Abstract
Background
While immediate benefits of levodopa–carbidopa intestinal gel (LCIG) are evident in patients with Parkinson’s disease (PD), long-term LCIG effects require further study.
Objectives
We explored long-term LCIG on motor symptoms, nonmotor symptoms (NMS), and LCIG treatment settings in patients with advanced PD (APD).
Methods
Data were obtained (medical records and patient visit) from COSMOS, a multinational, retrospective, cross-sectional post-marketing observational study in patients with APD. Patients were stratified into 5 groups based on LCIG treatment duration at the patient visit, from 1–2 to > 5 years LCIG. Between-group differences were assessed for changes from baseline in LCIG settings, motor symptoms, NMS, add-on medications, and safety.
Results
Out of 387 patients, the number of patients per LCIG group was: > 1– ≤ 2 years LCIG (n = 156); > 2– ≤ 3 years LCIG (n = 80); > 3– ≤ 4 years LCIG (n = 61); > 4– ≤ 5 years LCIG (n = 30); > 5 years LCIG (n = 60). Baseline values were similar; data reported are changes from the baseline. There were reductions in “off” time, dyskinesia duration, and severity across LCIG groups. Prevalence, severity, and frequency of many individual motor symptoms and some NMS were reduced amongst all LCIG groups, with few differences between groups. Doses for LCIG, LEDD and LEDD for add-on medications were similar across groups both at LCIG initiation and patient visit. Adverse events were similar across all LCIG groups and consistent with the established safety profile of LCIG.
Conclusions
LCIG may provide sustained, long-term symptom control, while potentially avoiding increases in add-on medication dosages.
Trial registration
ClinicalTrials.gov Identifier: NCT03362879. Number and date: P16-831, November 30, 2017.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Levodopa is the current gold standard therapy for the improvement of motor symptoms in patients with Parkinson’s disease (PD) [1]. However, long-term oral levodopa administration is associated with irregular, pulsatile dopamine stimulation and insufficient symptom control in patients with PD [2,3,4,5,6,7]. Progressive loss of striatal dopamine neurons and compromised dopamine signaling over time can contribute to greater motor complications in some patients with PD [2, 4]. Specifically, long-term use of oral levodopa (4–6 years) can be associated with up to a 40–75% likelihood of developing disabling motor complications and a 40% dyskinesia risk [1, 8, 9]. Further, the short levodopa half-life and irregular gastric emptying can lead to erratic plasma levodopa levels [2, 4, 10, 11]. Without consistent levodopa brain influx, patients are less likely to achieve a clinical response with oral levodopa over time [1]. Continuous dopaminergic stimulation produces several advantages over pulsatile dopaminergic stimulation [12]. Taken together, oral levodopa may not provide sufficient symptom control or clinical response as motor symptoms worsen over time in patients with PD.
Motor disturbances with long-term levodopa are also associated with a variety of nonmotor symptoms (NMS), all of which contribute to reduced health-related quality of life [1, 2, 13]. Treatment regimens include multiple medications aimed to increase dopamine neurotransmission (eg, addition of dopamine agonists) or to prolong the oral levodopa effect (eg, addition of monoamine oxidase [MAO] or catecholamine-O-methyltransferase [COMT] inhibitors) [14, 15]. These treatment regimens may provide symptom control for a period of time, but are often associated with drug-drug interactions, risk for medication errors, and reduced treatment adherence [14, 16, 17]. Additionally, add-on medications can have undesired side effects, such as impulse control disorders with dopamine agonists or diarrhea with COMT inhibitors [18, 19]. Likewise, medication non-adherence is prevalent in patients with PD, especially those with cognitive impairment or swallowing difficulties, leading to further motor dysfunction, as well as increased healthcare costs [1, 16, 20,21,22,23,24].
Levodopa–carbidopa intestinal gel (LCIG) allows individualized doses of levodopa to be infused continuously into the absorption site at the small intestine to maintain stable physiological dopamine levels [2, 25]. In clinical trials and real-world studies, LCIG leads to significantly greater improvements in PD symptom control and health-related quality of life, as compared to levodopa [2, 4, 26,27,28,29,30,31]. Likewise, a randomized, crossover design comparator study found that patients generally preferred infusion therapy with LCIG versus oral administration with levodopa (n = 24) [32]. Indeed, meta-analyses have found significant improvements in off-time and quality of life or comparable benefits with LCIG versus deep brain stimulation, continuous subcutaneous apomorphine infusion, and best medical treatment [33,34,35], and similar cost between LCIG and standard of care [36].
Studies on efficacy and safety supporting the use of LCIG vary in design, duration (3 weeks to 12 months), and patient population, with a few studies spanning over the course of years (2–5 years) [2, 4, 26,27,28,29,30]. More specifically, few studies have investigated the long-term use of LCIG monotherapy, with the longest durations being 2 years in the Global Long-term Registry on Efficacy and Safety of LCIG in patients with APD (GLORIA) study [37, 38], 3 years in the DUOGLOBE multinational real-world observational study [39], and in one study for up to 10 years (n = 37) [40]. There is a need for further clinical evidence on the long-term use of LCIG in patients with APD.
The COmedication Study assessing Mono- and cOmbination therapy with levodopa–carbidopa inteStinal gel (COSMOS) is a large, multinational study dedicated to evaluating the use of LCIG as a monotherapy or combination therapy [41]. Here, we report on long-term LCIG effects on motor symptoms, NMS, patient-reported outcomes, LCIG treatment settings, and safety according to LCIG treatment duration.
Methods
Study design
COSMOS was a multinational, retrospective, and cross-sectional post-marketing observational study in patients with APD treated with LCIG in routine clinical care (Clinicaltrials.gov identifier: NCT03362879; Fasano et al. [41] for full methods). Briefly, data were collected retrospectively via chart review and cross-sectionally at a single study patient visit. Clinical data were entered into a web-based electronic data capture system for analysis.
Participants
Patients were eligible for inclusion if they had a diagnosis of APD and had received ongoing LCIG treatment for ≥ 12 months prior to the study visit. Patients were excluded if they had participated in a concurrent or prior clinical trial involving LCIG or were unable to complete the study questionnaire. Written informed consent was obtained by each patient or legally authorized representative prior to any data collection. For this analysis, patients were stratified into five groups based on LCIG treatment duration at the patient visit: 1–2 years LCIG (> 1 to ≤ 2 years LCIG), 2–3 years LCIG (> 2 to ≤ 3 years LCIG), 3–4 years LCIG (> 3 to ≤ 4 years LCIG), 4–5 years LCIG (> 4 to ≤ 5 years LCIG), and > 5 years LCIG.
Demographics and clinical characteristics
During the patient visit, physicians collected current patient demographic information and medical history. Medical history data included PD history, clinical PD status, and LCIG treatment settings.
Clinical assessments
At baseline, defined as immediately prior to LCIG initiation and at the patient visit, physicians used the Unified Parkinson’s Disease Rating Scale (UPDRS) to measure “off” time (Part IV item 39, modified) and dyskinesia duration (Part IV item 32, modified), as well as dyskinesia severity (Part IV item 33). The UPDRS was selected based on the feasibility of completion within the clinical practice setting. “Off” time and dyskinesia duration were documented as hours during the day prior to the clinical visit as reported by the patient. At the patient visit, physicians assessed the prevalence, severity rating (none, mild, moderate, or severe), and frequency rating (rarely, often, frequent, or very frequent) of motor symptoms, NMS, and symptoms related to treatment from both timepoints (at baseline and at the patient visit). Symptoms were defined as characterized by the PD composite scale [42]. In addition, patients were assessed with the NMS Scale (NMSS), PD Sleep Scale Version 2 (PDSS-2), and PD Quality of Life Questionnaire (PDQ-8) at the patient visit. LCIG dosing and infusion-related dosing parameters were collected from medical records and at the patient visit. Data on add-on medications were collected.
Safety
Data from safety assessments were previously collected by healthcare professionals and documented in medical records along with any prior suspected adverse reactions from the patients’ medical files. Adverse events (AEs) possibly related to treatment or device were documented.
Statistical analysis
Data from medical records and patient visits were analyzed using descriptive statistics. All statistical analyses were carried out using SAS® version 9.4 (SAS Institute, Inc., Cary, NC, USA). Prevalence, defined as the percentage of patients affected, was analyzed for motor symptoms, NMS, and symptoms related to treatment. For motor symptoms, NMS, and treatment-related symptoms, ratings of frequency and severity were transformed into numerical scores and analyzed. For each symptom, variables ‘severity’ and ‘frequency’ were transformed as follows: for the transformed severity variable 'no symptom' was 0, mild = 1, moderate = 2, and severe = 3, and unknown was set to missing. For the transformed frequency variable 'no symptom' was 0, rarely = 1, often = 2, frequent = 3, very frequent = 4, and unknown was set to missing. A chi-squared test was used to compare categorical data (eg, PD motor phenotype, monotherapy, and motor symptom and NMS prevalence when possible). The Kappa test was used to analyze motor symptoms and NMS prevalence (within-group differences). The Wilcoxon test was used to analyze quantitative comparisons of continuous, non-normally distributed data (eg, between-group and within-group differences of severity and frequency scores for NMSS, PDSS-2, and PDQ-8).
Results
Participants
The COSMOS study included 409 patients from 49 sites in 14 countries [41]. A total of 387 patients were included in this retrospective analysis based on data availability from medical records at the patient visit date (ie, 22 patients were omitted due to lack of data on the LCIG initiation date in case report forms and the inability to calculate LCIG duration). The number of patients per LCIG duration were as follows: 1–2 years LCIG (n = 156); 2–3 years LCIG (n = 80); 3–4 years LCIG (n = 61); 4–5 years LCIG (n = 30); > 5 years LCIG (n = 60). Demographics and clinical characteristics at baseline were generally similar among groups (Table 1). The majority of patients were male with a mean ± standard deviation (SD) age of patients at baseline ranging from 64.5 ± 7.6 years of age (4–5 years LCIG) to 67.9 ± 7.4 years of age (1–2 years LCIG) (Table 1). Across all groups, the most common reason for LCIG initiation was due to disabling motor fluctuations/off periods (≥ 83.3%) (Table 1). The other most common reasons for LCIG initiation were decreased quality of life, uncontrolled dyskinesia, and lack of efficacy of previous treatment. The time from PD diagnosis to LCIG initiation ranged from a mean ± SD of 12.1 ± 5.4 years (1–2 years LCIG) to 13.8 ± 5.1 years (> 5 years LCIG) (Table 1). At baseline, patients in the 4–5 years LCIG group had lower mean ± SD dyskinesia duration (2.7 ± 2.6 h) as compared to other groups, ranging from mean ± SD of 3.1 ± 2.9 to 4.9 ± 4.5 h (Table 1).
Clinical assessments
“Off” time, “on” time, and dyskinesia severity
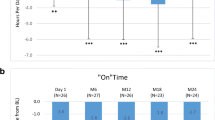
At the time of the patient visit, the duration of “off” time was reduced from baseline in all groups (p < 0.001) (Fig. 1). The duration of “on” time with dyskinesia was reduced from baseline in all (p < 0.001), with the exception of the 4–5 years LCIG group (p = 0.1378). “On” time without dyskinesia was increased from baseline in all groups (p < 0.0001 for all groups except 4–5 years LCIG [p = 0.0002]) (Fig. 1). Reductions from baseline were found in dyskinesia severity: mean ± SD, ranging from − 0.7 ± 1.3 to − 0.9 ± 1.3 (p < 0.0001 for all groups except 4–5 years LCIG [p = 0.0284] and > 5 years LCIG [p = 0.0003]).
Change from Baseline of “Off” Time and “On” Time with and without Dyskinesia During Waking Hours from LCIG Initiation to Patient Visit According to Duration of LCIG Treatment. Significance for change from baseline as follows: NS: p > 0.05; **p < 0.001; ***p < 0.0001. LCIG levodopa–carbidopa intestinal gel, NS not significant, SD standard deviation
Motor symptoms, NMS, and treatment-related symptoms
For many of the individual motor symptoms, reductions from baseline in prevalence were found in all groups for tremor and nocturnal/morning akinesia, while dysphagia increased (all groups except 4–5 years LCIG) and hypophonia increased (4–5 years LCIG only) (Fig. 2A). Some individual NMS showed similar reductions from baseline in all groups for the prevalence of anxiety, pain, constipation, gambling, and dopamine dysregulation syndrome, with the exception of constipation (> 5 years LCIG) and dopamine dysregulation syndrome (1–2 years LCIG and > 5 years LCIG) (Fig. 2B). There were no significant changes from baseline in the prevalence of treatment-related symptoms (Supplemental Fig. 1).
Reductions from baseline in severity and frequency were seen in individual motor symptoms, specifically for bradykinesia, rigidity, tremor, dystonia/cramps, gait impairment, nocturnal/morning akinesia, and freezing of gait for all groups (Supplemental Figs. 2A and 3A). Similarly, patients in all groups had reductions from baseline in the severity and frequency of some of the individual NMS and treatment-related symptoms assessed (Supplemental Figs. 2B, C and 3B, C). Differences between groups are noted in Supplemental Figs. 2 and 3.
NMSS, PDSS-2, and PDQ-8
NMSS scores were lower with 1–2 and 2–3 years LCIG vs > 5 years LCIG (mean ± SD, 1–2 years LCIG [55.3 ± 43.8]; 2–3 years LCIG [52.6 ± 38.3] vs > 5 years LCIG [68.9 ± 37.4], p = 0.0068 and p = 0.0057) (Table 2). PDSS-2 total scores were similar across groups at the patient visit (mean ± SD, ranging from 18.9 ± 9.1 to 22.0 ± 11.9) (Table 2). PDQ-8 was lower with 1–2 years and 2–3 years LCIG as compared to > 5 years LCIG (mean ± SD, 1–2 years LCIG [36.5 ± 19.0] and 2–3 years LCIG [38.9 ± 18.5] vs > 5 years LCIG [46.3 ± 16.5]; p = 0.0003 and p = 0.0076) (Table 2). Differences between groups with p < 0.05 are as follows: NMSS, 1–2 years vs > 5 years (p = 0.0068) and 2–3 years vs > 5 years (p = 0.0057); PDQ-8, 1–2 years vs 3–4 years (p = 0.0052), 1–2 years vs > 5 years (p = 0.0003), 2–3 years vs > 5 years (p = 0.0076). No differences between groups were found in PDSS-2.
LCIG dosage and add-on medications
LCIG total doses and LEDD were similar across groups at both LCIG initiation and at the patient visit (Table 3). Add-on medications were reduced in most groups at the patient visit as compared to baseline (Fig. 3A). Additionally, the number of add-on medication intakes were reduced at the patient visit as compared to baseline, independent of LCIG duration (p < 0.0001) (Fig. 3B). At 12 months after LCIG initiation, the percentages of patients receiving LCIG as monotherapy ranged from 22.4 to 46.7%, while the percentages of those receiving LCIG monotherapy plus night medication ranged from 10.0 to 30.3% and those receiving polytherapy ranged from 40.1 to 52.6% (Table 3).
Percentage of Patients Receiving Add-on Medications (A) and Add-on PD Medication Intakes (B) at Baseline and Patient Visita. aLess than 10% of patients had any intake of apomorphine, anticholinergics, and other add-on medications (not shown). No changes were found with NMDA antagonists as add-on medications (not shown). bLevodopa treatment included nocturnal levodopa, among other reasons, at the patient visit. COMT catecholamine-O-methyltransferase, LCIG levodopa–carbidopa intestinal gel, MAO monoamine oxidase, NMDA N-methyl-d-aspartate, PD Parkinson’s disease
Safety
A total of 109 AEs of any type were recorded via medical records during LCIG initiation and LCIG maintenance treatment for 387 patients, ranging across groups from 24% (37/156) in 1–2 years LCIG to 36% (22/61) in 3–4 years LCIG. The most common AEs reported were stoma site infection (> 5 years LCIG: 4/60 [7%] and 3–4 years LCIG: 3/61 [5%]) and unintentional medical device removal (3–4 years LCIG: 3/61 [5%]).
Discussion
In this post hoc analysis of the retrospective, cross-sectional, multinational COSMOS study, we reported LCIG treatment benefits present in the short-term and sustained through long-term LCIG treatment. Motor fluctuations, “off” time, dyskinesia, and motor symptoms improved from baseline for all groups, independent of LCIG duration. With continuous levodopa infusion and subsequent continuous dopaminergic stimulation, patients were able to maintain symptom control beyond 5 years with a stable levodopa requirement while reducing the burden of a complex add-on medication regimen. Approximately 20–45% of patients were on LCIG monotherapy at least 12 months after LCIG initiation, with 39.7% receiving LCIG monotherapy (and another 17.2% receiving LCIG monotherapy plus nighttime medication) after more than 5 years of treatment. The improvement of dyskinesia duration and severity, as well as “off” time, and the large percentage of patients on LCIG monotherapy, as found in the COSMOS primary analysis [41], support the potential use of LCIG for long-term symptom control.
A major strength of this study was the use of real-world, multinational, large-cohort clinical data. This post-hoc analysis of the COSMOS study is one of the few analyses to evaluate long-term LCIG therapy in patients with APD, and the first to systematically compare outcomes according to treatment duration, as well as long-term data for the rate of monotherapy, LEDD, and a number of intakes. The improvements in motor symptoms and NMS are supported by previous observational studies, as well as randomized controlled trials that have identified improvements in motor complications and NMS associated with LCIG treatment [2, 4, 26,27,28,29,30, 38].
The study included a partially retrospective design and observational analyses, without randomization at the study start, which limited data collection and interpretation. In addition, this study included only patients treated with ongoing LCIG who were able to sustain LCIG treatment for at least 12 months. Thus, the results of this analysis are not representative of all patients who initiate LCIG, possibly introducing an unintended bias. However, given the nature of the study, it is conceivable that patients with suboptimal outcomes over time discontinued LCIG and were not included in the analysis. In addition, recall bias at the patient visit may also influence the data upon which this post hoc analysis is based, wherein events that happened long ago are more likely to be misreported and thus potentially introducing systematic error across the treatment duration groups. Finally, the study was unable to achieve extended, long-term follow-up much beyond 5 years due to the low number of patients tracked for longer durations. However, this study design allows for a larger patient cohort than one would be able to track in a long-term treatment prospective design.
The data from this real-world analysis of patients with APD demonstrate patient outcome profiles with long-term LCIG treatment. Treatment with LCIG was associated with a sustained improvement in both dyskinesia duration and severity, independent of LCIG treatment duration. Stable levodopa delivery via LCIG reduced add-on medications. These results are in line with the EAN MDS guidelines for invasive therapies which identified improved time and quality of life in patients with LCIG versus oral therapy and shed light on potential patient selection for long-term LCIG treatment [43]. LCIG may be a long-term solution for carefully selected patients based on patient preferences and clinical characteristics (e.g., those without dementia) such that the benefits outweigh any potential risks [34, 44, 45]. Due to the long-term progressive nature of PD, as well as the aging process and potential treatment risks, patients with APD receiving treatments like LCIG may require consistent monitoring for worsening symptoms related to cognitive decline, vitamin B12 deficiency, or polyneuropathy [46, 47].
The study provides evidence that supports LCIG as an option to maintain adequate symptom control in the long term, despite the continued progression of PD. Adverse events were consistent with the known LCIG safety profile [2, 26, 41, 48]. Furthermore, LCIG can be a long-term solution for patients with memory or swallowing difficulties associated with APD who follow multi-faceted therapeutic regimens requiring many pills per day and may even eliminate the need to use add-on medications, as monotherapy was an option for many patients. Full understanding of the potential long-term benefits, beyond symptom control, with continuous dopaminergic stimulation via continuous levodopa delivery will require additional studies. In conclusion, long-term use of LCIG maintains reductions in “off” time, dyskinesia duration and severity, reduces the burden of several motor symptoms and NMS, and lessens the need for add-on medications, with a stable requirement of LCIG.
Data availability
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-andinformation-sharing/data-and-information-sharing-with-qualified-researchers.html.
References
Hauser RA (2009) Levodopa: past, present, and future. Eur Neurol 62:1–8
Antonini A, Poewe W, Chaudhuri KR, Jech R, Pickut B, Pirtosek Z, Szasz J, Valldeoriola F, Winkler C, Bergmann L, Yegin A, Onuk K, Barch D, Odin P, co-investigators Gs (2017) Levodopa–carbidopa intestinal gel in advanced Parkinson’s: final results of the GLORIA registry. Parkinsonism Relat Disord 45:13–20
Brooks DJ (2008) Optimizing levodopa therapy for Parkinson’s disease with levodopa/carbidopa/entacapone: implications from a clinical and patient perspective. Neuropsychiatr Dis Treat 4:39–47
Wang L, Li J, Chen J (2018) Levodopa–carbidopa intestinal gel in Parkinson’s disease: a systematic review and meta-analysis. Front Neurol 9:620
Contin M, Riva R, Albani F, Baruzzi A (1996) Pharmacokinetic optimisation in the treatment of Parkinson’s disease. Clin Pharmacokinet 30:463–481
Jourdain VA, Tang CC, Holtbernd F, Dresel C, Choi YY, Ma Y, Dhawan V, Eidelberg D (2016) Flow-metabolism dissociation in the pathogenesis of levodopa-induced dyskinesia. JCI Insight 1:e86615
Tambasco N, Romoli M, Calabresi P (2018) Levodopa in Parkinson’s disease: current status and future developments. Curr Neuropharmacol 16:1239–1252
Ahlskog JE, Muenter MD (2001) Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 16:448–458
Fahn S (1999) Parkinson disease, the effect of levodopa, and the ELLDOPA trial. Earlier vs Later L-DOPA. Arch Neurol 56:529–535
Hardoff R, Sula M, Tamir A, Soil A, Front A, Badarna S, Honigman S, Giladi N (2001) Gastric emptying time and gastric motility in patients with Parkinson’s disease. Mov Disord 16:1041–1047
Kurlan R, Rothfield KP, Woodward WR, Nutt JG, Miller C, Lichter D, Shoulson I (1988) Erratic gastric emptying of levodopa may cause “random” fluctuations of parkinsonian mobility. Neurology 38:419–421
van Wamelen DJ, Grigoriou S, Chaudhuri KR, Odin P (2018) Continuous drug delivery aiming continuous dopaminergic stimulation in Parkinson’s disease. J Parkinsons Dis 8:S65–S72
Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR, Group NV (2011) The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord 26:399–406
Hayes MT (2019) Parkinson’s disease and Parkinsonism. Am J Med 132:802–807
Song Z, Zhang J, Xue T, Yang Y, Wu D, Chen Z, You W, Wang Z (2021) Different catechol-o-methyl transferase inhibitors in Parkinson’s disease: a bayesian network meta-analysis. Front Neurol 12:707723
Davis KL, Edin HM, Allen JK (2010) Prevalence and cost of medication nonadherence in Parkinson’s disease: evidence from administrative claims data. Mov Disord 25:474–480
Wimmer BC, Bell JS, Fastbom J, Wiese MD, Johnell K (2016) Medication regimen complexity and polypharmacy as factors associated with all-cause mortality in older people: a population-based cohort study. Ann Pharmacother 50:89–95
Choi J, Horner KA (2022) Dopamine agonists. In: StatPearls. Treasure Island
Haasio K (2010) Toxicology and safety of COMT inhibitors. Int Rev Neurobiol 95:163–189
Feldmann F, Zipprich HM, Witte OW, Prell T (2020) Self-reported nonadherence predicts changes of medication after discharge from hospital in people with Parkinson’s disease. Parkinsons Dis 2020:4315489
Mendorf S, Witte OW, Grosskreutz J, Zipprich HM, Prell T (2020) What predicts different kinds of nonadherent behavior in elderly people with Parkinson’s disease? Front Med (Lausanne) 7:103
Mendorf S, Witte OW, Zipprich H, Prell T (2020) Association between nonmotor symptoms and nonadherence to medication in Parkinson’s disease. Front Neurol 11:551696
Cereda E, Cilia R, Klersy C, Canesi M, Zecchinelli AL, Mariani CB, Tesei S, Sacilotto G, Meucci N, Zini M, Isaias IU, Cassani E, Goldwurm S, Barichella M, Pezzoli G (2014) Swallowing disturbances in Parkinson’s disease: a multivariate analysis of contributing factors. Parkinsonism Relat Disord 20:1382–1387
Daley DJ, Myint PK, Gray RJ, Deane KH (2012) Systematic review on factors associated with medication non-adherence in Parkinson’s disease. Parkinsonism Relat Disord 18:1053–1061
Kruger R, Lingor P, Doskas T, Henselmans JML, Danielsen EH, de Fabregues O, Stefani A, Sensken SC, Parra JC, Onuk K, Yegin A, Antonini A (2017) An observational study of the effect of levodopa–carbidopa intestinal gel on activities of daily living and quality of life in advanced Parkinson’s disease patients. Adv Ther 34:1741–1752
Standaert DG, Rodriguez RL, Slevin JT, Lobatz M, Eaton S, Chatamra K, Facheris MF, Hall C, Sail K, Jalundhwala YJ, Benesh J (2017) Effect of levodopa–carbidopa intestinal gel on non-motor symptoms in patients with advanced Parkinson’s disease. Mov Disord Clin Pract 4:829–837
Fabbri M, Zibetti M, Calandra-Buonaura G, Contin M, Sambati L, Mohamed S, Romagnolo A, Berchialla P, Imbalzano G, Giannini G, Rizzone MG, Artusi CA, Cortelli P, Lopiano L (2020) Levodopa/carbidopa intestinal gel long-term outcome in Parkinson’s disease: focus on dyskinesia. Mov Disord Clin Pract 7:930–939
Fernandez HH, Standaert DG, Hauser RA, Lang AE, Fung VS, Klostermann F, Lew MF, Odin P, Steiger M, Yakupov EZ, Chouinard S, Suchowersky O, Dubow J, Hall CM, Chatamra K, Robieson WZ, Benesh JA, Espay AJ (2015) Levodopa–carbidopa intestinal gel in advanced Parkinson’s disease: final 12-month, open-label results. Mov Disord 30:500–509
Olanow CW, Kieburtz K, Odin P, Espay AJ, Standaert DG, Fernandez HH, Vanagunas A, Othman AA, Widnell KL, Robieson WZ, Pritchett Y, Chatamra K, Benesh J, Lenz RA, Antonini A, Group LHS (2014) Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol 13:141–149
Slevin JT, Fernandez HH, Zadikoff C, Hall C, Eaton S, Dubow J, Chatamra K, Benesh J (2015) Long-term safety and maintenance of efficacy of levodopa–carbidopa intestinal gel: an open-label extension of the double-blind pivotal study in advanced Parkinson’s disease patients. J Parkinsons Dis 5:165–174
Wirdefeldt K, Odin P, Nyholm D (2016) Levodopa–carbidopa intestinal gel in patients with Parkinson’s disease: a systematic review. CNS Drugs 30:381–404
Nyholm D, Nilsson Remahl AI, Dizdar N, Constantinescu R, Holmberg B, Jansson R, Aquilonius SM, Askmark H (2005) Duodenal levodopa infusion monotherapy vs oral polypharmacy in advanced Parkinson disease. Neurology 64:216–223
Antonini A, Pahwa R, Odin P, Isaacson SH, Merola A, Wang L, Kandukuri PL, Alobaidi A, Yan CH, Bao Y, Zadikoff C, Parra JC, Bergmann L, Chaudhuri KR (2022) Comparative effectiveness of device-aided therapies on quality of life and off-time in advanced Parkinson’s Disease: a systematic review and Bayesian Network meta-analysis. CNS Drugs 36:1269–1283
Nijhuis FAP, Esselink R, de Bie RMA, Groenewoud H, Bloem BR, Post B, Meinders MJ (2021) Translating evidence to advanced parkinson’s disease patients: a systematic review and meta-analysis. Mov Disord 36:1293–1307
Liu XD, Bao Y, Liu GJ (2019) Comparison between levodopa–carbidopa intestinal gel infusion and subthalamic nucleus deep-brain stimulation for advanced Parkinson’s disease: a systematic review and meta-analysis. Front Neurol 10:934
Chaudhuri KR, Pickard AS, Alobaidi A, Jalundhwala YJ, Kandukuri PL, Bao Y, Sus J, Jones G, Ridley C, Oddsdottir J, Najle-Rahim S, Madin-Warburton M, Xu W, Schrag A (2022) The cost effectiveness of levodopa–carbidopa intestinal gel in the treatment of advanced Parkinson’s disease in England. Pharmacoeconomics 40:559–574
Aldred J, Anca-Herschkovitsch M, Antonini A, Bajenaru O, Bergmann L, Bourgeois P, Cubo E, Davis TL, Iansek R, Kovacs N, Kukreja P, Onuk K, Pontieri FE, Robieson W, Siddiqui MS, Simu M, Standaert DG, Chaudhuri KR (2020) Application of the “5-2-1” screening criteria in advanced Parkinson’s disease: interim analysis of DUOGLOBE. Neurodegener Dis Manag 10:309–323
Poewe W, Bergmann L, Kukreja P, Robieson WZ, Antonini A (2019) Levodopa–carbidopa intestinal gel monotherapy: GLORIA registry demographics, efficacy, and safety. J Parkinsons Dis 9:531–541
Chaudhuri KR, Kovács N, Pontieri F, Aldred J, Bourgeois P, Davis T, Cubo E, Anca-Herschkovitsch M, Iansek R, Siddiqui M, Simu M, Bergmann L, Kukreja P, Ladhani O, Jia J, Standaert D (2022) P1.272 long-term motor and non-motor symptom benefits in patients with advanced Parkinson’s disease treated with levodopa–carbidopa intestinal gel: final analysis of the 36-month DUOGLOBE real-world multinational observational study. In: American Academy of Neurology. Seattle, Washington, USA
De Fabregues O, Dot J, Abu-Suboh M, Hernandez-Vara J, Ferre A, Romero O, Ibarria M, Seoane JL, Raguer N, Puiggros C, Gomez MR, Quintana M, Armengol JR, Alvarez-Sabin J (2017) Long-term safety and effectiveness of levodopa–carbidopa intestinal gel infusion. Brain Behav 7:e00758
Fasano A, Gurevich T, Jech R, Kovacs N, Svenningsson P, Szasz J, Parra JC, Bergmann L, Johnson A, Sanchez-Solino O, Tang Z, Vela-Desojo L (2021) Concomitant medication usage with levodopa–carbidopa intestinal gel: results from the COSMOS study. Mov Disord 36:1853–1862
Parkinson's Disease Composite scale. https://www.epda.eu.com/get-involved/the-parkinsons-disease-composite-scale/#:~:text=Developed%20by%20the%20My%20PD,with%20Parkinson's%20in%20a%20timely, Accessed 5 Oct 2022.
Deuschl G, Antonini A, Costa J, Smilowska K, Berg D, Corvol JC, Fabbrini G, Ferreira J, Foltynie T, Mir P, Schrag A, Seppi K, Taba P, Ruzicka E, Selikhova M, Henschke N, Villanueva G, Moro E (2022) European academy of neurology/movement disorder society-European section guideline on the treatment of Parkinson’s disease: I. Invasive therapies. Mov Disord 37:1360–1374
Burack M, Aldred J, Zadikoff C, Vanagunas A, Klos K, Bilir B, Fernandez HH, Standaert DG (2018) Implementing levodopa–carbidopa intestinal gel for Parkinson disease: insights from US Practitioners. Mov Disord Clin Pract 5:383–393
Catalan MJ, Antonini A, Calopa M, Bajenaru O, de Fabregues O, Minguez-Castellanos A, Odin P, Garcia-Moreno JM, Pedersen SW, Pirtosek Z, Kulisevsky J (2017) Can suitable candidates for levodopa/carbidopa intestinal gel therapy be identified using current evidence? eNeurol Sci 8:44–53
Merola A, Romagnolo A, Zibetti M, Bernardini A, Cocito D, Lopiano L (2016) Peripheral neuropathy associated with levodopa–carbidopa intestinal infusion: a long-term prospective assessment. Eur J Neurol 23:501–509
Pauls KAM, Toppila J, Koivu M, Eerola-Rautio J, Udd M, Pekkonen E (2021) Polyneuropathy monitoring in Parkinson’s disease patients treated with levodopa/carbidopa intestinal gel. Brain Behav 11:e2408
Kovács N, Szasz J, Vela-Desojo L, Svenningsson P, Femia S, Parra JC, Sanchez-Solino O, Bergmann L, Gurevich T, Fasano A (2022) Motor and nonmotor symptoms in patients treated with 24-hour daily levodopa–carbidopa intestinal gel infusion: analysis of the COmedication Study assessing Mono- and cOmbination therapy with levodopa–carbidopa inteStinal gel (COSMOS). Parkinsonism Relat Disord 105:139–144
Acknowledgements
Medical writing support was provided by Caryne Craige, Ph.D., of Fishawack Communications, Ltd, part of Fishawack Health, which was funded by AbbVie. AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized, individual, and trial-level data (analysis data sets), as well as other information (eg, protocols and Clinical Study Reports), so long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent, scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Funding
AbbVie sponsored the study; contributed to the design; participated in the collection, analysis, and interpretation of data; in writing, reviewing, and approval of the final version. No honoraria or payments were made for authorship.
Author information
Authors and Affiliations
Contributions
AF: Study design: conception, review and critique. Statistical analysis: review and critique. Manuscript: writing of the first draft, review and critique. RGR: Study design: review. Statistical analysis: review and critique. Manuscript: writing of the first draft, review and critique. TG: Study design: review. Statistical analysis: review and critique. Manuscript: writing of the first draft, review and critique. RJ: Study design: review. Statistical analysis: review and critique. Manuscript: writing of the first draft, review and critique. LB: Statistical analysis: design, execution, review and critique. Manuscript: writing of the first draft, review and critique. OSS: Study design: conception. Statistical analysis: design, execution. Manuscript: writing of the first draft, review and critique. JCP: Study design: conception. Statistical analysis: design, execution, review and critique. Manuscript: writing of the first draft, review and critique. MS: Study design: review. Statistical analysis: review and critique. Manuscript: writing of the first draft, review and critique.
Corresponding author
Ethics declarations
Conflicts of interest
The authors also declare that there are no conflicts of interest relevant to this work.
Ethical standards
All procedures were completed in accord with the ethical standards of the Independent Ethics Committees or Institutional Review Boards of the institution where data were collected.
Financial disclosures for the previous 12 months
Dr. Fasano received research support from Medtronic, Boston Scientific, University of Toronto, Michael J. Fox Foundation for Parkinson’s Research and Dystonia Medical Research Foundation, and honoraria from Abbott, Brainlab, UCB pharma, Medtronic, Novartis, Chiesi, Boston Scientific, AbbVie, Ipsen, and Sunovion for serving as a speaker. Dr. García-Ramos received honoraria for advisor, consultant, and/or speaker engagements from AbbVie, Allergan, Italfarmaco, Merz Pharma, Teva, UCB, and Zambon. Dr. Gurevich was a study investigator, received honoraria from AbbVie, Neuroderm, Medison, Truemed and Teva, research support from Parkinson’s Foundation, University Tel-Aviv and International Movement Disorders Society and travel support from AbbVie, Medison, Medtronic and Allergan. Dr. Jech received honoraria from AbbVie, Medtronic, Ipsen, Allergan, Cardion for consultancies and lectures. Dr. Simu received honoraria for lecturing at symposia and consultancy from AbbVie, AOP Orphan, Boehringer Ingelheim, Krka, Merck, Sanofi, Servier Pharma, Teva, and UCB Pharma. Drs. Bergmann, Sanchez-Soliño, Parra, are employees of AbbVie and may hold stock and/or stock options.
Ethical approval
All procedures of the COSMOS study (NCT03362879) were completed in accordance with the ethical standards of the Independent Ethics Committees or Institutional Review Boards of the institution where data were collected. Written informed consent was obtained by each patient or legal authorized representative prior to any data collection. The authors confirm that the approval of an institutional review board and informed consent were not required for this work, as it consists exclusively of secondary analysis of fully de-identified and publicly available data. We confirm that we have read the journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fasano, A., García-Ramos, R., Gurevich, T. et al. Levodopa–carbidopa intestinal gel in advanced Parkinson’s disease: long-term results from COSMOS. J Neurol 270, 2765–2775 (2023). https://doi.org/10.1007/s00415-023-11615-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-023-11615-3