Abstract
Multiple sclerosis (MS) treatment intervention with immunomodulating therapy at early disease stage improves short term clinical outcomes. The objective of this study is to describe the long-term outcomes and healthcare utilization of patients with clinically isolated syndrome (CIS) included in the Betaferon®/Betaseron® in Newly Emerging MS for Initial Treatment (BENEFIT) randomized, parallel group trial. In BENEFIT patients were assigned to “early” IFNB-1b treatment or placebo (“delayed” treatment). After 2 years or conversion to clinically definite multiple sclerosis (CDMS), all patients were offered IFNB-1b and were reassessed 15 years later. Of 468 patients, 261 (55.8%) were enrolled into BENEFIT 15 (161 [55.1%] from the early, 100 [56.8%] from the delayed treatment arm). In the full BENEFIT analysis set, risk of conversion to CDMS remained lower in the early treatment group ( – 30.5%; hazard ratio 0.695 [95% CI, 0.547–0.883]; p = 0.0029) with a 15.7% lower risk of relapse than in the delayed treatment group (p = 0.1008). Overall, 25 patients (9.6%; 9.9% early, 9.0% delayed) converted to secondary progressive multiple sclerosis. Disability remained low and stable with no significant difference between groups in Expanded Disability Status Scale score or MRI metrics. Paced Auditory Serial Addition Task-3 scores were better in the early treatment group (p = 0.0036 for treatment effect over 15 years). 66.3% of patients were still employed at Year 15 versus 74.7% at baseline. In conclusion, results 15 years from initial randomization support long-term benefits of early treatment with IFNB-1b.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is the most common cause of chronic neurological disability in young adults [1, 2]. Over the past 25 years, increased understanding of MS and availability of disease-modifying therapies (DMTs) has improved options to manage the disease [1]. Typically MS lasts several decades [2,3,4]. Prolonged follow-up of well characterized patients provides critical information on the implications of early treatment intervention.
The Betaferon®/Betaseron® in Newly Emerging MS for Initial Treatment (BENEFIT) trial investigated the effects of interferon beta-1b (IFNB-1b) treatment at or shortly after clinically isolated syndrome (CIS)—the first neurological episode suggestive of MS—on clinical and MRI outcomes [5,6,7,8]. The BENEFIT trial has been recognized as the most comprehensive follow-up study of patients who received early intervention with IFNB-1b, and has contributed to the broad consensus to offer patients early treatment to optimize disease management [1, 9]. Early study results demonstrated that initiating interferon beta-1b at CIS, compared with those who started treatment after a first relapse or after two years was associated with improved clinical and MRI outcomes beyond the 2-year double-blind randomized trial and through the 5-year rater-blinded study period.
Additional follow-up with a prospective, cross-sectional assessment at 11 years post baseline showed that early treatment with IFNB-1b remained beneficial up to 11 years after randomization. Patients who started IFNB-1b at CIS retained a lower risk for conversion to clinically definite multiple sclerosis (CDMS) and a lower annualized relapse rate (ARR) compared with those who started IFNB-1b after a short delay (mean 1.5 years) [5]. Disability remained low and stable in both treatment groups at the 11-year assessment.
This study aims to further extend the long-term information on this cohort and describe the course of this well-characterized inception cohort by an additional prospectively planned assessment 15 years after randomization. Data is reported from patient visits at Year 15 and from integrated analyses of the full BENEFIT cohort.
Methods
Patients with CIS and ≥ 2 brain MRI lesions suggestive of MS were randomly assigned in a 5:3 ratio to receive IFNB-1b (“early treatment”) or placebo. [8] After conversion to CDMS (defined as a second clinical attack or confirmed disease worsening), patients were offered open-label treatment with IFNB-1b without disclosing the initial randomization. At 2 years, all patients (including those who had not converted to CDMS) were offered to continue taking interferon beta-1b. The “delayed treatment” group included patients who were initially randomized to placebo. IFNB-1b was administered at a dose of 250 µg subcutaneously, every other day.
Prospective assessments (blinded as for the initial randomization) continued for 5 years following randomization. At 15 years, all initially randomized patients were asked to participate in a comprehensive clinical and MRI reassessment. Patients could be evaluated at their original center or, if their original study center did not participate in BENEFIT 15, at a different, participating center. Clinical outcomes included assessment of conversion to CDMS, progression to secondary progressive multiple sclerosis (SPMS), ARR, Expanded Disability Status Scale (EDSS), Paced Auditory Serial Addition Task – 3 s (PASAT-3), and employment status. CDMS was reached if a new neurological event (relapse) occurred, ie, the appearance of new neurological abnormality or reappearance of a neurological abnormality, separated by at least 30 days from onset of a preceding clinical demyelinating event, or by sustained worsening of > = 1.5 points on the EDSS and a total EDSS of > = 2.5. HRQoL was assessed by EuroQol 5-Dimension (EQ-5D), including the Visual Analog Scale (VAS) and Functional Assessment of Multiple Sclerosis (FAMS – total score and Trial Outcome Index (TOI)). Standardized questions on employment situation and resource utilization were asked. If a visit at the study center was not possible, patients were offered a structured telephone evaluation that also included a validated patient reported EDSS assessment tool for which an overall high correlation with EDSS as determined by physical examination had been demonstrated (Pearson’s correlation coefficient -0.95), across all functional scores and degrees of disability.[10, 11].
Investigators collected MRI data at study sites according to the BENEFIT MRI standardized protocol. Scans were analyzed at a central reading site (Institutes of Neurology and Healthcare Engineering, UCL, London, UK). Trained readers manually identified and quantified lesions using a local-intensity thresholding technique. Neuroradiological assessments included number of new T2 lesions, T2 lesion volume, T1 lesion volume, normalized brain volume, normalized thalamic volume, cortical thickness, and mean upper cervical cord area [5].
Analyses were performed for the BENEFIT 15 cohort. Integrated analyses were carried out using the full BENEFIT cohort, allowing for time to event calculations. Outcomes included time to conversion to CDMS, time to first relapse, time to recurrent relapse, number of patients with confirmed and sustained 1-point EDSS progression, and number of patients with confirmed 2.5-point EDSS progression. The number of patients with diagnosis of MS according to the McDonald 2001 and 2010 criteria at fifteen years after occurrence of CIS was determined in patients who had an MRI performed at Year 15. Patients who developed CDMS or McDonald MS by Year 11 of follow-up were also included in this analysis.
Safety was also assessed at Year 15. An adverse event (AE) was defined as any untoward medical occurrence (ie, any unfavorable and unintended sign, symptom, or disease temporally associated with the use of a medicinal product, whether or not considered related to the medicinal product, also covering laboratory findings or results of other diagnostic procedures considered clinically relevant). Conditions were documented that started or deteriorated after signing of informed consent.
Statistical procedures
Statistical modeling was used to estimate treatment effects, and to explore the relationship between target variables and treatment. The study was exploratory in nature, with conversion to CDMS and/or SPMS, relapse rate, EDSS, PASAT-3, resource utilization, and employment/retirement status defined as variables of primary interest. All variables were analyzed descriptively with appropriate statistical methods. Particular outcomes (time to CDMS, time to first relapse, time to use of an ambulatory device, and time to dependence on an ambulatory device) were evaluated using Kaplan–Meier (KM) methods, log-rank tests and proportional hazards regression for time-to-event outcomes and a generalized linear regression model for ARR, with steroid use during the first event (yes or no); multifocal or monofocal onset of disease, number of T2 lesions at screening (2 to 4, 5 to 8, or ≥ 9); number of gadolinium-enhancing (Gd +) lesions at screening; age; and sex included as the set of covariates in the proportional hazards regression model and the generalized linear regression model. For PASAT-3 distribution over time a parametric longitudinal linear mixed model (with baseline PASAT-3 as the covariate) was established. The statistical evaluation was performed using software package SAS® (Statistical Analysis System) release 9.2 (SAS Institute Inc., Cary, North Carolina, United States).
Standard protocol approvals, registrations, and patient consents
The institutional review boards of participating institutions approved the protocol for the study. Patients provided informed consent at enrollment of the trial. The BENEFIT 15 trial is listed on clinicaltrials.gov under NCT03269175.
Results
Patient disposition
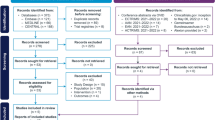
Of the 468 patients initially randomized in the BENEFIT trial, 261 (55.8%) were enrolled in BENEFIT 15 (early treatment: 161 of 292 patients [55.1%], delayed treatment: 100 of 176 patients [56.8%]) between September 2017 and May 2018 at the 69 sites contributing in this 15-year follow-up study (Fig. 1). Sites included those actively participating in the BENEFIT 15 trial, as well as non-participating sites who had their patients enrolled for assessments at one of the active sites. Overall, 66.4% of the patients originally randomized and treated in these participating sites were included. Of the 261 patients enrolled in BENEFIT 15, a total of 199 patients (76.25%) attended the study centers in person; 62 patients (23.75%) were unable to attend study centers and were instead evaluated remotely by telephone interview.
Study profile for the entire BENEFIT Study. a Includes one patient randomized to receive interferon beta-1b but treated with placebo. b Includes one patient randomized to receive placebo but treated with interferon beta-1b. c Includes one patient entered into the BENEFIT follow-up study after premature discontinuation of the BENEFIT Study. d Four lost to follow-up, 2 missing data, 1 noncompliance, 1 treatment failure, 2 refused final visit. e Three lost to follow-up, 1 relocated away from site, 1 pregnancy, 1 unable to attend visit because of job. f To be eligible for the 11-year or the 15-year cross-sectional follow-up study, patients only needed to be randomized and treated in the original BENEFIT Study (i.e., they did not need to be included in the previous BENEFIT analyses). BENEFIT Betaferon/Betaseron in Newly Emerging MS for Initial Treatment, CDMS clinically definite multiple sclerosis, DMT disease-modifying therapy
The baseline characteristics of participants in the 15-year follow-up study did not differ from those in the initial BENEFIT trial cohort (Table 1). At the 15-year visit, 161 patients (61.7%; 61.5% of the early, 62% of the delayed group) were receiving a DMT, of which 37.3% (n = 60) were administering IFNB, with no difference in current use observed between the randomization groups (early treatment: 37,4%, delayed: 37.10%). Also escalation therapy use at the time of the study visit was similar (Step 1 escalation in 16.8% (n = 27) of the early and in 16.1% (n = 16) of the delayed treatment group, Step 2 escalation in 6.2% (n = 10) of the early and 8% (n = 8) of the delayed treatment group) Any previous or current Step 1 escalation therapy use was recorded for 29.2% (n = 47) of the patients in the early and by 25% (n = 25) in the delayed treatment group, and any Step 2 escalation therapy use for 14.9% of the early and 16% of the delayed treatment group. Mean follow-up time was 15.2 years and the mean (standard deviation [SD]) age in this cohort was 46.7 (7.3) years, with 8 patients (3.1%) ≥ 60 years of age. The mean delay in initiation of therapy in the delayed treatment group was 1.53 years.
Clinical outcomes
Over the 15-year study period, the risk of conversion to CDMS among patients in the early treatment arm was 30.5% lower compared with the delayed treatment arm (hazard ratio 0.695 [95% confidence interval, 0.547–0.883] p = 0.0029). Kaplan–Meier (KM) estimate for conversion to CDMS by Year 15 was 69.8% in the overall study cohort (N = 468). Per KM estimate, 169 patients in the early treatment group (67.6% of the total early treatment group) and 118 patients in the delayed treatment group (73.5% of the total delayed treatment group) had converted to CDMS until Year 15 (Fig. 2). The risk of relapse was 15.7% lower in the early relative to the delayed treatment group (p = 0.1008). The mean ARR in the full BENEFIT cohort analysis set was 0.2083 over 15 years, and the median time to first relapse was 1888 days in the early treatment group compared to 931 days in the delayed treatment group. There was no significant difference between treatment groups regarding time to recurrent relapse (hazard ratio 0.842 [95% confidence interval, 0.671–1.056]).
In the BENEFIT 15 cohort, the KM estimate for the rate of conversion to SPMS at 15 years was 10.0% overall. No differences were observed in rates of conversion to SPMS between the two treatment groups (early treatment: 16/161 patients [9.9%, KM estimate: 10.2%]; delayed treatment: 9/100 patients [9.0%, KM estimate: 9.9%]; log-rank test p = 0.9713). SPMS occurrence was weakly associated with higher age (HR 1.056 [p = 0.0484]. In the delayed treatment group, KM estimate for SPMS was 4.0% for younger patients (< 30 years at study entry) versus 14.3% for those who entered BENEFIT at the age of 30 years or above. However, in the early treatment group, KM estimate for conversion to SPMS in patients below 30 years of age at BENEFIT study entry was similar (10.5%) to patients > = 30 years (9.9%).
At the Year 15 follow-up assessment, the mean (SD) EDSS score was 2.5 (1.76), and the median EDSS score (Q1, Q3) was 2.0 (1.5, 3.5). The mean (SD) EDSS scores in the early treatment and delayed treatment groups were 2.55 (1.76) and 2.43 (1.76), respectively; the median EDSS score [Q1, Q3] was 2.0 (1.5, 3.5) and 2.0 (1.0, 3.5), respectively. Overall, at the Year 15 follow-up, the majority of patients in both treatment arms presented with minimal disability, corresponding to an EDSS score of ≤ 2.5 (Fig. 3); 2.7% of the patients participating in the 15-year follow-up had an EDSS of ≥ 7 and depended on using a wheelchair as mobility aid. The numbers of patients with confirmed and sustained 1-point EDSS worsening were 91 (56.5%) and 47 (29.2%), respectively, in the early treatment group and 49 (49%) and 34 (34%), respectively, in the delayed treatment group. Within the same cohort, 32 (19.9%) patients had confirmed 2.5-point EDSS worsening in the early treatment group compared to 18 (18%) patients in the delayed treatment group.
Mean (SD) PASAT-3 score at Year 15 was similar in both treatment groups: 51.4 (10.7) in the early treatment group and 51.1 (8.8) in the delayed treatment group. The median (Q1, Q3) PASAT-3 score at 15 years after the subject´s first clinical event was 55.0 (50.0–58.0) in the early treatment group and 54.0 (49.0–57.0) in the delayed treatment group. For PASAT-3 score over the 15-year period a positive treatment effect could be observed (p = 0.0036 for treatment effect, adjusted for baseline PASAT score; Fig. 4).
HRQoL outcomes
At Year 15, the median (Q1, Q3) EQ-5D index score was 0.7960 (0.6910, 1.000; n = 259) in the overall cohort (Fig. 5). Median (Q1, Q3) EQ-5D change from baseline was 0.00 ( – 0.1880, 0.00). The EQ-5D VAS scores showed a similar pattern (85.50 [75.00, 93.00] at baseline (n = 186) and at Year 15 (n = 199; 80.00 [65.00, 90.00]). Although no statistical testing was conducted, the EQ-5D scores were generally similar between treatment groups. Median (Q1, Q3) FAMS-TOI was 114.50 (90.50, 133.00) at Year 15 (n = 260); median (Q1, Q3) change from baseline was – 7 ( – 28, 2.54). FAMS total score (Q1, Q3) was 139.00 (111.50, 159.00) at Year 15 and the median (Q1, Q3) change from baseline was – 5 ( – 27, 5.00).
Employment and resource utilization
Employment status information was available for 257/261 patients (98.5%) participating in the BENEFIT 15 study. Overall, 173/261 patients (66.3%) remained employed at Year 15, compared to 195/261 (74.7%) employed at baseline (Fig. 6). At Year 15, 143 patients (54.8%) were working > 20 h per week, 30 patients (11.5%) were working < 20 h per week. A total of 155 patients (59.4%) reported MS as having no impact on their working ability and employment status and 162 patients (62.1%) reported experiencing no periods of being unable to work because of MS in the past 12 months. There were no reports of hospitalizations during the past 12 months in 245/261 patients (93.9%). The use of adaptations in the past 6 months because of MS were reported by 29 patients (11.1%) with walking aids being most commonly utilized (17 patients; 6.5%). Other adaptation included: wheelchairs (8 patients; 3.1%), special hygiene utensils (8 patients; 3.1%), adaptation for car (5 patients; 1.9%), ramps (4 patients, 1.5%), adaptation at work (4 patients, 1.5%), special kitchen utensils (4 patients, 1.5%), spectacles (2 patients, 0.8%), alarms (1 patient, 0.8%) and stair lift (1 patient, 0.4%). Employment and resource utilization were generally similar between the treatment groups.
MRI Outcomes
MRI data were available for 110 patients (68.3%) in the early treatment group and 58 patients (58.0%) in the delayed treatment group. No consistent differences were seen between the treatment groups in MRI outcomes. The number of patients with any new T2 lesion since the patient’s last scan at Year 11 was 52 (47.3%) in the early and 30 (51.7%) in the delayed treatment arm. In the overall cohort, 41 patients (24.4%) experienced no new T2 lesions since their last analysis (data missing for 45 patients [26.8%]).
The McDonald criteria (2001 and 2010, respectively) were applied to identify patients who developed MS within 15 years after CIS. The 2010 McDonald criteria was applied to 255 patients (157 in the early treatment group, 98 in the delayed treatment group); in the early treatment group 149 (94.9%) met the criteria compared to 94 (95.9%) in the delayed treatment group. The 2001 McDonald criteria were applied in 252 patients (155 in the early treatment group, 97 in the delayed treatment group); 146 (94.2%) patients in the early treatment group met criteria compared to 92 (94.8%) in the delayed treatment group.
Safety
None of the participants who received IFNB-1b therapy experienced an AE during the BENEFIT 15 study period, and none of the AEs reported in a previous BENEFIT study were ongoing at the BENEFIT 15 visit. No deaths, serious AEs, or pregnancies were reported during this study. Changes in vital signs and body mass index since BENEFIT study baseline were negligible.
Discussion
BENEFIT 15 is a unique study cohort with 15-year follow-up data from randomization that compared early vs delayed treatment with IFNB-1b in patients presenting with CIS. Considering the time elapsed since the initial randomization, the number of patients enrolled in BENEFIT 15 is a respectable proportion—55.8%—of randomized patients, thus providing a unique opportunity to determine long-term outcomes of early treatment with IFNB-1b. The possibility that patients with a more severe disease course dropped out before this 15 year follow-up study cannot entirely be ruled out although such selective drop out may also have occurred with asymptomatic participants. However, the 15-year follow-up population did not appear to have a selection bias, as baseline characteristics were very similar to those of the original BENEFIT population, with a T2 lesion load at baseline that reflected substantial disease burden. Patients from sites that participated in this 15-year assessment were invited irrespective of their current medication, and great effort was made to reach more severely disabled patients with reduced mobility. In those cases where a clinic visit was not feasible, remote standardized telephone assessments were conducted. It is therefore remarkable that the conversion rates to CDMS after 15 years from initial randomization still favour the early treatment group (67.6% vs 73.5% with delayed treatment, by KM estimates). The overall relatively low EDSS and high PASAT-3 scores suggest limited disease progression by Year 15. EDSS scores compared favorably with natural history populations with shorter duration of follow-up [12, 13]. This, along with the low SPMS conversion rate after 15 years of follow up in both the early treatment and delayed but still early treatment groups, supports the overall beneficial effect of early initiation of DMTs. With an average of 1.5 years until the delayed treatment groups received treatment, both groups initiated treatment relatively early.
Patients in this study generally reported good HRQoL across both measures. Population surveys have demonstrated a relationship between age and HRQoL measures, and the mean EQ-5D VAS in the BENEFIT 15 cohort was comparable to that of EQ-5D VAS norm in an international general population for its respective age group [14].
Employment findings of the patients enrolled in the BENEFIT study at Year 15 reflect patients’ ability to accomplish daily professional tasks. These tasks often can be limited by MS symptoms and the disabilities inherent to the disease. The Global MS Employment Report demonstrated that 43% of unemployed patients with MS discontinued work within 3 years of diagnosis, and 70% after 10 years of diagnosis [15]. In fact, results from a prospective observational study showed that, in patients with MS, accumulation of disability and increase in relapse at 5 years following first episode of central nervous system demyelination were each associated with a decline in employment trajectory [16]. In the context of BENEFIT 15, the majority of patients (66.3%) were employed at Year 15 and had not taken days off from work in the past 12 months because of MS, regardless of whether they were in the early treatment or the delayed treatment arm of the initial BENEFIT cohort. This result supports the value of IFNB-1b treatment initiation at an early stage, especially when considering that the burden of MS can be substantial, throughout adult life.[17] In 2017, a survey of 4590 working-age patients with MS in Germany (mean (SD) age: 51.8 (11.0) years; mean (SD) age at diagnosis: 36.3 (10.6) years) revealed that 51% were employed or self-employed [18]. Despite their patient population having a shorter average disease duration, the findings from our study compare favorably with these results, with 66.3% of patients in our study being employed at Year 15.
Another survey of 1727 patients with MS in New Zealand, with a similar age and disease duration as our patient population, found that more than half (54%) were not working [19], while the study population in BENEFIT 15 including patients who either attended study centers or were evaluated via telephone interview had a non-employment rate of only 32.2%. This although great effort was taken to include all types of patients with varying disability, as patients with higher disability may be prone to non-employment. HRQoL data from the BENEFIT 15 study also compared favorably with population-based values and with data from other MS cohorts [20,21,22].
No new safety signals were detected with IFNB-1b long-term treatment in the current cross-sectional study. Clinical outcomes from the 15-year BENEFIT trial follow-up further support initiating DMT at or shortly after CIS. In the follow up of a placebo controlled CIS study comparing early interferon beta intramuscular treatment 10 years after randomization with delayed treatment Kinkel et al. reported that 18.4% had an EDSS ≥ 3.0.[23] In a study by Chung et al. conducted in a British cohort of untreated patients 30 years after CIS only 42% had remained ambulatory (EDSS scores of ≤ 3.5) and 34% developed SPMS [22].
With a follow-up of 15 years, the BENEFIT-15 cohort offers the longest duration of follow-up in a randomized population treated at or shortly after CIS, thus suggesting that many patients who start treatment with IFNB-1b at an early disease stage can retain clinical and social wellbeing in the future.
Data availability
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the EFPIA/PhRMA “Principles for responsible clinical trial data sharing”. This pertains to scope, timepoint and process of data access. As such, Bayer commits to sharing upon request from qualified scientific and medical researchers’ patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States (US) and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 01, 2014. Interested researchers can use www.vivli.org to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the member section of the portal. Data access will be granted to anonymized patient-level data, protocols and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
References
Reder AT, Oger JF, Kappos L, O’Connor P, Rametta M (2014) Short-term and long-term safety and tolerability of interferon beta-1b in multiple sclerosis. Mult Scler Relat Disord. 3(3):294–302. https://doi.org/10.1016/j.msard.2013.11.005
Compston A, Coles A (2002) Multiple sclerosis. Lancet. 359(9313):1221–31. https://doi.org/10.1016/S0140-6736(02)08220-X
Solaro C, Ponzio M, Moran E, Tanganelli P, Pizio R, Ribizzi G et al (2015) The changing face of multiple sclerosis: Prevalence and incidence in an aging population. Mult Scler. 21(10):1244–50. https://doi.org/10.1177/1352458514561904
Lunde H, Assmus J, Myhr KM, Bø L, Grytten N (2017) Survival and cause of death in multiple sclerosis: a 60-year longitudinal population study. J Neurol Neurosurg Psychiatry. 88(8):621–5. https://doi.org/10.1136/jnnp-2016-315238
Kappos L, Edan G, Freedman MS, Montalban X, Hartung HP, Hemmer B et al (2016) The 11-year long-term follow-up study from the randomized BENEFIT CIS trial. Neurology. 87(10):978–87. https://doi.org/10.1212/WNL.0000000000003078
Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP, Miller DH et al (2007) Effect of early versus delayed interferon beta-1b treatment on disability after a first clinical event suggestive of multiple sclerosis: a 3-year follow-up analysis of the BENEFIT study. Lancet. 370(9585):389–97. https://doi.org/10.1016/S0140-6736(07)61194-5
Kappos L, Freedman MS, Polman CH, Edan G, Hartung HP, Miller DH et al (2009) Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol. 8(11):987–97. https://doi.org/10.1016/S1474-4422(09)70237-6
Kappos L, Polman CH, Freedman MS, Edan G, Hartung HP, Miller DH et al (2006) Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 67(7):1242–9. https://doi.org/10.1212/01.wnl.0000237641.33768.8d
Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D et al (2018) ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 24(2):96–120. https://doi.org/10.1177/1352458517751049
Collins CD, Ivry B, Bowen JD, Cheng EM, Dobson R, Goodin DS et al (2016) A comparative analysis of Patient-Reported Expanded Disability Status Scale tools. Mult Scler. 22(10):1349–58. https://doi.org/10.1177/1352458515616205
Lechner-Scott J, Kappos L, Hofman M, Polman CH, Ronner H, Montalban X et al (2003) Can the Expanded Disability Status Scale be assessed by telephone? Mult Scler. 9(2):154–9. https://doi.org/10.1191/1352458503ms884oa
Karampampa K, Gustavsson A, Miltenburger C, Eckert B (2012) Treatment experience, burden and unmet needs (TRIBUNE) in MS study: results from five European countries. Mult Scler. 18(2 Suppl):7–15. https://doi.org/10.1177/1352458512441566
Karampampa K, Gustavsson A, Miltenburger C, Kindundu CM, Selchen DH (2012) Treatment experience, burden, and unmet needs (TRIBUNE) in multiple sclerosis: the costs and utilities of MS patients in Canada. J Popul Ther Clin Pharmacol 19(1):e11–e25
Szende A, Janssen B, Cabases J. Self-Reported Population Health: An International Perspective based on EQ-5D. Heidelberg, New York, London: Springer Open; 2014. https://doi.org/10.1007/978-94-007-7596-1
Jones N. Global MS Employment Report. London: Multiple Sclerosis International Federation 2016 [1–20]
Minneboo A, Barkhof F, Polman CH, Uitdehaag BM, Knol DL, Castelijns JA (2004) Infratentorial lesions predict long-term disability in patients with initial findings suggestive of multiple sclerosis. Archives of neurology. 61(2):217–21. https://doi.org/10.1001/archneur.61.2.217
GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019 Mar;18(3):269–285. 10.1016/ S1474–4422(18)30443–5
Flachenecker P, Kobelt G, Berg J, Capsa D, Gannedahl M. New insights into the burden and costs of multiple sclerosis in Europe: Results for Germany. Mult Scler. 2017;23(2_suppl):78–90. https://doi.org/10.1177/1352458517708141
Pearson JF, Alla S, Clarke G, Mason DF, Anderson T, Richardson A et al (2017) Multiple Sclerosis impact on employment and income in New Zealand. Acta neurologica Scandinavica. 136(3):223–32. https://doi.org/10.1111/ane.12714
Reese JP, Wienemann G, John A, Linnemann A, Balzer-Geldsetzer M, Mueller UO et al (2013) Preference-based Health status in a German outpatient cohort with multiple sclerosis. Health and quality of life outcomes. 11:162. https://doi.org/10.1186/1477-7525-11-162
Yalachkov Y, Soydaş D, Bergmann J, Frisch S, Behrens M, Foerch C et al (2019) Determinants of quality of life in relapsing-remitting and progressive multiple sclerosis. Mult Scler Relat Disord. 30:33–7. https://doi.org/10.1016/j.msard.2019.01.049
Chung KK, Altmann D, Barkhof F, Miszkiel K, Brex PA, O’Riordan J et al (2020) A 30-Year Clinical and Magnetic Resonance Imaging Observational Study of Multiple Sclerosis and Clinically Isolated Syndromes. Annals of neurology. 87(1):63–74. https://doi.org/10.1002/ana.25637
Kinkel RP, Dontchev M, Kollman C, Skaramagas TT, O’Connor PW, Simon JH (2012) Archives of neurology. 69(2):183–90. https://doi.org/10.1001/archneurol.2011.1426
Acknowledgements
The authors thank the patients and the BENEFIT 15 investigators for their continuing contributions to the study. VMLY&R provided writing support that included medical editing, English-language assistance, formatting of text and figures, and coordinating reviews, which was funded by Bayer AG. BENEFIT and BENEFIT 15 Study group. Principal investigators: Austria – S Strasser-Fuchs (Graz), T Berger (Innsbruck), K Vass (Vienna)*; Belgium – C Sindic, V van Pesch (Brussels), B Dubois (Leuven), D Dive, V Delvaux (Liège), J Debruyne, G Laureys (Ghent); Canada – L Metz (Calgary), G Rice (London), P Duquette, Y Lapierre (Montreal), M Freedman (Ottawa), A Traboulsee (Vancouver), P O’Connor (Toronto)*; Czech Republic – P Štourač (Brno), R Taláb, M Valis (Hradec Kralove); O Zapletalová (Ostrava), I Kovářová, E Medová (Prague), J Fiedler (Plzen); Denmark – J Frederiksen (Glostrup); France – B Brochet (Bordeaux), T Moreau (Dijon), P Vermersch (Lille), J Pelletier (Marseille), G Edan (Rennes), M Clanet (Toulouse), P Clavelou (Clermont Ferrand), C Lebrun-Frenay (Nice), O Gout (Paris); Finland M Kallela, J Erälinna (Helsinki), T Pirttilä, (Kuopio), J Ruutiainen (Turku), K Koivisto (Seinäjoki), M Reunanen, AM Keskinarkaus (Oulu), I Elovaara, M Sumelahti (Tampere); Germany – A Villringer, H Altenkirch, S Brockhaus, F Paul (Berlin), K Wessel (Braunschweig), H-P Hartung, W Steinke (Düsseldorf), H Kölmel (Erfurt), P Oschmann, M Berghoff (Giessen), R Diem, M Weber (Göttingen), A Dressel (Greifswald), F Hoffmann (Halle/Saale), K Baum (Hennigsdorf), S Jung (Homburg/Saar), H Felicitas Petereit, D Reske (Cologne), M Sailer (Magdeburg), J Köhler, F Zipp (Mainz), N Sommer, B Tackenberg (Marburg), R Hohlfeld, T Kuempfel (Munich), K-H Henn (Offenbach), A Steinbrecher (Regensburg), H Tumani (Ulm), R Gold, P Rieckmann, (Würzburg); Hungary – R Komoly, G Gács, G Jakab, P Salacz (Budapest), L Csiba, T Csepany (Debrecen), L Vécsei (Szeged); Israel – A Miller (Haifa), D Karussis, P Petrou (Jerusalem), J Chapman (Tel-Hashomer); Italy – A Ghezzi, M Zaffaroni (Gallarate), G Comi, V Martinelli (Milan), P Gallo (Padua), V Cosi, R Bergamaschi (Pavia), L Durelli, M Clerico (Turin); Netherlands – B Anten (Sittard), L Visser (Tilburg); Norway – K-M Myhr (Bergen); Poland – A Szczudlik (Kraków), K Selmaj, M Stasiolek (Łódź), Z Stelmasiak, H Bartosik-Psujek (Lublin), R Podemski, M Ejma (Wrocław), Z Maciejek, S Wawrzyniak (Bydgoszcz); Portugal – L Cunha, L Sousa (Coimbra); Slovenia – S Sega-Jazbec (Ljubljana); Spain – X Montalbán, T Arbizu, A Saiz, S Martinez Yelamos (Barcelona), J Bárcena (Barakaldo), R Arroyo, J Matias-Guiu (Madrid), O Fernández, P Serrano (Málaga), G Izquierdo (Seville), B Casanova (Valencia); Sweden – J Lycke, (Mölndal); Switzerland – L Kappos, J. Kuhle (Basel), A Chan (Bern), K Beer, (St Gallen); UK – R Coleman (Aberdeen), J Chataway (London), J O’Riordan (Dundee), S Howell (Sheffield). *This contributor has passed away.
Funding
Open access funding provided by University of Basel. This study was funded by Bayer AG.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical statement
L Kappos received no personal compensation. His institutions (University Hospital Basel/Foundation Clinical Neuroimmunology and Neuroscience Basel) have received the following exclusively for research support: steering committee, advisory board and consultancy fees (AbbVie, Actelion, Auriga Vision AG, Bayer HealthCare, Biogen, Celgene, Dörries Frank-Molnia & Pohlman, Eli Lilly, EMD Serono, Genentech, Genzyme, Glaxo Smith Kline, Janssen, Merck, Minoryx, Novartis, Roche, Sanofi, Santhera, Senda Biosciences, Shionogi and Wellmera AG); speaker fees (Bristol Myers Squibb, Celgene, Janssen, Merck, Novartis, and Roche); support for educational activities (Biogen, Desitin, Novartis, Sanofi and Teva); license fees for Neurostatus products; and grants (European Union, Innosuisse, Novartis, Roche, Swiss MS Society and Swiss National Research Foundation). F Barkhof has received compensation for consultancy from Bayer, Biogen Idec, Merck, Novartis, Roche, Combinostics, and IXICO, and has received research support from the Dutch MS Research Foundation, NIHR biomedical research center at UCLH and the EU (FP7 and IMI). MS Freedman has received compensation from Alexion, Atara Biotherapeutics, Bayer HealthCare, Beigene, BMS/Celgene, EMD Inc./EMD Serono and Merck Serono, Hoffman La-Roche, Janssen (J&J), Novartis, Pendopharm, Sanofi-Genzyme for consulting services. He also participates in a Sanofi-Genzyme and EMD Serono-sponsored speakers bureaus. G Edan has received honoraria for consulting from Biogen, Merck, Novartis, Sanofi, Roche, and LFB, and has received personal compensation for serving on the BENEFIT scientific advisory board and for speaking from Bayer AG. His institution has also received research support from Novartis, Sanofi, Merck, Biogen, Roche, and Teva. H-P Hartung has received honoraria for consulting and speaking at symposia from Bayer AG, Biogen Idec, BMS Celgene, GeNeuro, Merck, Novartis, Roche, Sanofi, and TG Therapeutics, with approval by the rector of Heinrich-Heine University. X Montalbán has received speaking honoraria and travel expenses for participation in scientific meetings and has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past years with Actelion, Bayer, Biogen, Celgene, Genzyme, Merck, Novartis, Roche, Sanofi-Genzyme, Teva Pharmaceutical, Excemed, MSIF and NMSS. R Koelbach is an employee of Ingress-Health HWM GmbH (a Cytel company) and was an employee of Parexel International GmbH at the time of this study. DG MacManus has nothing to disclose. EM Wicklein is a salaried employee of Bayer AG.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kappos, L., Edan, G., Freedman, M.S. et al. Long-term clinical outcomes in patients with CIS treated with interferon beta-1b: results from the 15-year follow up of the BENEFIT trial. J Neurol (2024). https://doi.org/10.1007/s00415-024-12417-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00415-024-12417-x