Abstract
Background
Tuberculosis (TB) is the second most common cause of death due to a single infectious agent worldwide after COVID-19. Up to 15% of the cases are extrapulmonary, and if it is located in the central nervous system (CNS-TB), it presents high morbidity and mortality. Still, the global epidemiology of CNS-TB remains unknown.
Aim
To estimate the global prevalence and incidence of CNS-TB based on the available literature.
Methods
We systematically searched in MEDLINE, Cochrane Central, Scopus, and LILACS databases (April 2020) and included observational studies evaluating the epidemiology of CNS-TB. Two independent researchers selected and assessed the quality of the studies and extracted relevant data. We performed random-effects model meta-analysis of proportions to estimate the pooled prevalence. The protocol of this study was registered in PROSPERO (CRD 42018103946).
Results
We included 53 studies from 28 countries, representing 12,621 patients with CNS-TB. The prevalence of CNS-TB was 2 per 100,000 inhabitants. According to the clinical setting, the prevalence of CNS-TB represented the 13.91% of all cases of meningitis and 4.55% of all cases of TB. The mortality was calculated by tuberculous meningitis due to the lack of data of other presentation, and it rose up to 42.12% in hospitalized patients. The burden of countries’ TB, Human Development Index (HDI), and the prevalence of HIV were the most important prevalence moderators, especially in patients with TB. No data on incidence were found.
Conclusion
The prevalence and mortality of CNS-TB remain high, and TB meningitis is the most frequent presentation. The highest prevalence was reported in developing countries, and its main moderators were the countries’ HDI and HIV infection. Our study was limited by high heterogeneity, risk of bias, and potential data under registration from developing countries. The integration of CNS-TB early detection and management into national TB programs and population-based studies from developing countries are needed for better global estimation and response.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Currently, tuberculosis (TB) is the second most common cause of death for a single infectious agent worldwide after COVID-19 [58]. In 2020, due to the ongoing pandemic, the number of patients diagnosed and reported dropped approximately 1 million, resulting in increased mortality and burden, especially for low-income countries [57]. In 2015, the World Health Organization (WHO) implemented the End TB Strategy, whose principal targets were reducing the TB incidence and mortality rates by 20% and 35%, respectively, between 2015 and 2020 [56]. As stated by the latest Global Tuberculosis Report, less than one-third of the targets have been achieved. As a result, countries with a high TB burden are no longer on track to reach the 2020 targets [60].
Although the main manifestation of TB is pulmonary, cases of extrapulmonary TB (EPTB) are not rare, representing 15% of all TB reported cases [60]. Central nervous system TB (CNS-TB) is one of the most challenging clinical diagnoses, and it is associated with a high morbidity and mortality. A high risk of CNS-TB is described in children aged < 5 years and patients under immunosuppression [39, 40, 55]. CNS-TB is classified according to its anatomical localization (intracranial and spinal), tuberculous meningitis (TB meningitis) being the most common manifestation [28, 75].
Previous research has described the prevalence of CNS-TB according to presentation. A systematic review of patients in Africa reported that TB meningitis represented on average 15.3% of all patients with meningitis, associated with a higher mortality in settings with a prevalence higher than 20% [94]. This high mortality rate of TB meningitis was confirmed by a later systematic review of worldwide cases and reported as 22.8%, and the pooled risk of neurological sequelae was as high as 28.7% [93], representing a high burden.
Despite epidemiological data on TB, the worldwide prevalence of CNS-TB remains unknown. For this reason, this systematic review and meta-analysis summarizes the existing data on the prevalence of CNS-TB to provide accurate data for a more effective TB burden reduction strategy.
Methods
We conducted a systematic review of the literature with meta-analysis following the recommendation of the PRISMA guidelines [18]. The study protocol was registered in PROSPERO (number CRD42018103946). Additionally, we followed the Meta-analysis Of Observational Studies in Epidemiology checklist [82], which is found in Supplementary Table 1.
Literature search and study selection
We systematically searched MEDLINE, Scopus, Central Cochrane Library, and LILACS from inception to April 2020, using a search strategy with the following search terms: “Tuberculosis, Central Nervous System” OR “Tuberculoma, Intracranial” OR “Tuberculosis, Meningeal” AND “Prevalence” OR “Incidence” OR “Risk factors.” The complete search strategy for each database is available in Supplementary Table 2.
We included cross-sectional, case–control, and cohort studies. Other researches that assessed the prevalence of CNS-TB were also included. No restrictions in language or publication date were employed. We excluded animal studies, letters, news, case series with small sample size (< 30 participants), clinical trials, and literature reviews (unless original data were described). Duplicate records were removed using EndNote software before selection.
The study selection process was performed independently by two reviewers with a standard approach. For the first step, titles and abstracts were screened to identify potentially relevant articles for inclusion. After that, these relevant articles were full text assessed to evaluate their eligibility. Disagreement was resolved by a third reviewer.
Data extraction
Two independent researchers extracted the following information from each of the included studies into a Microsoft Excel sheet, in the cases of disagreements, a third reviewer was consulted. General variables were extracted as author, year of publication, year(s) of the study, country, national income defined by the World Bank [5], countries’ Human Development Index (HDI), burden of TB by country [59], and prevalence of HIV in the sample. For the denominators, we classified the groups as follows: (1) general population, the assessment came from the epidemiological surveillance of entire population, as a city or a country (not restricted to hospital), and (2) hospital setting, the total sample including the cases and the non-cases came from institutionalized patients. Additionally, specifications according to the type of diagnosis were made as follows: (a) TB, the sample of the study came from patients with TB (pulmonary or extrapulmonary); (b) meningitis, the sample came from patients diagnosed with meningitis; and (c) general hospitalization: patients admitted to general wards or internal medicine wards, also as the entire surveillance from a hospital.
For the numerators, we extracted tuberculous meningitis, Tuberculoma, and CNS-TB. The latter was the summation of the first two conditions unless the study reported an overall value of CNS-TB. Additionally, we extracted the number of CNS-TB-related deaths.
Risk of bias (quality) assessment
To assess the risk of bias in prevalence studies, we used the tool developed by Loney et al. [45]. This instrument contains eight items: a score of 1 (yes) or 0 (no) was assigned for each item, and then, the scores were summed to obtain the overall score, which ranged from 0 to 8. The items were adapted to our study as follows: (1) Study design and methods: One point was given if it was observational with census or random sampling. (2) Sampling frame: The recruitment list came from a census data. (3) Sampling size: The sample size was estimated with a 95% confidence interval (CI) or if it included 195 subjects. This value was estimated with the previous prevalence of TB meningitis [94] and with an online software (http://www.raosoft.com/samplesize.html). (4) Appropriate measurement: Bacteriological or molecular confirmation was used as an inclusion criterion of the study. (5) Unbiased measurement: It was yes if bacteriological confirmation was made through bacteriological or clinical records. (6) Response rate: The response rate was equal to or higher than 70%, or it was a national surveillance. (7) Results: Estimates were given with 95% CI and subgroup analysis. (8) Study subjects: The sociodemographic characteristics of study subjects were explained in detail: at least sex, age, and prevalence/incidence of TB in the country.
Subsequently, studies were classified as a low (7 and 8), moderate (5 and 6), or high (≤ 4) risk of bias. Two review authors independently assessed the study’s methodological quality. Disagreements were resolved by a third reviewer.
Statistical analyses
We calculated the pooled CNS-TB (TB meningitis and tuberculoma) prevalence and mortality rates with their corresponding 95% CI using a binomial model. Before pooling the estimates, we performed the Freeman–Tukey double arcsine transformation to stabilize the proportion variability [25]. Due to the expected between-study heterogeneity, the exploratory meta-analysis was performed by a prespecified random-effects model according to the DerSimonian and Laird method [19], and the CI calculation was based on the exact method [6]. Additionally, we computed a random-effects cumulative meta-analysis using the data collection year of each study to assess the trend across time of the pooled CNS-TB estimate.
Furthermore, subgroup meta-analyses were conducted to estimate the prevalence of meningitis TB according to the setting (general population or hospital), burden of the TB, country, and prevalence of HIV. We also conducted a sensitivity analysis to assess the robustness of the estimates by evaluating the influence of any individual study. We assessed heterogeneity using the I2 statistic, which estimates the percentage of total variation across studies due to true between-study differences rather than chance. We considered low heterogeneity when the I2 value was < 35%. Publication bias was checked by visual inspection of funnel plots and tested for significance using Egger’s regression test.
Moreover, we conducted univariate random-effects meta-regression to test potential between-study moderators [32]. As recommended by Thompson and Higgins [84], we performed meta-regression when at least eight studies were included in the meta-analysis [84]. We used the following criteria to select the best model: the residual percentage of heterogeneity, proportion of between-study variance explained (adjusted R2), and significant criterion of p < 0.05 per each moderator. We used the categorical outcomes from the subgroup and sensitivity analyses in addition to the continuous estimates of the HDI and prevalence of HIV. Subsequently, we performed Monte Carlo permutation tests (i.e., repeated random sampling) using 10,000 random iterations in order to account for the high false-positive rates that are associated with meta-regression models. The data were analyzed using Stata v15.0 software (College Station, TX).
Evidence certainty
The certain of evidence assessment was conducted as in our previously published articles [2, 65, 99]. We adapted the evaluation for prevalence meta-analysis using the five domains described in the GRADE handbook as follows: study limitations (the risk of bias of the primary studies), imprecision (appropriateness of the sample size and width of the CI), indirectness (generalizability of results), inconsistency (between-study heterogeneity), and publication bias [76]. Following the GRADE recommendations, the evidence was classified as of high, moderate, low, or very low certainty [4]. We manually adapted a summary of findings table (SoF) from the GRADE online tool (http://gradepro.org) to display the results.
Result
Characteristics of the studies
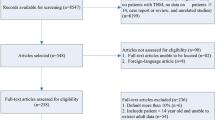
A total of 3,173 studies were identified in the search, and 58 were included after full text assessment [1, 3, 7, 9,10,11,12,13,14,15,16,17, 20,21,22, 24, 26, 30, 31, 34,35,36,37,38, 41, 44, 47,48,49,50,51,52,53,54, 62,63,64, 68,69,70,71,72,73,74, 77,78,79,80,81, 83, 86, 88,89,90, 92, 95, 97, 98]. The details of the selection process are presented in Fig. 1. Additionally, the list of excluded studies with reasons for the decision is presented in Supplementary Table 3.
We included studies from 31 countries located in the five continents worldwide. The most common country was South Africa (n = 10), followed by China (n = 6), India (n = 6), Spain (n = 3), the United States (n = 3), Ethiopia (n = 2), Iran (n = 2), and Peru (n = 2). The total number of subjects varied according to the denominator. We included 230,735,358 individuals from populational studies, 14,409 patients from general hospital wards, 202,265 patients with TB (pulmonary and/or extrapulmonary), and 17,1619 patients with meningitis. For the cases, we included 11,165 patients with TB meningitis, 162 patients with tuberculomas, and overall (included not specified cases) 11,515 patients with CNS-TB. The mean prevalence of HIV among the studies was 72%, ranging from 0.5 to 100%. The mean age of the participants of the included studies was 30 (range, 1.3 to 52) years, and there were 15 studies conducted only in children, 11 only in adults, and 24 in both. According to the countries’ income, there were 12 studies conducted in high-income economies, 23 in the upper middle-income economies, 20 in the lower-middle-income economies, and 3 in the low-income economies. The characteristics of the included studies are depicted in Supplementary Table 4.
Quality of studies
The risk of bias was high in 23 studies, moderate in 18 studies, and low in 17 studies. Most of the studies complied with item 1, except from Mbuh et al., which used a convenience sample [49]. The results were presented with 95% CIs only in 26 (49%) of 58 studies, and only 9 (15.5%) of 58 studies presented enough description of the studied population. The complete scores for each study are presented in Supplementary Table 5.
Prevalence of CNS-TB
According to the source of data, the prevalence of CNS-TB in the general population was 2.11 per 100,000 inhabitants (95% CI 0.81 to 4.03; I2, 99.91%), from 11 studies including 7979 cases and a population of 230 million inhabitants [1, 7, 13, 17, 20,21,22, 52, 63, 68, 70]. For that analysis, values were only available from patients with TB meningitis since no data were found for tuberculoma. In hospitalized patients (general hospitalization), the prevalence of CNS-TB was 8.64% (95% CI 5.34–12.55; I2, 97.10%) based on 13 studies (14,409 patients and 448 cases) [15, 62, 64, 73, 74, 77,78,79,80,81, 92, 95, 98]. On the other hand, the prevalence of CNS-TB was 13.91% of all patients with meningitis (95% CI 10.40–17.81; I2, 99.70%; 33 studies, 171,619 patients, and 6460 cases) [9, 11,12,13,14,15,16, 20,21,22, 26, 30, 31, 34,35,36,37,38, 41, 44, 47,48,49,50,51,52, 54, 70, 79, 83, 86, 88], and it represented the 4.55% of patients with TB (95% CI 2.63–6.97; I2, 98.81%; 11 studies, 202,265 patients, and 4043 cases) [7, 11, 17, 63, 68, 69, 71, 72, 90, 92, 98]. The forest plots for these analyses are presented in Fig. 2.
Forest plots of the prevalence of CNS-TB. a CNS-TB in the general population, b CNS-TB in general hospitalized patients, c CNS-TB in patients with meningitis, and d CNS-TB in patients with TB. ES Effect Size (prevalence estimates), CI Confidence Interval, %W primary studies’ relative weights, I2 estimate of heterogeneity, p p -value of heterogeneity assessment
Prevalence of TB meningitis
The overall population-based estimate was the same as for CNS-TB because only TB meningitis was reported in populational studies. The prevalence in general hospitalization was 7.40% (95% CI 4.50–10.91; I2, 96.77%), from 12 studies including 419 cases and 14,309 total patients [15, 62, 64, 73, 77,78,79,80,81, 92, 95, 97]. In patients with meningitis, TB meningitis represented the 14.63% (95% CI 10.95–18.73; I2, 99.71%) calculated from 32 studies including 169,538 total patients and 6322 cases [9, 11,12,13,14,15,16, 20,21,22, 24, 26, 30, 31, 34,35,36,37,38, 41, 44, 47,48,49, 51, 52, 54, 70, 79, 83, 86, 88]. Finally, in patients with TB, TB meningitis represented the 3.67% (95% CI 2.16–5.56; I2, 99.71%), from 10 studies including 201,223 total patients and 3875 cases [7, 11, 17, 63, 68, 69, 71, 72, 92, 98]. The forest plots for these analyses are depicted by Fig. 3.
Forest plots of the prevalence of TB meningitis. a TB meningitis in general hospitalized patients, b TB meningitis in patients with TB, and c TB meningitis in patients with meningitis. ES: Effect Size (prevalence estimates), CI Confidence Interval, %W primary studies’ relative weights, I2 estimate of heterogeneity, p p value of heterogeneity assessment
Prevalence of tuberculoma
The pooled prevalence of tuberculoma was not possible to calculate according to setting because there was only one study for the general population [63]. In patients with TB, tuberculoma represented the 0.52% (95% CI 0.05–1.32; I2, 0.00%), from 2 studies including 675 patients and 4 cases [11, 63], while in patients with meningitis, it represented 0.156% (95% CI 0.130–0.186; I2, 0.00%), from 2 studies including 81,841 patients and 137 cases [37, 50]. The forest plots for these analyses are displayed in Fig. 4.
Mortality
Mortality was estimated only by the values of TB meningitis due to the lack of data. The mortality of TB meningitis in general hospitalized patients was 42.12% (95% CI 26.46–58.53; I2, 72.53%), from 6 studies including 663 patients and 265 fatalities [15, 79, 81, 92, 95, 97]. In patients with meningitis, the mortality of TB meningitis was 41.06% (95% CI 29.39–53.20; I2, 75.42%), from 10 studies including 432 patients and 164 fatalities [9,10,11, 15, 79, 81, 83, 92, 95, 97]. Cumulative meta-analysis confirmed that these estimates were as high as 40% since the first report in 1996. The plots for these analyses are shown in Supplementary Figs. 1,2,3.
Subgroup analysis
In CNS-TB
According to setting in patients with TB, the prevalence was higher in studies from hospitalized patients than from the general population (6.49 [95% CI 2.03–13.17] vs. 1.77 [95% CI 1.71–1.83]). According to the countries’ TB burden, it was higher in countries with a high TB burden in the general population and hospitalized patients. Finally, the analysis by the prevalence of HIV in the sample showed that the prevalence of CNS-TB was higher in samples with a high prevalence of HIV in hospitalized patients but not in the general population. A summary of the estimates is presented in Table 1. The forest plots of the subanalyzes are displayed in Supplementary Figs. 4,5,6,7,8,9,10,11,12,13.
In TB meningitis
According to countries’ TB burden, in countries with a higher burden, the prevalence was higher in hospitalized patients and patients with TB. In the analysis by the prevalence of HIV in the sample, the prevalence of TB meningitis was higher in settings with a higher prevalence of HIV for hospitalized patients. A summary of the estimates is presented in Table 2. The forest plots of the subanalyzes are displayed in Supplementary Figs. 14,15,16,17,18,19,20,21.
Publication bias
For CNS-TB, the visual inspection of the funnel plots showed asymmetry in the analysis of hospitalized patients and patients with meningitis, which was corroborated by the Egger test resulting p = 0.008 and p = 0.008, respectively. The funnel plots are presented in Supplementary Figs. 22,23,24,25.
Similarly, for TB meningitis, the visual inspection of the funnel plots showed asymmetry, which was corroborated in the analyses of hospitalized patients (Egger test, p = 0.017) and patients with meningitis (Egger test, p = 0.007). The funnel plots are presented in Supplementary Figs. 26,27,28,29.
Meta-regression
The prevalence of HIV in study sample was positively correlated with the prevalence of CNS-TB and TB meningitis. Likewise, the study design (retrospective) was positively associated with both the prevalences of CNS-TB and TB meningitis, and the countries’ burden of TB was also positively correlated with the prevalence of CNS-TB. Furthermore, in patients with TB, the countries’ HDI was negatively correlated with the prevalences of CNS-TB and TB meningitis. The estimates are presented in Table 3.
Evidence certainty
We judged the certainty of the evidence of the meta-analyses of CNS-TB and its subtypes. For CNS-TB in the general population we found a moderate level of certainty. We started the evaluation from a high level of certainty since only population-based studies were included. Then, we downgraded the evidence one level due to imprecision since the CI was wide. The rest of the meta-analyses presented a very low level of evidence. Details of the process are presented in Table 4.
Discussion
In this systematic review, we found that the estimated global prevalence of CNS-TB in the general population is 2 cases per 100,000 inhabitants. Additionally, we found a prevalence of 8% in hospitalized patients. The CNS-TB morbidity was influenced mainly by country’s TB burden, HDI, and prevalence of HIV. Furthermore, we calculated a pooled mortality of approximately 40% in hospitalized patients, which reflects the clinical challenge of these conditions. These estimates are mainly based only on TB meningitis data, which is the most important clinical presentation of CNS-TB potentially due to contiguous or hematologic dissemination, most frequently occurring in infants [100]. To the best of our knowledge, no previous systematic reviews have quantitatively addressed this question.
The most common reported presentation is TB meningitis (ranging from 3 to 14%). Tuberculoma was reported in few studies with a frequency of < 1%. We found a prevalence of TB meningitis in patients with meningitis of 14.63%, and this estimate also came exclusively from high and upper-middle-income countries with low TB burden; therefore, it is possible that our calculation is underestimated due to the lack of data from low-income countries. We were unable to identified other populational meta-analysis of the prevalence of TB meningitis or any other CNS-TB presentation; however, a previous meta-analysis of African studies that found a prevalence of TB meningitis in patients with meningitis was 15.3% [94]. Although very similar, this could suggest higher values from low- and low-middle-income countries. Additionally, according to the WHO annual report on TB, the scenario of underreport and neglect of treatment had increased since the COVID-19 pandemic [57] and could affect future epidemiological estimation of worldwide CNS-TB cases.
The pooled frequency of CNS-TB in hospitalized patients is approximately 9% (14% in patients with meningitis and 5% in patients with TB), which is significantly higher than in the general population. This could be explained by the fact that CNS-TB is a disease of hospital diagnosis and treatment; hence, the proportion would be higher in using that setting. Still, more surveillance reports of general population are needed, especially from countries where TB produces a high burden and subacute and chronic CNS-TB could pass underdiagnosed or misdiagnosed.
The estimated mortality of CNS-TB is approximately 42% in hospitalized patients, which is calculated based on meningitis-related deaths. After a cumulative analysis, we found that these estimates are still higher (> 40%) since the first epidemiological reports in 1996. This value is higher than the global estimate previous reported (22.8%) [93] but smaller than the estimation from African countries (60%) [94]. Previous studies have reported a two times higher mortality of CNS-TB cases than that of other EPTB; thus, CNS-TB is the most lethal clinical presentation of this infection [22]. In addition, El Sahly et al. [22] reported that the main risk factors for mortality are older age, other extrapulmonary site infection, and hydrocephalus. The current management approaches to reduce mortality, apart from the TB-specific therapy, include steroids to reduce neuroinflammation; nonetheless, the evidence is heterogeneous, especially for long-term outcomes and neurological sequelae [85, 87]. Considering that hydrocephalus is associated with a higher mortality, close monitoring of this complication and potential lumbar puncture approach (i.e., frequent and controlled lumbar punctures similar to the one used in the management of cryptococcal meningitis) could be included in the patient care. However, the validation of the best management protocol requires further studies.
We found that the main moderators were the HDI and country’s burden of TB, specifically for the prevalence of CNS-TB in patients with TB. The economic growth of a country as an independent marker does not seem to be correlated with the prevalence of TB since many upper-middle economies still have a high burden of TB (i.e. China, Peru, and South Africa) just as countries with lower-middle and low incomes [59]. The HDI is a measure that represents the individual’s capacity of consumption and, hence, their standard of living [66]. Still, that concept is limited, and accordingly, we found a weak correlation in the meta-regression. Hence, other metrics related to describe health system efficiencies could be used to understand the populational determinants of CNS-TB, such as coverage, countries’ overall mortality, economics in health, and user satisfaction. These metrics can serve as an indirect measure of the countries’ ability to implement public health policies designed for reducing TB [42]. However, the level of inequities, normalization of corruption at different levels of structure, and quality of the care provided by public or private sectors in the country play a significant role [23, 27, 67]. Therefore, reducing the burden of TB requires a joint effort of economic investment, healthcare policies, and social interventions [29].
The role of surveillance of EPTB (including CNS-TB) is crucial for controlling the prevalence of active cases of TB; however, most of the largest preventive programs—the majority in countries with a high TB burden—provide active monitoring almost exclusively for pulmonary TB [46]. Additional interventions could be included to address the most important risk factors for EPTB as clinical HIV status, patients receiving immunological treatment, lung cancer, and diagnosis of diabetes mellitus [8, 96]. Taking into consideration those vulnerable groups, the early identification of latent TB and role of prophylactic treatment at that disease stage are interventions to be prioritized, being as accessible as oral isoniazid [8].
The sociodemographic factors, such as being young, female sex, from a low-resource setting, and non-white ethnicity, including other inequities should be part of the core target for interventions, especially in developing countries [8]. In developed countries, most of the patients with TB are migrants (including those with EPTB) [33]. Therefore, focused interventions designed for migrants and other high risk groups (as refugees) that cover the surveillance of EPTB and latent TB are providing excellent results and could serve as a model for other countries [43, 91].
Finally, the prevalence of HIV is a moderator in the prevalence of CNS-TB in hospitalized patients, which suggests that the comorbidity increases the prevalence of CNS-TB only in hospitalized and possible uncontrolled HIV cases. HIV plays a pivotal role in the burden of TB, being historically higher in sub-Saharan African countries and low-income settings but recently also increased in more developed areas of the world [61].
Limitations and strengths
The main limitations of our analysis are as follows: (a) lack of information in several regions of the world, especially in low-income countries, (b) lack of studies for other presentations of CNS-TB (tuberculoma) and with information of other denominators (general population) to estimate the population prevalence of the disease, (c) lack of prospective cohort studies, and (d) heterogeneity of the prevalence estimates among the included studies. The findings were based on demographic statistics, which may not be accurate for several parts of the world, especially for extreme age groups or due to premature death of cases without defining the diagnosis. Finally, we assume that the specific prevalence would remain constant over time, but changes in risk exposure can increase or decrease the prevalence over time, just as specific therapies, socioeconomic improvements, and healthcare can estimate the prevalence.
On the other hand, given the importance for public health and for decision-making on the distribution of resources, this meta-analysis provides valuable information that can be useful for health policies in the regions and countries reported.
Conclusions
The global prevalence of CNS-TB in the general population is 2 cases per 100,000 inhabitants, based mainly on TB meningitis cases from high- and upper-middle-income countries with a low TB burden. In hospitalized patients, it is present in approximately 9%. This frequency is around 14% in patients with meningitis and 5% in patients with TB. Tuberculoma was reported in few studies with a frequency of < 1%. The estimated mortality of CNS-TB was approximately 42% in hospitalized patients (based on meningitis-related deaths), which remains high since the early epidemiological reports in 1996. The main moderator factors were the HDI, country’s burden of TB, and prevalence of HIV. The integration of CNS-TB early detection and management into national TB programs and population-based studies from developing countries are needed for better global estimation and response.
Availability of data and material
Additional information is shared in the Supplementary Material. For more details, please contact the corresponding author.
Code availability
Not applicable.
References
Acevedo-Mendoza WF, Buitrago Gomez DP, Atehortua-Otero MA, Paez MA, Jimenez-Rincon M, Lagos-Grisales GJ, Rodriguez-Morales AJ (2017) Influence of socio-economic inequality measured by the Gini coefficient on meningitis incidence caused by Mycobacterium tuberculosis and Haemophilus influenzae in Colombia, 2008–2011. Le infezioni in medicina : rivista periodica di eziologia, epidemiologia, diagnostica, clinica e terapia delle patologie infettive 25:8–12
Alva-Díaz C, Navarro-Flores A, Rivera-Torrejon O, Huerta-Rosario A, Molina RA, Velásquez-Rimachi V, Morán-Mariños C, Farroñay C, Pacheco-Mendoza J, Metcalf T, Burneo JG, Pacheco-Barrios K (2021) Prevalence and incidence of epilepsy in Latin America and the Caribbean: a systematic review and meta-analysis of population-based studies. Epilepsia 62:984–996
Asselman V, Thienemann F, Pepper DJ, Boulle A, Wilkinson RJ, Meintjes G, Marais S (2010) Central nervous system disorders after starting antiretroviral therapy in South Africa. AIDS (London, England) 24:2871–2876
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH (2011) GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 64:401–406
Bank W (2021) World Bank Country and Lending Groups—World Bank Data Help Desk.
Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T (2013) Meta-analysis of prevalence. J Epidemiol Community Health 67:974
Bello-López JM, León-García G, Rojas-Bernabé A, Fernández-Sánchez V, García-Hernández O, Mancilla Rámirez J, Ibáñez-Cervantes G (2019) Morbidity trends and risk of tuberculosis: Mexico 2007–2017. Can Respir J 2019:8295261–8295261
Ben Ayed H, Koubaa M, Marrakchi C, Rekik K, Hammami F, et al. (2018) Extrapulmonary tuberculosis: update on the epidemiology, risk factors and prevention strategies. Int J Trop Dis 1(1):006
Bergemann A, Karstaedt AS (1996) The spectrum of meningitis in a population with high prevalence of HIV disease. QJM Monthly J Assoc Phys 89:499–504
Berhe T, Melkamu Y, Amare A (2012) The pattern and predictors of mortality of HIV/AIDS patients with neurologic manifestation in Ethiopia: a retrospective study. AIDS Res Ther 9:11
Bhagwan S, Naidoo K (2011) Aetiology, clinical presentation, and outcome of meningitis in patients coinfected with human immunodeficiency virus and tuberculosis. AIDS Res Treat 2011:180352
Bokade C, Gulhane R, Bagul A, Thakre S (2014) Acute febrile encephalopathy in children and predictors of mortality. J Clin Diag Res JCDR 8:Pc09-11
Britz E, Perovic O, von Mollendorf C, von Gottberg A, Iyaloo S, Quan V, Chetty V, Sriruttan C, Ismail NA, Nanoo A, Musekiwa A, Reddy C, Viljoen K, Cohen C, Govender NP (2016) The epidemiology of meningitis among adults in a South African province with a high HIV prevalence, 2009–2012. PLoS ONE 11:e0163036
Caliman-Sturdza OA, Mihalache D, Luca CM, Petrovici C, Dorobat CMJTM-SJ (2010) Utilitatea testului quantiferon TB Gold in diagnosticul meningitei tuberculoase la copil. Med-Surg J 114:1048–1052
Chapp-Jumbo E (2006) Neurologic infections in a Nigerian university teaching hospital. Afr Health Sci 6:55–58
Chaya S, Dangor Z, Solomon F, Nzenze S, Izu A, Madhi SJ, Disease L (2016) Incidence of tuberculosis meningitis in a high HIV prevalence setting: time-series analysis from 2006 to 2011. Int J Tuberc Lung Dis 20:1457–1462
Che D, Bitar DJ (2005) Situation épidémiologique de la tuberculose en France en 2003. Bull Acad Natl Med 189:1257–1270
David M, Alessandro L, Jennifer T, Douglas A, Altman DAG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. https://doi.org/10.1371/journal.pmed.1000097
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Ducomble T, Tolksdorf K, Karagiannis I, Hauer B, Brodhun B, Haas W, Fiebig L (2013) The burden of extrapulmonary and meningitis tuberculosis: an investigation of national surveillance data, Germany, 2002 to 2009. Eur Commun Dis Bull. https://doi.org/10.2807/ese.18.12.20436-en
Duque-Silva A, Robsky K, Flood J, Barry PMJP (2015) Risk factors for central nervous system tuberculosis. Pediatrics 136:e1276–e1284
El Sahly HM, Teeter LD, Pan X, Musser JM, Graviss EA (2007) Mortality associated with central nervous system tuberculosis. J Infect 55:502–509
Emadi M, Delavari S, Bayati M (2021) Global socioeconomic inequality in the burden of communicable and non-communicable diseases and injuries: an analysis on global burden of disease study 2019. BMC Public Health 21:1771
Ford H, Wright JJ, Health C (1994) Bacterial meningitis in Swaziland: an 18 month prospective study of its impact. J Epidemiol Community Health 48:276–280
Freeman MF, Tukey JW (1950) Transformations related to the angular and the square root. Ann Math Stat 1:607–611
Ganiem AR, Parwati I, Wisaksana R, van der Zanden A, van de Beek D, Sturm P, van der Ven A, Alisjahbana B, Brouwer AM, Kurniani N, de Gans J, van Crevel R (2009) The effect of HIV infection on adult meningitis in Indonesia: a prospective cohort study. AIDS (London, England) 23:2309–2316
García PJ (2019) Corruption in global health: the open secret. Lancet (London, England) 394:2119–2124
Garg RK (1999) Tuberculosis of the central nervous system. Postgrad Med J 75:133–140
Grobusch MP, Kapata N (2018) Global burden of tuberculosis: where we are and what to do. Lancet Infect Dis 18:1291–1293
Gupta S, Shah D, Shah I (2009) Neurological disorders in HIV-infected children in India. Ann Tropical Paediatr 29:177–181
Hakim JG, Gangaidzo IT, Heyderman RS, Mielke J, Mushangi E, Taziwa A, Robertson VJ, Musvaire P, Mason PRJA (2000) Impact of HIV infection on meningitis in Harare, Zimbabwe: a prospective study of 406 predominantly adult patients. AIDS 14:1401–1407
Harbord RM, Higgins JPT (2008) Meta-regression in Stata. Stata J 8:493–519
Hayward SE, Rustage K, Nellums LB, van der Werf MJ, Noori T, Boccia D, Friedland JS, Hargreaves S (2021) Extrapulmonary tuberculosis among migrants in Europe, 1995 to 2017. Clin Microbiol Infect 27:1347.e1341-1347.e1347
Helbok R, Pongpakdee S, Beer R, Lackner P, Vejjajiva A, Schmutzhard E (2008) Chronic meningitis in Thailand clinical characteristics, laboratory data and outcome with specific reference to tuberculosis and cryptococcosis. LIPPINCOTT WILLIAMS & WILKINS TWO COMMERCE SQ, PHILADELPHIA, pp A101–A102
Hui A, Ng K, Tong P, Mok V, Chow K, Wu A, Wong L (2005) Bacterial meningitis in Hong Kong: 10-years’ experience. Clin Neurol Neurosurg 107:366–370
Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS (2010) Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis 10:1–6
Jeri F, Castañeda MA, Yalán F, Heinicke H (1999) Tuberculosis del sistema nervioso. Observaciones sobre 1360 pacientes estudiados en tres centros asistenciales de Lima. Rev Neuropsiquiatr 62:28–50
Jowi J, Mativo P, Musoke S (2007) Clinical and laboratory characteristics of hospitalised patients with neurological manifestations of HIV/AIDS at the Nairobi hospital. East Afr Med J 84:67–76
Joseph RZ (2018) Tuberculosis of the central nervous system. Contin Lifelong Learn Neurol 24(5):1422–1438
Kiran T, Das M, Dooley KE, Gupta A (2018) The Global Neurological Burden of Tuberculosis. Semin Neurol 38(2):226–237
Kozko VM, Bondarenko AV, Gavrylov AV, Shevchenko OS, Gargin VV (2017) Pathomorphological peculiarities of tuberculous meningoencephalitis associated with HIV infection. Intervent Medi Appl Sci 9:144–149
Kruk ME, Gage AD, Arsenault C, Jordan K, Leslie HH, Roder-DeWan S, Adeyi O, Barker P, Daelmans B, Doubova SV, English M, García-Elorrio E, Guanais F, Gureje O, Hirschhorn LR, Jiang L, Kelley E, Lemango ET, Liljestrand J, Malata A, Marchant T, Matsoso MP, Meara JG, Mohanan M, Ndiaye Y, Norheim OF, Reddy KS, Rowe AK, Salomon JA, Thapa G, Twum-Danso NAY, Pate M (2018) High-quality health systems in the Sustainable Development Goals era: time for a revolution. Lancet Glob Health 6:e1196–e1252
Kunin M, Timlin M, Lemoh C, Sheffield DA, Russo A, Hazara S, McBride J (2022) Improving screening and management of latent tuberculosis infection: development and evaluation of latent tuberculosis infection primary care model. BMC Infect Dis 22:49
Liew F, Ang LW, Cutter J, James L, Goh KT (2010) Evaluation on the effectiveness of the national childhood immunisation programme in Singapore, 1982–2007. Ann Acad Med Singapore 39:532–510
Loney PL, Chambers LW, Bennett KJ, Roberts JG, Stratford PW (1998) Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can 19:170–176
Lönnroth K, Migliori GB, Abubakar I, D’Ambrosio L, De Vries G, Diel R, Douglas P, Falzon D, Gaudreau M-A, Goletti D (2015) Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J 45:928–952
Marais S, Meintjes G, Pepper DJ, Dodd LE, Schutz C, Ismail Z, Wilkinson KA, Wilkinson RJ (2013) Frequency, severity, and prediction of tuberculous meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis 56:450–460
Marais S, Pepper DJ, Schutz C, Wilkinson RJ, Meintjes G (2011) Presentation and outcome of tuberculous meningitis in a high HIV prevalence setting. PLoS ONE 6:e20077
Mbuh TP, Ane-Anyangwe I, Adeline W, Pokam BDT, Meriki HD, Mbacham WF (2019) Bacteriologically confirmed extra pulmonary tuberculosis and treatment outcome of patients consulted and treated under program conditions in the littoral region of Cameroon. BMC Pulm Med 19:1–7
Meshkini A, Shahzadi S, Alikhah H, Naghavi-Behzad M (2013) Role of stereotactic biopsy in histological diagnosis of multiple brain lesions. Asian J Neurosurg 8:69–73
Mihret W, Zenebe G, Bekele A, Abebe M, Wassie L, Yamuah LK, Woldemeskel D, Kassahun Y, Medhin G, Engers H, Aseffa A (2014) Chronic meningitis in immunocompromised adult Ethiopians visiting Tikur Anbessa Teaching Hospital and Ye’huleshet Clinic from 2003–2004. Ethiop Med J Suppl 1:43–48
Mitchell HK, Mokomane M, Leeme T, Tlhako N, Tsholo K, Ramodimoosi C, Dube B, Mokobela KO, Tawanana E, Chebani T (2019) Causes of pediatric meningitis in Botswana: results from a 16-year national meningitis audit. Pediatr Infect Dis J 38:906–911
Modi A, Atam V, Jain N, Gutch M, Verma R (2012) The etiological diagnosis and outcome in patients of acute febrile encephalopathy: a prospective observational study at tertiary care center. Neurol India 60:168
Moghtaderi A, Alavi-Naini R, Rashki S (2013) Cranial nerve palsy as a factor to differentiate tuberculous meningitis from acute bacterial meningitis. Acta Med Iran 11:113–118
Muzumdar D, Vedantam R, Muzumder D (2018) Tuberculosis of the central nervous system in children. Child’s Nerv Syst 34:1925–1935
Organization WH (2015) The end TB strategy 2015. World Health Assembly
Organization WH (2021) Global Tuberculosis Report 2021; WHO In, Geneva, Switzerland, 2021
Organization WH (2021) Tuberculosis deaths rise for the first time in more than a decade due to the COVID-19 pandemic. In:
Organization WH (2015) Use of high burden country lists for TB by WHO in the post-2015 era: Summary. In, Geneva
Organization WH World Health Statistics 2019: Monitoring health for the SDGs. WHO 2019
Pandey A, Galvani AP (2019) The global burden of HIV and prospects for control. Lancet HIV 6:e809–e811
Patel VB, Singh R, Connolly C, Kasprowicz V, Zumla A, Ndungu T, Dheda KJPo, (2010) Comparison of a clinical prediction rule and a LAM antigen-detection assay for the rapid diagnosis of TBM in a high HIV prevalence setting. PLoS ONE 5:e15664
Paulsrud C, Poulsen A, Vissing N, Andersen PH, Johansen IS, Nygaard UJID (2019) Think central nervous system tuberculosis, also in low-risk children: a Danish nationwide survey. Infect Dis 51:368–372
Per H, Unal E, Poyrazoglu HG, Ozdemir MA, Donmez H, Gumus H, Uzum K, Canpolat M, Akyildiz BN, Coskun A (2014) Childhood stroke: results of 130 children from a reference center in Central Anatolia, Turkey. Pediatr Neurol 50:595–600
Pinedo-Torres I, Flores-Fernández M, Yovera-Aldana M, Gutierrez-Ortiz C, Zegarra-Lizana P, Intimayta-Escalante C, Moran-Mariños C, Alva-Diaz C, Pacheco-Barrios K (2020) Prevalence of diabetes mellitus and its associated unfavorable outcomes in patients with acute respiratory syndromes due to coronaviruses infection: a systematic review and meta-analysis. Clin Med Insights Endocrinol Diabetes 13:1179551420962495
Programe UND (2020) Human Development Reports. In:
Reid MJA, Goosby E (2020) Improving quality is necessary to building a TB-free world: Lancet Commission on Tuberculosis. J Clin Tuberc Other Mycobact Dis 19:100156
Reinhard C, Paul WS, Mcauley JB (1997) Epidemiology of pediatric tuberculosis in Chicago, 1974 to 1994: a continuing public health problem. Am J Med Sci 313:336–340
Russell G, Merle C, Cooke G, Casas E, Silveira da Fonseca M, du Cros P (2013) Towards the WHO target of zero childhood tuberculosis deaths: an analysis of mortality in 13 locations in Africa and Asia. Int J Tuberc Lung Dis 17:1518–1523
Saeed N, Al Ansari H, Al Khawaja S, Nasser K, Al Yousef E (2016) Trend of bacterial meningitis in Bahrain from 1990 to 2013 and effect of introduction of new vaccines. East Mediterr Health J 22:175–182
Salgueiro Rodríguez M, González Barcala J, Zamarrón Sanz C, Pombo Pasin M, Ricoy Gabaldón J, Garazo P, Calvo Álvarez U, Perez Del Molino M, Antúnez López J, Durán Rivas J (2004) Tuberculosis en el área de Santiago de Compostela durante los años 1999, 2000, 2001 y 2002: Un estudio epidemiológico. An Med Interna. https://doi.org/10.4321/S0212-71992004000500003
Salgueiro Rodríguez M, Zamarrón C, González Barcala J, Vilas Iglesias A, Suárez Antelo J, Durán Rivas J, Pavón G, Vieites Pérez-Quintela M, Rodríguez Suárez J (2001) Estudio epidemiológico de la tuberculosis en el Área Sanitaria de Santiago de Compostela durante los años 1995, 1996, 1997 y 1998. An Med Interna. https://doi.org/10.4321/S0212-71992001000100005
Sanchez-Portocarrero J, Perez-Cecilia E, Jimenez-Escrig A, Martin-Rabadan P, Roca V, Yague MR, Romero-Vivas J, Palau E, Picazo J (1996) Tuberculous meningitis: clinical characteristics and comparison with cryptococcal meningitis in patients with human immunodeficiency virus infection. Arch Neurol 53:671–676
Satishchandra P, Nalini A, Gourie-Devi M, Khanna N, Santosh V, Ravi V, Desai A, Chandramuki A, Jayakumar P, Shankar S (2000) Profile of neurologic disorders associated with HIV/AIDS from Bangalore, south India (1989–96). Indian J Med Res 111:14–23
Schaller MA, Wicke F, Foerch C, Weidauer S (2019) Central nervous system tuberculosis: etiology, clinical manifestations and neuroradiological features. Clin Neuroradiol 29:3–18
Schunemann H (2008) GRADE handbook for grading quality of evidence and strength of recommendation. Version 3.2.http://www cc-ims net/gradepro
Shaikh Samiullah MH, Hanif G, Ghouri AA, Shaikh K (2010) Etiological patterns of stroke in young patients at a tertiary care hospital. Age 50(6):5622
Sharma SR, Hussain M, Habung H (2017) Neurological manifestations of HIV-AIDS at a tertiary care institute in North Eastern India. Neurol India 65:64
Silber E, Sonnenberg P, Ho KC, Koornhof HJ, Eintracht S, Morris L, Saffer D (1999) Meningitis in a community with a high prevalence of tuberculosis and HIV infection. J Neurol Sci 162:20–26
Singh R, Kaur M, Arora D (2011) Neurological complications in late-stage hospitalized patients with HIV disease. Ann Indian Acad Neurol 14:172–177
Soumare M, Seydi M, Ndour C, Fall N, Dieng Y, Sow A, Diop B (2005) Epidemiological, clinical, etiological features of neuromeningeal diseases at the Fann Hospital Infectious Diseases Clinic, Dakar (Senegal). Med Maladies Infect 35:383–389
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283:2008–2012
Sung RY, Senok AC, Ho A, Oppenheimer SJ, Davies DP (1997) Meningitis in Hong Kong children, with special reference to the infrequency of haemophilus and meningococcal infection. J Paediatr Child Health 33:296–299
Thompson SG, Higgins JP (2002) How should meta-regression analyses be undertaken and interpreted? Stat Med 21:1559–1573
Thwaites GE, Bang ND, Dung NH, Quy HT, Oanh DTT, Thoa NTC, Hien NQ, Thuc NT, Hai NN, Lan NTN (2004) Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med 351:1741–1751
Thwaites GE, Chau TT, Stepniewska K, Phu NH, Chuong LV, Sinh DX, White NJ, Parry CM, Farrar JJ (2002) Diagnosis of adult tuberculous meningitis by use of clinical and laboratory features. Lancet (London, England) 360:1287–1292
Thwaites GE, Macmullen-Price J, Chau TTH, Mai PP, Dung NT, Simmons CP, White NJ, Hien TT, Summers D, Farrar JJ (2007) Serial MRI to determine the effect of dexamethasone on the cerebral pathology of tuberculous meningitis: an observational study. Lancet Neurol 6:230–236
Tian L, Zhang Z, Sun ZY (2019) Pathogen analysis of central nervous system infections in a Chinese teaching hospital from 2012–2018: a laboratory-based retrospective study. Curr Med Sci 39:449–454
Tshimangani T, Pongo J, Mabiala JB, Yotebieng M, O’Brien NF (2018) Pediatric acute severe neurologic illness and injury in an urban and a rural hospital in the Democratic Republic of the Congo. Am J Trop Med Hygiene 98:1534
Vera CA, Patron-Ordoñez G, Verastegui-Diaz A, Mejia CR (2019) Factores sociodemográficos y fisiopatológicos asociados a la tuberculosis del sistema nervioso central en un Hospital Público de Lima-Perú, 2014–2017. Infect 23:155–160
Verrall AJ, Hill PC, Thorburn D, Maze M, Perumal L, Grimwade K, Thornley CN, Freeman J, Nisbet M, Blackmore TK (2020) Towards elimination of tuberculosis in New Zealand. N Z Med J 133:89–96
Watch V, Aipit J, Kote-Yarong T, Rero A, Bolnga JW, Lufele E, Laman M (2017) The burden of presumed tuberculosis in hospitalized children in a resource-limited setting in Papua New Guinea: a prospective observational study. Int Health 9:374–378
Wen L, Li M, Xu T, Yu X, Wang L, Li K (2019) Clinical features, outcomes and prognostic factors of tuberculous meningitis in adults worldwide: systematic review and meta-analysis. J Neurol 266:3009–3021
Woldeamanuel YW, Girma B (2014) A 43-year systematic review and meta-analysis: case-fatality and risk of death among adults with tuberculous meningitis in Africa. J Neurol 261:851–865
Xiao J, Gao G, Li Y, Zhang W, Tian Y, Huang Y, Su W, Han N, Yang D, Zhao H (2013) Spectrums of opportunistic infections and malignancies in HIV-infected patients in tertiary care hospital. China. Transl Lung Cancer Res 8:e75915
Xiong K, Sun W, He Y, Fan L (2021) Advances in molecular mechanisms of interaction between Mycobacterium tuberculosis and lung cancer: a narrative review. Transl Lung Cancer Res 10:4012–4026
Yang CD, Wang XD, Ye S, Gu YY, Bao CD, Wang Y, Chen SL (2007) Clinical features, prognostic and risk factors of central nervous system infections in patients with systemic lupus erythematosus. Clin Rheumatol 26:895–901
Yang J, Chen J, Yue J, Liu L, Han M, Wang H (2014) Relationship between human LTA4H polymorphisms and extra-pulmonary tuberculosis in an ethnic Han Chinese population in Eastern China. Tuberculosis 94:657–663
Yovera-Aldana M, Velásquez-Rimachi V, Huerta-Rosario A, More-Yupanqui MD, Osores-Flores M, Espinoza R, Gil-Olivares F, Quispe-Nolazco C, Quea-Vélez F, Morán-Mariños C, Pinedo-Torres I, Alva-Diaz C, Pacheco-Barrios K (2021) Prevalence and incidence of diabetic peripheral neuropathy in Latin America and the Caribbean: a systematic review and meta-analysis. PLoS ONE 16:e0251642
Zunt JR (2018) Tuberculosis of the central nervous system. Continuum (Minneapolis, Minn) 24:1422–1438
Funding
The present study was self-funded by the authors.
Author information
Authors and Affiliations
Contributions
N-FA: Conceptualization, data curation, data analysis, data visualization production, drafting of the manuscript, and supervision of data extraction. F-CJE: Conceptualization, protocol writing, data extraction, risk of bias assessment, and drafting of the manuscript. P-BN: Protocol writing, data extraction, risk of bias assessment, and drafting of the manuscript. SR: Data extraction and risk of bias assessment. P-BK: Conceptualization, protocol writing, data analysis, drafting of the manuscript, and supervision of all processes. All authors provided critical revisions to the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Ethical approval
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Navarro-Flores, A., Fernandez-Chinguel, J.E., Pacheco-Barrios, N. et al. Global morbidity and mortality of central nervous system tuberculosis: a systematic review and meta-analysis. J Neurol 269, 3482–3494 (2022). https://doi.org/10.1007/s00415-022-11052-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11052-8