnosis of PD from atypical and secondary Parkinsonian syndromes,
-
(ii)
the early diagnosis of PD in clinically unclear cases with Parkinsonian motor signs, and
-
(iii)
the detection of subjects at risk for PD,
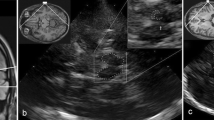
Transcranial sonography (TCS) in the diagnosis of Parkinson’s disease (PD). A MRI of midbrain axial transection corresponding to the TCS images shown in (B) and (C). B Axial TCS scan at midbrain level in an individual with normal aspect of substantia nigra (small echogenic area, SN–). This finding is typical for essential tremor and multiple system atrophy. C Axial TCS scan at midbrain level in an individual with enlarged echogenic size of substantia nigra (hyperechogenicity, SN +). This finding is typical for PD. D Position of the ultrasound transducer for diagnostic TCS in PD. E Zoomed TCS image of midbrain shown in (B). The echoic area of substantia nigra is traced for automated measurement. F Zoomed TCS image of midbrain shown in (C). The echoic area of substantia nigra is traced for automated measurement. G MRI of basal-ganglia axial transection corresponding to the TCS images shown in (H) and (I). C caudate nucleus, L lenticular nucleus, T thalamus, arrow head: pineal gland. H Axial TCS scan at basal-ganglia level in an individual with normal aspect of lenticular nucleus (weakly echogenic, LN−; arrow). This finding is typical for PD. I Axial TCS scan at basal-ganglia level in an individual with increased echogenicity of lenticular nucleus (LN + ; arrow). This finding is frequent in atypical Parkinsonian syndromes
including asymptomatic mutation carriers for monogenic forms of PD, with preferably combining TCS with other screening procedures for (iii) [17]. In the clinical guideline of the German Society of Neurology (DGN), TCS was listed already in 2008 as a facultative diagnostic method for supporting the diagnosis of PD at early motor disease stages [18]. The subsequent German guideline 2012 listed TCS as a facultative diagnostic method for (i) the discrimination of PD from atypical Parkinsonian syndromes and (ii) supporting the diagnosis of PD at early motor stages [19]. In the updated German guideline 2016, TCS was recommended as an optional method for the premotor, early and differential diagnosis of PD, based on systematic review of studies published until 2010 [20]. The guideline required an experienced TCS investigator, and proposed a first criterion for this, i.e. an investigator having performed TCS on 100 individuals with or without PD [20]. Still, these guidelines did not exactly define the diagnostic questions and respective brain structures to be assessed on TCS, nor the requirements in education and training for being regarded as qualified TCS investigator [17,18,19,20]. These needs have now been addressed with the recently issued 2023 update of the German clinical guideline (https://register.awmf.org/de/leitlinien/detail/030-010) [21]. In the present article, the specified diagnostic questions for TCS, the results of systematic literature reviews and the new guideline recommendations are reported.
Methods
Definition of the diagnostic questions
The guideline coordinating committee initially set one diagnostic question for TCS:
-
(1)
What is the accuracy of TCS in the diagnosis of PD compared with the diagnosis established by long-term clinical follow-up?
The drafting authors of the chapter “Brain parenchyma sonography” (UW, KL) were requested in December 2020 to confirm or revise the diagnostic question. Considering the previous guidelines [17,18,19,20], and the scientific evidence available in early 2021, the following more detailed diagnostic questions were proposed by the chapter authors and confirmed by the coordinating committee in May 2021:
-
(1)
What is the accuracy of TCS in the differential diagnosis of PD versus atypical and secondary Parkinsonian syndromes?
-
(2)
What is the accuracy of TCS in the differential diagnosis of PD versus essential tremor?
-
(3)
What is the accuracy of TCS in the diagnosis of PD in persons with typical early symptoms*, compared with the diagnosis established by clinical follow-up? (*early motor signs of PD, hyposmia, depression, REM sleep behavior disorder)
The key questions were converted into the “PICOS” format for further search (see below). For each of these diagnostic questions, a systematic review was conducted in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for diagnostic test accuracy [22, 23]. These systematic reviews were not registered.
Data sources and search strategy
Systematic literature searches were performed through PubMed to identify studies eligible for inclusion. All records published until 31.12.2021 were included in the initial search. Search terms are outlined in Table S1 in the supplementary materials (Online Resource 1). No restrictions on language, or study type were specified on the search protocol. The following PICOS criteria were used as a framework to formulate the literature search strategies to ensure comprehensive searches:
-
P (Population): adults (>18y) with (suspected) PD and/or (if applicable) atypical/secondary Parkinsonian disorders and/or (if applicable) essential tremor.
-
I (Intervention): transcranial B-mode sonography (TCS) of brain parenchyma.
-
C (Comparison): clinical diagnosis of PD established by movement disorder specialists based on international consensus criteria (established/confirmed at follow-up visits).
-
O (Outcomes): detection/discrimination of PD on TCS.
-
S (Studies): original articles (including observational studies, randomized control trials), systematic reviews, meta-analyses, and case series.
-
The title and abstract of the detected records were screened for relevance and full text articles were retrieved for those that passed the inclusion criteria. For articles from the same group containing a search period overlap and similar data sets, only the most recent article was included to avoid duplication of data. Narrative reviews, editorials, short communications, case studies, and articles for which full text was not retrievable were excluded. The studies identified on the initial search were entered in an Excel sheet (Microsoft, Redmond, Washington, USA).
Study analysis and guideline establishment
The studies listed in the Excel sheet were then further assessed for their relevance and reported data by the chapter authors, who in the first step independently evaluated the reports. A backward citation and a forward citation (dating until 30th July 2023) were used when appropriate to include pertinent articles. Subsequently, consensus was achieved by the chapter authors on which of the identified studies were regarded as relevant for the systematic review. The variables for which data were sought were: the number of participants per diagnostic group undergoing TCS, participant characteristics (age, gender, disease duration), the ultrasound system applied for TCS, the qualification of investigator, degree of blinding of investigators to diagnosis, the definition (cut-off criterion on planimetry) of substantia nigra hyperechogenicity, the diagnostic gold standard, the duration of follow-up, and the number of cases who met the diagnostic endpoint(s). The data analysis of each finally approved study included the assessment of sensitivity, specificity, positive and negative predictive values (PPV, NPV), as well as positive and negative likelihood ratios (LR + , LR – ) with respect to the referring diagnostic question. Strengths and weaknesses of each study were commented. These data and comments were listed in a table, separately for each diagnostic question. Based on this, a concise report on the levels of evidence of the relevant studies was compiled, and the resulting recommendations were formulated. The chapter tables and reports were then included in the draft of the complete guideline and send out for reading to all guideline chapter lead authors (n = 34) and delegates of involved medical societies (n = 20), who formed the consensus voting committee. In 3 web-based consensus meetings all guideline chapters had to be presented and defeated by the referring lead authors, and the strength level of each recommendation and, if applicable, the required further editions of the referring chapter were consented by committee. Considering all requested revisions, a final voting was performed in the committee web-conference, and the degree (percentage) of consensus was documented.
Results
Identification of relevant studies
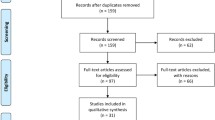
For the diagnostic question 1, the PubMed search identified 11 records, out of which three studies were included after screening, and another 11 studies were included from citation search. Details are given in Figure S1A in the supplementary materials (Online Resource 2). For the diagnostic question 2, the PubMed search identified five records, out of which none were included after screening, however, another five studies were included from citation search (Figure S1B, Online Resource 2). For the diagnostic question 3, the PubMed search identified 30 records, out of which two studies were included after screening, and another three from citation search (Figure S1C, Online Resource 2).
Guideline report and recommendations
-
(1)
What is the accuracy of TCS in the differential diagnosis of PD versus atypical and secondary Parkinsonian syndromes?
Background: Particularly in the early motor disease stages the clinical discrimination of PD from atypical and secondary Parkinsonian syndromes may be difficult. Therefore, there is a strong need of additional diagnostic tools to increase diagnostic certainty.
Evidence basis: In this literature search, one large longitudinal cohort study, one systematic review with meta-analysis, nine cross-sectional studies, two small longitudinal cohort studies and two reviews, but no studies with histologically proven diagnosis were identified.
Result: TCS supports the diagnostic discrimination of PD versus atypical and secondary Parkinsonian syndromes in the first years of (motor) disease since the TCS findings are specific already in this disease stage [10]. The reliability of TCS in this application is dependent on the qualification of the investigator [16, 24]. Therefore, TCS is to be performed by a qualified investigator. If TCS is performed only of substantia nigra, PD (typically hyperechoic substantia nigra, SN +) can be discriminated best from multiple system atrophy (MSA; typically normal substantia nigra, SN-) [3, 25]. If also other atypical Parkinson syndromes are considered (progressive supranuclear palsy, PSP, corticobasal degeneration, CBD), TCS only of substantia nigra is less specific since in PSP and CBD SN + is more frequent [25, 26]. A meta-analysis of 71 studies on more than 5,000 patients yielded only a sensitivity of 75% and a specificity of 70% of substantia nigra TCS in the discrimination of PD from atypical Parkinsonian syndromes [27]. However, several longitudinal and cross-sectional studies have demonstrated that the additional TCS assessment of lenticular nucleus and third-ventricle width increases the diagnostic accuracy (Table 1) [10, 28,29,30,31,32,33]. The combined finding of hyperechoic lenticular nucleus (LN +) with at least one of two other (SN- or third-ventricle width > 10 mm) discriminates multiple system atrophy and progressive supranuclear palsy best from PD [29]. For the discrimination of PD and dementia with Lewy bodies TCS of substantia nigra is helpful, TCS of basal ganglia or ventricles does not add diagnostic value [32, 34]. The characteristic TCS finding in dementia with Lewy bodies is a bilateral-symmetric SN + (asymmetry index < 1.15, individual ratio of the larger echogenic size divided by the lesser echogenic size); the diagnostic specificity, however, is limited. In patients with vascular Parkinsonism typically bilateral SN- is found [4, 35, 36]. It has been discussed that patients with suspected vascular Parkinsonism who, however, exhibit SN + on TCS might have PD [36]; this remains to be studied systematically. In general, TCS does not completely discriminate PD from other Parkinsonian syndromes, therefore the individual clinical course and other diagnostic findings need to be considered.
Table 1 Studies on TCS for the differential diagnosis of PD versus atypical and secondary Parkinsonian syndromes Justification of recommendation: The body of evidence is largest for the use of substantia nigra TCS in the discrimination of PD versus atypical Parkinsonian syndromes; sensitivity and specificity (75%; 70%) were rated as being suboptimal in a high-quality meta-analysis [27]. The evidence on combined TCS of substantia nigra, lenticular nucleus and (optionally) third ventricle is based on a larger longitudinal study and seven cross-sectional, case–control, or smaller longitudinal studies with diverse quality of study design as well as two reviews with analysis of pooled study data. The evidence on TCS in dementia with Lewy bodies and vascular Parkinsonism is limited (altogether five studies on small cohorts). In a recent position paper of the responsible medical societies (German Society of Ultrasound in Medicine, DEGUM; German Society for Clinical Neurophysiology and Functional Imaging, DGKN) it is outlined how the status of qualified investigator for TCS in the diagnosis of PD can be achieved within the curricular educational concept of the DEGUM/DGKN (Table 2) [37].
Table 2 Minimum educational requirements for an investigator being regarded as ‘qualified’ for TCS in the early and differential diagnosis of PD [37]
Recommendation (new in German guideline, 2023):
TCS performed by a qualified investigator can be considered for supporting the differential diagnosis of PD versus atypical and secondary Parkinsonian syndromes. | |
TCS for the discrimination of PD from atypical Parkinsonian syndromes shall include assessment of substantia nigra, lenticular nucleus and third ventricle. | |
Level of consensus: 97.4%, strong consensus. |
-
(2)
What is the accuracy of TCS in the differential diagnosis of PD versus essential tremor?
Background: The discrimination between essential tremor and early tremor-predominant PD by clinical investigation only is sometimes impossible. Therefore, there is a strong need of additional diagnostic tools to increase diagnostic certainty.
Evidence basis: In this literature search one high-quality systematic review with meta-analysis of studies on TCS only, and three cross-sectional studies on the combination of TCS and olfactory testing, but no studies with histologically proven diagnosis were identified.
Result: A systematic review with meta-analysis involved 18 appropriate TCS studies on 1264 patients with PD and 824 patients with essential tremor [38]. The meta-analysis found a sensitivity of 85% (95% confidence interval, 79.4–88.6%) and a specificity of 84% (78.4–88.2%) of TCS in the differentiation of PD versus essential tremor. A subgroup analysis of three out of the 18 studies showed in addition that diagnostic sensitivity and specificity are similar to that of dopamine transporter scintigraphy (DaTSCAN) [38]. For this, TCS of substantia nigra is sufficient, TCS of basal ganglia or ventricles does not add diagnostic value [39]. Diagnostic specificity is increased by combining TCS and screening for hyposmia (e.g., with Sniffin’ Sticks; Table 3) [36, 40, 41]. Patients with essential tremor and the TCS finding of SN + maybe at an increased risk of later developing PD [42]. TCS of SN is to be performed by a qualified investigator [16, 24].
Table 3 Studies on TCS for the differential diagnosis of PD versus essential tremor Justification of recommendation: The evidence on TCS of substantia nigra is based on a high-quality systematic review with meta-analysis of 18 appropriate studies. The diagnostic reliability of TCS (sensitivity, 85%, specificity, 84%) is comparable to that of dopamine transporter scintigraphy (DaTSCAN). The evidence for combining TCS and olfactory screening is based on three smaller cross-sectional and cohort studies. In a recent position paper it is outlined how the status of qualified investigator for TCS in the diagnosis of PD can be achieved within the curricular educational concept of the DEGUM/DGKN (Table 2) [37].
Recommendation (new in German guideline, 2023):
TCS performed by a qualified investigator can be considered for supporting the differential diagnosis of PD versus essential tremor. | |
TCS for the discrimination of PD from essential tremor can be combined with a screening test for hyposmia to increase diagnostic certainty. | |
Level of consensus: 97.1%, strong consensus. |
-
(3)
What is the accuracy of TCS in the diagnosis of PD in persons with typical early symptoms*, compared with the diagnosis established by clinical follow-up? (*early motor signs of PD, hyposmia, depression, REM sleep behavior disorder).
Background: The diagnosis of incident PD may allow for the early initiation of upcoming neuroprotective/neuro-restorative therapies. So far, there is no possibility of diagnosing incident PD by a single test. Therefore, there is a need of additional diagnostic tools to increase diagnostic certainty, especially in individuals with early symptoms of PD.
Evidence basis: In this literature search one longitudinal study on a large German cohort of persons at risk of developing PD, a pooled analysis of five German longitudinal studies on risk cohorts, and three small longitudinal studies of risk cohorts, but no studies with histologically proven diagnosis were identified.
Result: TCS shows, depending on the applied cut-off value, moderate to marked SN + at least unilaterally in 9–22% (on average, 13%) of adult healthy population, which is detected in 75–90% (on average, 83%) of patients with PD [43]. TCS of SN requires a qualified investigator [16, 24]. The finding of SN + in healthy subjects aged > 50 years indicates a 20-fold increased risk of subsequently developing PD, however the positive predictive value is low (6%) [44]. Longitudinal studies on populations with epidemiologically increased risk of subsequent PD (mild motor signs of PD, hyposmia, depression, idiopathic REM sleep behavior disorder) were found to have higher positive predictive values of SN + for indicating incident PD (Table 4) [45,46,47,48,49]. A pooled analysis of five German follow-up studies on risk cohorts suggests that SN + may be of higher diagnostic value in women (compared to men) and individuals at age < 65 years [48]. In cohorts with REM sleep behavior disorder proven on polysomnography, rather high positive predictive values of about 50% for incident PD or dementia with Lewy bodies have been reported [46, 49]. The predictive value for incident PD in risk cohorts can be increased by combining TCS of substantia nigra with a screening test for hyposmia (e.g. Sniffin’ Sticks) [45, 49]. Currently the combined assessment of a bunch of risk markers (including e.g. substantia nigra TCS) and prodromal markers is recommended to enhance the diagnostic certainty in an individual [50, 51]. It is to be expected that a more precise prediction of incident PD will be possible in the future through the additional inclusion of novel laboratory and genetic markers [52].
Table 4 Studies on TCS in comparison with clinical follow-up for the diagnosis of PD in persons with typical early symptoms of PD Justification of recommendation: The evidence on TCS in comparison with clinical follow-up is based on a study with pooled data analysis of five German follow-up studies in three large and two small risk cohorts, and another two small longitudinal studies on risk cohorts with an approximately 10-year follow-up. In a recent position paper it is outlined how the status of qualified investigator for TCS in the diagnosis of PD can be achieved within the curricular educational concept of the DEGUM/DGKN (Table 2) [37].
Recommendation (new in German guideline, 2023):
TCS performed by a qualified investigator can indicate an increased individual risk of subsequent PD, however, the predictive value of stand-alone TCS is low. | |
In individuals aged > 50 years with REM sleep behavior disorder proven on polysomnography, the combined findings of substantia nigra hyperechogenicity (SN +) on TCS and verified hyposmia should be regarded as indication of presence of PD. | |
Level of consensus: 97.0%, strong consensus. |
Discussion
The present updated German guideline on PD includes a newly formulated chapter on the clinical diagnostic uses of TCS (brain parenchyma sonography) in PD [21]. The analysis of scientific evidence on the three diagnostic questions was based on the results of PubMed search of records until end of 2021 and forward citation search until 30th July 2023. A repeat of the PubMed searches on 7th January 2024 yielded the same records as in the search performed during the guideline development process which makes it unlikely that a relevant recent study was missed. Compared with the previously issued versions of the German and European guidelines [17,18,19,20], the discrimination between PD and essential tremor on TCS has been addressed here for the first time in a separate section. Another new element of the present guideline is the definition of the educational requirements for being regarded as ‘qualified TCS investigator’ in PD, established by the responsible German medical societies (DEGUM, neurology section; DGKN) [37]. This definition comprises separate qualification criteria for TCS of substantia nigra and TCS of basal ganglia since the latter requires longer training. It can be expected that the present guideline recommendations will promote the diagnostic use of TCS in clinical practice.
Still, the recommendations for the diagnostic use of TCS in PD are formulated with a rather moderate degree of strength which deserves comment. There are several reasons for this. First, there are no studies so far comparing the diagnoses obtained by TCS with post-mortem histopathological investigation which, however, remains the diagnostic gold standard in Parkinsonian syndromes. For other neuroimaging methods (MRI, radionuclide scan) used in this context there are study data with post-mortem verification available [53, 54]. Even though early TCS findings have been validated by investigating asymptomatic and symptomatic gene mutation carriers in families with mono-genetically caused PD [55,56,57,58], and agreement of MRI and TCS localization of SN has been shown [59], there is a need of studies comparing TCS with post-mortem findings in PD and atypical Parkinsonian syndromes.
Second, in Germany and most European countries TCS is usually performed by neurologists, but rarely by radiologists. While transcranial color-coded duplex sonography (TCCS) of intracranial arteries is an obligatory part of residency training in neurology in Germany and many other European countries [60], TCS in movement disorders is not obligatory. Even though special courses are regularly offered by the DEGUM/DGKN and the European Society of Neurosonology and Cerebral Hemodynamics (ESNCH; https://esnch.org/), the number of physicians who fulfil the qualification criteria (Table 2) is rather limited. The now clearly defined qualification criteria for TCS in PD, along with the accompanying recommendation how the special training can be realized in the curricular concept of DEGUM/DGKN [37], may enhance the offer of such opportunities. On the other hand, there are specific advantages of TCS (compared to MRI or molecular imaging) that deserve to be made more known: TCS can be performed by the movement disorder specialists themselves, and is applicable at any location using portable ultrasound systems (e.g. in neurological practices). Moreover, the diagnostic precision of TCS is nowadays enhanced by technologies such as real-time MRI-ultrasound fusion imaging and integral digitized image analysis [61,62,63,64,65]. New attractive TCS applications in PD, such as time-saving postoperative control of deep brain electrode position, especially in patients with subthalamic nucleus stimulation [64, 65], may further increase interest in learning this application.
Third, characteristic findings in PD, including alteration of substantia nigra, can nowadays be visualized and quantified on MRI [53, 59, 66]. Compared with MRI, the resource of qualified TCS is less widely available, and TCS image quality may be affected by temporal skull bone thickness and experience of the investigator. Nevertheless, current evidence supports the view that elaborate MRI imaging and TCS disclose different aspects of substantia nigra pathology [67]. Since the typical TCS findings in PD (SN +) and atypical Parkinsonian disorders (LN +) are present in the early disease stages [29, 44,45,46,47,48,49, 55,56,57,58], TCS can well be employed for diagnostic screening. It should be stressed that for the planimetric measurement of substantia nigra echogenicity, which is the current standard of grading its echogenicity on TCS, high intra- and inter-rater reliability has been demonstrated with experienced investigators [16]. The potential increase of diagnostic validity of substantia nigra and basal-ganglia TCS by digitized image analysis [13, 61,62,63], especially if TCS is performed by less experienced investigators, remains to be proven in prospective studies. Ongoing advances in TCS technology could even reduce the impact of the main obstacle to TCS in the coming years, namely the inter-individually variable skull bone thickness [68, 69].
In conclusion, the updated guideline underpins the use of TCS in PD. The strength of recommendation of TCS in PD may potentially increase in future guideline issues. For this, TCS studies in Parkinsonian patients with subsequently autopsy-proven diagnosis as well as the adherence of potential investigators to standardized educational curricula are desired.
Data sharing and data accessibility
Systematic literature search data will be shared by request from any qualified investigator. Data sharing requests are made in writing through Dr. Walter (uwe.walter@med.uni-rostock.de) and require a formal data sharing agreement with approval from the Rostock University Medical Center and the guideline office of the German Society of Neurology (DGN). Data sharing agreements must include details on how the data will be stored, who will have access to the data and intended use of the data, and agreements as to the allocation of intellectual property.
References
Becker G, Seufert J, Bogdahn U, Reichmann H, Reiners K (1995) Degeneration of substantia nigra in chronic Parkinson’s disease visualized by transcranial color-coded real-time sonography. Neurology 45:182–184. https://doi.org/10.1212/wnl.45.1.182
Berg D, Siefker C, Becker G (2001) Echogenicity of the substantia nigra in Parkinson’s disease and its relation to clinical findings. J Neurol 248:684–689. https://doi.org/10.1007/s004150170114
Walter U, Niehaus L, Probst T, Benecke R, Meyer BU, Dressler D (2003) Brain parenchyma sonography discriminates Parkinson’s disease and atypical parkinsonian syndromes. Neurology 60:74–77. https://doi.org/10.1212/wnl.60.1.74
Tsai CF, Wu RM, Huang YW, Chen LL, Yip PK, Jeng JS (2007) Transcranial color-coded sonography helps differentiation between idiopathic Parkinson’s disease and vascular parkinsonism. J Neurol 254:501–507. https://doi.org/10.1007/s00415-006-0403-9
Stockner H, Sojer M, Seppi K et al (2007) Midbrain sonography in patients with essential tremor. Mov Disord 22:414–417. https://doi.org/10.1002/mds.21344
Mijajlović M, Dragasević N, Stefanova E, Petrović I, Svetel M, Kostić VS (2008) Transcranial sonography in spinocerebellar ataxia type 2. J Neurol 255:1164–1167. https://doi.org/10.1007/s00415-008-0862-2
Doepp F, Plotkin M, Siegel L et al (2008) Brain parenchyma sonography and 123I-FP-CIT SPECT in Parkinson’s disease and essential tremor. Mov Disord 23:405–410. https://doi.org/10.1002/mds.21861
Budisic M, Trkanjec Z, Bosnjak J, Lovrencic-Huzjan A, Vukovic V, Demarin V (2009) Distinguishing Parkinson’s disease and essential tremor with transcranial sonography. Acta Neurol Scand 119:17–21. https://doi.org/10.1111/j.1600-0404.2008.01056.x
Walter U, Behnke S, Eyding J, Niehaus L, Postert T, Seidel G, Berg D (2007) Transcranial brain parenchyma sonography in movement disorders: state of the art. Ultrasound Med Biol 33:15–25. https://doi.org/10.1016/j.ultrasmedbio.2006.07.021
Berg D, Godau J, Walter U (2008) Transcranial sonography in movement disorders. Lancet Neurol 7:1044–1055. https://doi.org/10.1016/S1474-4422(08)70239-4
Berg D (2011) Hyperechogenicity of the substantia nigra: pitfalls in assessment and specificity for Parkinson’s disease. J Neural Transm (Vienna) 118:453–461. https://doi.org/10.1007/s00702-010-0469-5
Walter U (2013) How to measure substantia nigra hyperechogenicity in Parkinson disease: detailed guide with video. J Ultrasound Med 32:1837–1843. https://doi.org/10.7863/ultra.32.10.1837
Walter U, Školoudík D (2014) Transcranial sonography (TCS) of brain parenchyma in movement disorders: quality standards, diagnostic applications and novel technologies. Ultraschall Med 35:322–331. https://doi.org/10.1055/s-0033-1356415
Yilmaz R, Berg D (2018) Transcranial B-Mode Sonography in Movement Disorders. Int Rev Neurobiol 143:179–212. https://doi.org/10.1016/bs.irn.2018.10.008
Walter U, Kanowski M, Kaufmann J, Grossmann A, Benecke R, Niehaus L (2008) Contemporary ultrasound systems allow high-resolution transcranial imaging of small echogenic deep intracranial structures similarly as MRI: a phantom study. Neuroimage 40:551–558. https://doi.org/10.1016/j.neuroimage.2007.12.019
van de Loo S, Walter U, Behnke S et al (2010) Reproducibility and diagnostic accuracy of substantia nigra sonography for the diagnosis of Parkinson’s disease. J Neurol Neurosurg Psychiatry 81:1087–1092. https://doi.org/10.1136/jnnp.2009.196352
Berardelli A, Wenning GK, Antonini A et al (2013) EFNS/MDS-ES/ENS recommendations for the diagnosis of Parkinson’s disease. Eur J Neurol 20:16–34. https://doi.org/10.1111/ene.12022
Oertel WH, Reichmann H et al (2008) Parkinson-Syndrome: Diagnostik und Therapie, 2008. In: Deutsche Gesellschaft für Neurologie (ed) Leitlinien für Diagnostik und Therapie in der Neurologie. https://dgn.org/leitlinie/parkinson-krankheit. Accessed 26 February 2024
Eggert KM, Oertel WH, Reichmann H et al (2012) Parkinson-Syndrome - Diagnostik und Therapie, 2012. In: Deutsche Gesellschaft für Neurologie (ed) Leitlinien für Diagnostik und Therapie in der Neurologie. https://dgn.org/leitlinie/parkinson-krankheit. Accessed 26 February 2024
Deuschl G, Oertel WH, Reichmann H et al (2016) Idiopathisches Parkinson-Syndrom, S3-Leitinie, 2016. In: Deutsche Gesellschaft für Neurologie (ed) Leitlinien für Diagnostik und Therapie in der Neurologie. https://dgn.org/leitlinie/parkinson-krankheit. Accessed 26 February 2024
Höglinger G, Trenkwalder C et al (2023) Parkinson-Krankheit, S2k-Leitlinie, 2023. In: Deutsche Gesellschaft für Neurologie (ed) Leitlinien für Diagnostik und Therapie in der Neurologie. https://dgn.org/leitlinie/parkinson-krankheit. Accessed 26 February 2024
McInnes MDF, Moher D, Thombs BD et al (2018) Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 319:388–396. https://doi.org/10.1001/jama.2017.19163
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Skoloudík D, Fadrná T, Bártová P et al (2007) Reproducibility of sonographic measurement of the substantia nigra. Ultrasound Med Biol 33:1347–1352. https://doi.org/10.1016/j.ultrasmedbio.2007.03.013
Gaenslen A, Unmuth B, Godau J et al (2008) The specificity and sensitivity of transcranial ultrasound in the differential diagnosis of Parkinson’s disease: a prospective blinded study. Lancet Neurol 7:417–424. https://doi.org/10.1016/S1474-4422(08)70067-X
Walter U, Dressler D, Wolters A, Probst T, Grossmann A, Benecke R (2004) Sonographic discrimination of corticobasal degeneration vs progressive supranuclear palsy. Neurology 63:504–509. https://doi.org/10.1212/01.wnl.0000133006.17909.32
Shafieesabet A, Fereshtehnejad SM, Shafieesabet A, Delbari A, Baradaran HR, Postuma RB, Lökk J (2017) Hyperechogenicity of substantia nigra for differential diagnosis of Parkinson’s disease: a meta-analysis. Parkinsonism Relat Disord 42:1–11. https://doi.org/10.1016/j.parkreldis.2017.06.006
Behnke S, Berg D, Naumann M, Becker G (2005) Differentiation of Parkinson’s disease and atypical parkinsonian syndromes by transcranial ultrasound. J Neurol Neurosurg Psychiatry 76:423–425. https://doi.org/10.1136/jnnp.2004.049221
Walter U, Dressler D, Probst T, Wolters A, Abu-Mugheisib M, Wittstock M, Benecke R (2007) Transcranial brain sonography findings in discriminating between parkinsonism and idiopathic Parkinson disease. Arch Neurol 64:1635–1640. https://doi.org/10.1001/archneur.64.11.1635
Hellwig S, Reinhard M, Amtage F et al (2014) Transcranial sonography and [18F]fluorodeoxyglucose positron emission tomography for the differential diagnosis of parkinsonism: a head-to-head comparison. Eur J Neurol 21:860–866. https://doi.org/10.1111/ene.12394
Li X, Xue S, Jia S et al (2017) Transcranial sonography in idiopathic REM sleep behavior disorder and multiple system atrophy. Psychiatry Clin Neurosci 71:238–246. https://doi.org/10.1111/pcn.12483
Monaco D, Berg D, Thomas A et al (2018) The predictive power of transcranial sonography in movement disorders: a longitudinal cohort study. Neurol Sci 39:1887–1894. https://doi.org/10.1007/s10072-018-3514-z
Alonso-Canovas A, Tembl Ferrairó JI, Martínez-Torres I et al (2019) Transcranial sonography in atypical parkinsonism: How reliable is it in real clinical practice? A multicentre comprehensive study. Parkinsonism Relat Disord 68:40–45. https://doi.org/10.1016/j.parkreldis.2019.09.032
Walter U, Dressler D, Wolters A, Wittstock M, Greim B, Benecke R (2006) Sonographic discrimination of dementia with Lewy bodies and Parkinson’s disease with dementia. J Neurol 253:448–454. https://doi.org/10.1007/s00415-005-0023-9
Venegas-Francke P (2010) Transcranial sonography in the discrimination of Parkinson’s disease versus vascular parkinsonism. Int Rev Neurobiol 90:147–156. https://doi.org/10.1016/S0074-7742(10)90010-X
Busse K, Heilmann R, Kleinschmidt S et al (2012) Value of combined midbrain sonography, olfactory and motor function assessment in the differential diagnosis of early Parkinson’s disease. J Neurol Neurosurg Psychiatry 83:441–447. https://doi.org/10.1136/jnnp-2011-301719
Walter U, Berg D, Behnke S, Schminke U, Ertl M, Witte OW, Krogias C (2023) Positionspapier der DEGUM, Sektion Neurologie, und der DGKN zur Untersucherqualifikation für die transkranielle B-Bild-Sonografie (TCS) des Gehirns in der Diagnostik der Parkinson-Krankheit. Klin Neurophysiol 54:245–248. https://doi.org/10.1055/a-2150-4468
Heim B, Peball M, Hammermeister J, Djamshidian A, Krismer F, Seppi K (2022) Differentiating Parkinson’s Disease from Essential Tremor Using Transcranial Sonography: A Systematic Review and Meta-Analysis. J Parkinsons Dis 12:1115–1123. https://doi.org/10.3233/JPD-213012
Alonso-Cánovas A, López-Sendón JL, Buisán J et al (2014) Sonography for diagnosis of Parkinson disease-from theory to practice: a study on 300 participants. J Ultrasound Med 33:2069–2074. https://doi.org/10.7863/ultra.33.12.2069
Chen W, Tan YY, Hu YY et al (2012) Combination of olfactory test and substantia nigra transcranial sonography in the differential diagnosis of Parkinson’s disease: a pilot study from China. Transl Neurodegener 1:25. https://doi.org/10.1186/2047-9158-1-25
López Hernández N, García Escrivá A, Shalabi Benavent M (2015) Diagnostic value of combined assessment of olfaction and sustantia nigra hyperechogenicity for Parkinson’s disease. Neurologia 30:496–501. https://doi.org/10.1016/j.nrl.2014.03.010
Cardaioli G, Ripandelli F, Paolini Paoletti F et al (2019) Substantia nigra hyperechogenicity in essential tremor and Parkinson’s disease: a longitudinal study. Eur J Neurol 26:1370–1376. https://doi.org/10.1111/ene.13988
Li DH, He YC, Liu J, Chen SD (2016) Diagnostic accuracy of transcranial sonography of the substantia nigra in Parkinson’s disease: a systematic review and meta-analysis. Sci Rep 6:20863. https://doi.org/10.1038/srep20863
Berg D, Behnke S, Seppi K et al (2013) Enlarged hyperechogenic substantia nigra as a risk marker for Parkinson’s disease. Mov Disord 28:216–219. https://doi.org/10.1002/mds.25192
Walter U, Heilmann R, Kaulitz L, Just T, Krause BJ, Benecke R, Höppner J (2015) Prediction of Parkinson’s disease subsequent to severe depression: a ten-year follow-up study. J Neural Transm (Vienna) 122:789–797. https://doi.org/10.1007/s00702-014-1313-0
Iranzo A, Stockner H, Serradell M et al (2014) Five-year follow-up of substantia nigra echogenicity in idiopathic REM sleep behavior disorder. Mov Disord 29:1774–1780. https://doi.org/10.1002/mds.26055
Pilotto A, Heinzel S, Suenkel U et al (2017) Application of the movement disorder society prodromal Parkinson’s disease research criteria in 2 independent prospective cohorts. Mov Disord 32:1025–1034. https://doi.org/10.1002/mds.27035
Heinzel S, Kasten M, Behnke S et al (2018) Age- and sex-related heterogeneity in prodromal Parkinson’s disease. Mov Disord 33:1025–1027. https://doi.org/10.1002/mds.27349
Miyamoto M, Miyamoto T (2020) Relationship of substantia nigra hyperechogenicity to risk of Lewy body disease in idiopathic REM sleep behavior disorder patients: a longitudinal study. Sleep Med 68:31–34. https://doi.org/10.1016/j.sleep.2019.09.008
Berg D, Postuma RB, Adler CH et al (2015) MDS research criteria for prodromal Parkinson’s disease. Mov Disord 30:1600–1611. https://doi.org/10.1002/mds.26431
Heinzel S, Berg D, Gasser T et al (2019) Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord 34:1464–1470. https://doi.org/10.1002/mds.27802
Mahlknecht P, Marini K, Werkmann M, Poewe W, Seppi K (2022) Prodromal Parkinson’s disease: hype or hope for disease-modification trials? Transl Neurodegener 11:11. https://doi.org/10.1186/s40035-022-00286-1
Wang JY, Zhuang QQ, Zhu LB et al (2016) Meta-analysis of brain iron levels of Parkinson’s disease patients determined by postmortem and MRI measurements. Sci Rep 6:36669. https://doi.org/10.1038/srep36669
Thomas AJ, Attems J, Colloby SJ et al (2017) Autopsy validation of 123I-FP-CIT dopaminergic neuroimaging for the diagnosis of DLB. Neurology 88:276–283. https://doi.org/10.1212/WNL.0000000000003512
Walter U, Klein C, Hilker R, Benecke R, Pramstaller PP, Dressler D (2004) Brain parenchyma sonography detects preclinical parkinsonism. Mov Disord 19:1445–1449. https://doi.org/10.1002/mds.20232
Schweitzer KJ, Brüssel T, Leitner P et al (2007) Transcranial ultrasound in different monogenetic subtypes of Parkinson’s disease. J Neurol 254:613–616. https://doi.org/10.1007/s00415-006-0369-7
Saunders-Pullman R, Hagenah J, Dhawan V et al (2010) Gaucher disease ascertained through a Parkinson’s center: imaging and clinical characterization. Mov Disord 25:1364–1372. https://doi.org/10.1002/mds.23046
Brüggemann N, Hagenah J, Stanley K et al (2011) Substantia nigra hyperechogenicity with LRRK2 G2019S mutations. Mov Disord 26:885–888. https://doi.org/10.1002/mds.23644
Ahmadi SA, Bötzel K, Levin J et al (2020) Analyzing the co-localization of substantia nigra hyper-echogenicities and iron accumulation in Parkinson’s disease: A multi-modal atlas study with transcranial ultrasound and MRI. Neuroimage Clin 26:102185. https://doi.org/10.1016/j.nicl.2020.102185
Baracchini C, Azevedo E, Walter U, Sargento-Freitas J, Malojcic B, Council of Nations of the European Society of Neurosonology and Cerebral Hemodynamics (ESNCH) (2024) Neurosonology Survey in Europe and Beyond. Ultrasound Int Open 10:a22439625. https://doi.org/10.1055/a-2243-9625
Skoloudík D, Jelínková M, Blahuta J et al (2014) Transcranial sonography of the substantia nigra: digital image analysis. AJNR Am J Neuroradiol 35:2273–2278. https://doi.org/10.3174/ajnr.A4049
Walter U, Skowrońska M, Litwin T et al (2014) Lenticular nucleus hyperechogenicity in Wilson’s disease reflects local copper, but not iron accumulation. J Neural Transm (Vienna) 121:1273–1279. https://doi.org/10.1007/s00702-014-1184-4
Kozel J, Školoudík D, Ressner P et al (2023) Echogenicity of brain structures in Huntington’s disease patients evaluated by transcranial sonography - magnetic resonance fusion imaging using virtual navigator and digital image analysis. Ultraschall Med 44:495–502. https://doi.org/10.1055/a-2081-1635
Walter U, Müller JU, Rösche J et al (2016) Magnetic resonance-transcranial ultrasound fusion imaging: a novel tool for brain electrode location. Mov Disord 31:302–309. https://doi.org/10.1002/mds.26425
Reese R, Kriesen T, Kersten M et al (2023) Combining ultrasound and microelectrode recordings for postoperative localization of subthalamic electrodes in Parkinson’s disease. Clin Neurophysiol 156:196–206. https://doi.org/10.1016/j.clinph.2023.11.001
He N, Chen Y, LeWitt PA, Yan F, Haacke EM (2023) Application of neuromelanin MR imaging in Parkinson disease. J Magn Reson Imaging 57:337–352. https://doi.org/10.1002/jmri.28414
Prasuhn J, Strautz R, Lemmer F et al (2022) Neuroimaging correlates of substantia nigra hyperechogenicity in Parkinson’s disease. J Parkinsons Dis 12:1191–1200. https://doi.org/10.3233/JPD-213000
Mozaffarzadeh M, Verschuur E, Verweij MD, Daeichin V, De Jong N, Renaud G (2022) Refraction-corrected transcranial ultrasound imaging through the human temporal window using a single probe. IEEE Trans Ultrason Ferroelectr Freq Control 69:1191–1203. https://doi.org/10.1109/TUFFC.2022.314812
Mazzotti M, Kohtanen E, Erturk A, Ruzzene M (2023) Optimizing transcranial ultrasound delivery at large incident angles by leveraging cranial leaky guided wave dispersion. Ultrasonics 128:106882. https://doi.org/10.1016/j.ultras.2022.106882
Acknowledgements
German Parkinson Guideline Group: Mathias Bähr, MD (Department of Neurology, University Medical Center Göttingen, Göttingen, Germany), Jos Becktepe, MD, Daniela Berg, MD, Günther Deuschl, MD, Gregor Kuhlenbäumer, MD, Walter Maetzler, MD and Kirsten Zeuner, MD (Department of Neurology, University Hospital Schleswig-Holstein, Campus Kiel, Kiel, Germany), Kathrin Brockmann, MD and Inga Liepelt-Scarfone, PhD (Department of Neurodegeneration, Hertie Institute for Clinical Brain Research, University of Tübingen, Tübingen, Germany), Carsten Buhmann, MD and Monika Pötter-Nerger, MD (Department of Neurology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany), Andrés Ceballos-Baumann, MD (Schön Klinik München Schwabing, München, Germany), Joseph Classen, MD (Department of Neurology, Leipzig University Medical Center, Leipzig, Germany), Cornelius Deuschl, MD (Department of Diagnostic and Interventional Radiology and Neuroradiology, Essen University Hospital, Essen, Germany), Georg Ebersbach, MD (Movement Disorders Clinic, Beelitz-Heilstätten, Beelitz, Germany), Carsten Eggers, MD (Department of Neurology, Knappschaftskrankenhaus Bottrop, Bottrop, Germany), Thilo van Eimeren, MD (Departments of Nuclear Medicine and Neurology, University of Cologne, Köln, Germany), Alessandra Fanciulli, MD, Florian Krismer, MD and Klaus Seppi, MD (Department of Neurology, Medical University of Innsbruck, Innsbruck, Austria), Bruno Fimm, PhD, and Kathrin Reetz, MD (Department of Neurology, University Hospital RWTH Aachen, Aachen, Germany), Ann-Kristin Folkerts, PhD and Elke Kalbe, PhD (Department of Medical Psychology, Neuropsychology and Gender Studies, Centre for Neuropsychological Diagnostics and Intervention, University Hospital Cologne, Köln, Germany), Madeleine Gausepohl (Neurological Center Segeberger Kliniken, Bad Segeberg, Germany), Alkomiet Hasan, MD (Department of Psychiatry, Psychotherapy and Psychosomatics, Faculty of Medicine, University of Augsburg, Augsburg, Germany), Wiebke Hermann, MD, Matthias Löhle, MD and René Reese, MD (Department of Neurology, Rostock University Medical Center, Rostock, Germany), Rüdiger Hilker-Roggendorf, MD (Department of Neurology, Klinikum Vest, Recklinghausen, Germany), Matthias Höllerhage, MD, Martin Klietz, MD, Christoph Schrader, MD and Florian Wegner, MD (Department of Neurology, Hannover Medical School, Hannover, Germany), Franziska Hopfner, MD, Thomas Köglsperger, MD and Johannes Levin, MD (Department of Neurology, LMU University Hospital, Ludwig-Maximilians University of Munich, München, Germany), Wolfgang Jost, MD (Parkinson-Klinik Ortenau, Wolfach, Germany), Jan Kassubek, MD (Department of Neurology, University Hospital Ulm, Ulm, Germany), Stephan Klebe, MD (Department of Neurology, Essen University Hospital, Essen, Germany), Christine Klein, MD (Institute of Neurogenetics, University of Lübeck, Lübeck, Germany), Andrea Kühn, MD (Department of Neurology with Experimental Neurology, Movement Disorders and Neuromodulation Unit, Charité—Universitätsmedizin Berlin, Berlin, Germany), Paul Krack, MD (Department of Neurology, Inselspital, University Hospital Bern, Bern, Switzerland), Paul Lingor, MD and Sylvia Maaß, MD (Department of Neurology, Klinikum rechts der Isar, Technical University Munich, München, Germany), Stefan Lorenzl, MD (Department of Neurology and Palliative Care, University Hospital Agatharied, Hausham, Germany), Regina Menzel (Department of Neurology, University of Heidelberg, Heidelberg, Germany), Philipp T. Meyer, MD (Department of Nuclear Medicine, Faculty of Medicine, University of Freiburg, Freiburg, Germany), Brit Mollenhauer, MD (Department of Neurology, University Medical Center Göttingen and Paracelsus-Elena-Klinik, Kassel, Germany), Manuela Neumann, MD (Department of Neuropathology, University of Tübingen, Tübingen, Germany), Per Odin, MD (Division of Neurology, Department of Clinical Sciences, Lund University, Lund, Sweden), Tiago F. Outeiro, PhD (Department of Experimental Neurodegeneration, Center for Biostructural Imaging of Neurodegeneration, University Medical Center Göttingen, Göttingen, Germany), Olaf Rieß, MD (Institute of Medical Genetics and Applied Genomics, University of Tübingen, Tübingen, Germany), Viktoria C. Ruf, MD (Center for Neuropathology and Prion Research, Ludwig-Maximilians University of Munich, München, Germany), Anja Schneider, MD (Department of Neurology, University Hospital Bonn, Bonn, Germany), Alfons Schnitzler, MD (Department of Neurology, Medical Faculty and University Hospital Düsseldorf, Düsseldorf, Germany), Friederike Sixel-Döring, MD (Department of Neurology, Philipps-University Marburg and Paracelsus-Elena-Klinik, Kassel, Germany), Lars Tönges, MD (Department of Neurology, St. Josef-Hospital, Ruhr-University, Bochum, Germany), Tobias Wächter, MD (Department of Neurology, Rehabilitation Centre Bad Gögging, Passauer Wolf, Bad Gögging, Germany), Tobias Warnecke, MD (Department of Neurology and Neurorehabilitation, Klinikum Osnabrück—Academic Teaching Hospital of the University of Münster, Osnabrück, Germany), Christian Winkler, MD (Department of Neurology, Lindenbrunn Hospital, Coppenbrügge, Germany), Karsten Witt, MD (Department of Neurology, School of Medicine and Health Sciences, University of Oldenburg, Oldenburg, Germany), Dirk Woitalla, MD (Department of Neurology, St. Josef-Krankenhaus Kupferdreh, Essen, Germany).
Funding
Open Access funding enabled and organized by Projekt DEAL. The administration of guideline development and the systematic literature search was supported by the German Society of Neurology (DGN). No direct financial funding was provided by the DGN or other commercial entities.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
U.W. has received speaker honoraria and travel grants from Bristol-Myers Squibb, Boehringer Ingelheim Pharma, Daiichi-Sankyo, Ipsen Pharma, Merz Pharmaceuticals, Pfizer Pharma, and an unrestricted research grant from Merz Pharmaceuticals outside the present study. He was funded by the German Federal Ministry of Education and Research (BMBF) outside the present study. He serves as Joint Editor-in-Chief of the European Journal of Ultrasound (Thieme, Stuttgart, Germany). K.L. has stated explicitly that there are no conflicts of interest in connection with this article. R.D. received research support from DGN, Faber-Stiftung Marburg, General Electric, LINF, and Roche. He serves as a consultant for Lilly. He received honoraria for scientific presentations from Eisai, Lilly, Roche and others but all honoraria were used for research (except travel costs). He is an investigator on three patents hold by the Philipps-University Marburg, Germany. A.S. has received funding from the Deutsche Forschungsgemeinschaft (German Research Association) and the Helmholtz-Association outside the present study. He has received honoraria for presentations/advisory boards/consultations from Esteve, Desitin, Lobsor Pharmaceuticals, STADA, Bial, RG Gesellschaft, Zambon, NovoNordisk and AbbVie outside the present study. He has received royalties from Kohlhammer Verlag and Elsevier Press. He serves as an editorial board member of Stem Cells International. C.T. has received funding from the DLR (German Research Association) by ERA-Net Project BRAVA (EU-Grant) outside the present study. She has received honoraria for presentations/advisory boards/consultations from Abbvie, UCB, STADA, Bial, Roche, Boehringer Ingelheim Pharma, Convatec, Ono Pharmaceuticals outside the present study. She has received royalties from Thieme Publisher and licence Fees for Parkinson Sleep Scale. She has received travel stipends from the Movement Disorder Society. She serves as an editorial board member for Sleep Medicine. G.H. received research support from Abbvie, UCB; has ongoing research collaborations with Roche, UCB, Abbvie; serves as a consultant for Abbvie, Alzprotect, Amylyx, Aprinoia, Asceneuron, Bayer, Bial, Biogen, Biohaven, Epidarex, Ferrer, Kyowa Kirin, Lundbeck, Novartis, Retrotope, Roche, Sanofi, Servier, Takeda, Teva, UCB; received honoraria for scientific presentations from Abbvie, Bayer, Bial, Biogen, Bristol Myers Squibb, Kyowa Kirin, Pfizer, Roche, Teva, UCB, Zambon. He holds a patent (Höglinger GU, Höllerhage M, Rösler T. Treatment of Synucleinopathies. United States Patent No.: US 10,918,628 B2, Date of Patent: Feb. 16, 2021; and European Patent No.: EP 17 787 904.6–1109 / 3 525 788). He received publication royalties from Academic Press, Kohlhammer, and Thieme. He was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy – ID 390857198); the German Federal Ministry of Education and Research (BMBF, CurePML EN2021-039); European Joint Programme on Rare Diseases (Improve-PSP); Deutsche Forschungsgemeinschaft (DFG, HO2402/18–1 MSAomics); and participated in industry-sponsored research projects from Abbvie, Biogen, Biohaven, Novartis, Roche, Sanofi, UCB.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Walter, U., Loewenbrück, K.F., Dodel, R. et al. Systematic review-based guideline “Parkinson’s disease” of the German Society of Neurology: diagnostic use of transcranial sonography. J Neurol (2024). https://doi.org/10.1007/s00415-024-12502-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00415-024-12502-1