Abstract
Background
Tuberculosis (TB)-associated mortality in South Africa remains high. This review aimed to systematically assess risk factors associated with death during TB treatment in South African patients.
Methods
We conducted a systematic review of TB research articles published between 2010 and 2018. We searched BioMed Central (BMC), PubMed®, EBSCOhost, Cochrane, and SCOPUS for publications between January 2010 and December 2018. Searches were conducted between August 2019 and October 2019. We included randomised control trials (RCTs), case control, cross sectional, retrospective, and prospective cohort studies where TB mortality was a primary endpoint and effect measure estimates were provided for risk factors for TB mortality during TB treatment. Due to heterogeneity in effect measures and risk factors evaluated, a formal meta-analysis of risk factors for TB mortality was not appropriate. A random effects meta-analysis was used to estimate case fatality ratios (CFRs) for all studies and for specific subgroups so that these could be compared. Quality assessments were performed using the Newcastle-Ottawa scale or the Cochrane Risk of Bias Tool.
Results
We identified 1995 titles for screening, 24 publications met our inclusion criteria (one cross-sectional study, 2 RCTs, and 21 cohort studies). Twenty-two studies reported on adults (n = 12561) and two were restricted to children < 15 years of age (n = 696). The CFR estimated for all studies was 26.4% (CI 18.1–34.7, n = 13257 ); 37.5% (CI 24.8-50.3, n = 5149) for drug-resistant (DR) TB; 12.5% (CI 1.1–23.9, n = 1935) for drug-susceptible (DS) TB; 15.6% (CI 8.1–23.2, n = 6173) for studies in which drug susceptibility was mixed or not specified; 21.3% (CI 15.3-27.3, n = 7375) for people living with HIV/AIDS (PLHIV); 19.2% (CI 7.7–30.7, n = 1691) in HIV-negative TB patients; and 6.8% (CI 4.9–8.7, n = 696) in paediatric studies. The main risk factors associated with TB mortality were HIV infection, prior TB treatment, DR-TB, and lower body weight at TB diagnosis.
Conclusions
In South Africa, overall mortality during TB treatment remains high, people with DR-TB have an elevated risk of mortality during TB treatment and interventions to mitigate high mortality are needed. In addition, better prospective data on TB mortality are needed, especially amongst vulnerable sub-populations including young children, adolescents, pregnant women, and people with co-morbidities other than HIV. Limitations included a lack of prospective studies and RCTs and a high degree of heterogeneity in risk factors and comparator variables.
Systematic review registration
The systematic review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under the registration number CRD42018108622. This study was funded by the Bill and Melinda Gates Foundation (Investment ID OPP1173131) via the South African TB Think Tank.
Similar content being viewed by others
Background
A tuberculosis (TB) death is defined as any death during an episode of TB, regardless of treatment or the underlying cause of death [1]. This may include death before or during TB treatment but does not include death after successful completion of antituberculosis treatment [1]. In 2021, the World Health Organization (WHO) estimated that 10.6 million people developed TB worldwide and approximately 1.6 million TB deaths occurred [2]. This included an estimated 304,000 incident cases of TB and 23,000 HIV-negative and 33,000 HIV-positive TB deaths in South Africa [2].
The End TB strategy aims to reduce the annual number of TB deaths (as reported in 2015) by 75% by 2025 and by 95% by 2035, for a reduction in TB CFR from 15% to 6.5% by 2025 [3]. Interim progress against these ambitious targets is measured by evaluating whether milestones have been reached within particular time frames [3]. By 2019, South Africa had reached milestones set for reducing TB incidence but not for reducing TB CFR [4], and a TB CFR of 19% was estimated for 2020 [2]. Although the proportion of all deaths due to TB in South Africa has declined from 6.5% in 2016 to 6% in 2018, TB remains the leading cause of death in South Africa [5].
In a systematic review of risk factors for mortality in people on TB treatment globally between 1966 and 2010, two distinct epidemics dependent on TB/HIV burden were described [6]. In high TB/HIV regions HIV positivity, the presence of atypical changes on X-ray, sputum smear-negative disease, advanced immunosuppression, and malnutrition were identified as risk factors for mortality. In settings with low TB/HIV prevalence, increased age, typical features of severe TB on chest radiograph, smear positivity and low socioeconomic status were identified as risk factors [6]. In South Africa, a country with a high burden of TB, HIV, and DR-TB, individual studies have identified co-infection with HIV [7,8,9,10,11,12]; failing to start treatment following a TB diagnosis [13]; undiagnosed TB (found at post mortem) [14, 15]; drug resistance [11, 16,17,18]; and co-morbidities like diabetes [19] as important risk factors for TB mortality. However, there is no comprehensive review of the risk of TB mortality or the relative importance of risk factors for TB mortality in South Africa. National TB policymakers have prioritised understanding TB mortality in South Africa and the analysis of data from 2010 onwards.
We aimed to identify risk factors for TB mortality during TB treatment in South Africa for patients with DS-TB, DR-TB, with and without HIV, and for adult and paediatric populations.
Methods
Identification and selection of papers
This systematic review is reported according to PRISMA guidelines (Additional file 1: PRISMA 2020 Checklist) [20,21,22]. An initial search using “Tuberculosis” AND [“treatment outcomes” OR “death” OR “mortality” OR “poor outcome”] AND [“risk factors” OR “determinants” OR “predictors” OR “contributing factors”] AND [“South Africa” OR “Southern Africa” OR “Sub-Saharan Africa” OR “Africa”] was run across BioMed Central (BMC), PubMed®, EBSCOhost, Cochrane, and SCOPUS for publications between January 2010 and December 2018. Searches were conducted between August 2019 and October 2019.
Titles were screened and duplicates identified and removed. Unique titles were reviewed, and studies excluded if they did not investigate risk factors for mortality in TB patients, were in vitro or animal studies, or were review, editorial, opinion, comment, response, or other non-data driven article-types. Concurrent work evaluating mortality using data from a comprehensive programmatic dataset of South African TB treatment registers was in progress at the time of the review [23, 24] and to prevent duplication of findings from the same data sources, studies based on the routine programmatic TB registers (ETR.Net or EDRWeb) were excluded from our review. During the abstract review process, two reviewers excluded further articles based on initial exclusion criteria used for screening titles: mortality not being a defined outcome for the study, a focus on all-cause mortality instead of mortality associated with TB, very narrow sampling criteria, or research designs other than clinical cohort, cross-sectional, or case comparison. We did not set any exclusion criteria for timing of death, before or during TB treatment. Studies were grouped for synthesis by subgroup and TB mortality risk factors to determine comparability.
Data abstraction and analysis
We conducted data abstraction via online survey using Research Electronic Data Capture (REDCap) [25]. Data included year of publication; TB drug susceptibility test (DST) pattern; eligible population; age groups; sampling strategy; sample size; definition of TB death or CFR; TB treatment and duration; risk factors for death including effect measures and 95% confidence intervals (CI); and the limitations of the study (full list presented in Additional file 1: Complete list of outcomes and variables for which data were sought). Each article was independently reviewed by two of 23 researchers, and any disagreement was resolved in discussion with the principal investigator.
Data extracted were tabulated and subgroups and comparability of risk factors assessed. Due to heterogeneity in effect measures and risk factors evaluated, a formal meta-analysis of risk factors for TB mortality was not appropriate. We conducted a random effects meta-analysis using the restricted maximum likelihood estimation method to establish CFRs for all studies and for specific subgroups to contribute to our understanding of mortality risk factors; however, this was not an original aim of the study. One author assessed the quality of evidence for case control and cohort studies using the Newcastle-Ottawa scale (NOS) [26] and used the Cochrane Risk of Bias Tool [27] to assess the quality of randomised control trials (RCTs).
Results
Literature search results
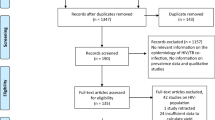
We identified 1995 titles for screening and 24 publications met our inclusion criteria (Fig. 1). Study designs included retrospective studies (n = 13), prospective cohort studies (n = 8), two RCTs and one case control study.
Methodological quality of studies included
Based on the NOS, most cohort studies (n = 21) rated well overall, as well as for selection criteria, comparability, and outcomes. However, in relation to loss to follow-up (LTFU), seven studies either reported attrition greater than 10% without demonstrating a lack of systematic difference between those followed and those LTFU or did not report the number of those LTFU (Additional file 1: Table 1). The two RCTs performed well overall and were rated as low risk, but a lack of blinding was a possible source of bias for both (Additional file 1: Table 2). The case control study scored six out of a maximum of eight points (Additional file 1: Table 3).
Study characteristics
There was heterogeneity in effect measures with risk factors reported as hazard ratios (HRs), incidence rate ratios (IRR), odds ratios (ORs), and risk ratios (RRs). Twelve studies reported exclusively on DR-TB, and this accounted for 39% of all patients (5149/13257). Of the three studies reporting exclusively on DS-TB, one was of miners and one of children. The remaining nine studies included four on patients with mixed DST patterns and five where the DST was not specified. Only two studies represented children, and these were restricted to very specific populations. The first was of children (defined as 0–13 years) with TB meningitis (TBM) [46] and the second of children (defined as 0–15 years) living with HIV but not on ART [49]. Nine of the studies were restricted to PLHIV and 14 of the remaining 15 studies included HIV as a variable. Only single studies evaluated factors like renal impairment [43], bilateral cavitary disease [28], and adverse events [35] (Table 1).
Reporting of TB diagnosis, treatment regimens and time to death across studies was inconsistent: all studies reported some element of laboratory confirmation, but this varied with the reporting of smear, culture, and GeneXpert MTB/RIF assay (Xpert; Cepheid, Sunnyvale, CA); 14 studies described treatment regimens; 13 the duration of treatment; 9 time from treatment initiation to death; 4 time from diagnosis to death; and 2 reported place of death (Table 2). The CFR estimated for all studies was 26.4% (CI 18.1–34.7, n = 13257); 37.5% (CI 24.8–50.3, n = 5149) for DR-TB; 12.5% (CI 1.1–23.9, n = 1935) for DS-TB; 15.6% (CI 8.1–23.2, n = 6173) for studies in which drug susceptibility was mixed or not specified and 6.8% (CI 4.9–8.7, n = 696) in paediatric studies. There was little difference in CFRs estimated for PLHIV (21.3%, CI 15.3–27.3, n = 7375) and HIV-negative TB patients (19.2%, CI 7.7–30.7, n = 1691); this is because five of six studies reporting on HIV-negative patients were restricted to DR-TB patients, elevating the CFR for this subgroup (Figs. 2 and 3).
Results of a random effects meta-analysis for case fatality ratios of specific populations in South Africa, 2010–2018, with Panel A: drug-resistant TB, Panel B: drug-susceptible TB, Panel C: drug susceptibility mixed or not specified, Panel D: HIV+, Panel E: HIV- (Note: Five of the six studies reported on drug-resistant TB, elevating the case fatality ratio for this group), Panel F: children
Case fatality ratios
In the three studies reporting on individuals with DS-TB, 67% (1302/1935) of patients were included from a single study of adult miners [48] and 33% (633/1935) were PLHIV. The highest CFR (26.7%, 16/60) was reported during hospitalisation for PLHIV with CD4 counts < 350 cells/mm3 and included death due to suspected bacterial sepsis and disseminated TB [34]. In that study, all patients had started TB treatment within a median of 1 day from diagnosis and 31% of TB patients who died were on ART [34]. In the single study of children with DS-TB the CFR was 6.5% and included seven children with HIV who died prior to initiating antiretroviral therapy (ART). These children died between 2004 and 2008 prior to universal ART access [49] (Table 3).
Twelve studies reported on individuals with DR-TB studies (n = 5149), the lowest CFRs were reported for a small sample of HIV-negative patients (CFR = 5.4%, 3/56) [44] and those receiving treatment at centralised hospital sites (CFR = 6.8%, 30/441) [45]. The highest CFR was reported for a cohort of people with extensively drug-resistant (XDR) TB diagnosed between 2002 and 2008 after 5 years of follow up (CFR = 72.9%, 78/107; Table 3) [17]. In studies restricted to PLHIV (n = 9) the highest CFRs were reported for a small sample of patients who had a positive urinary lipoarabinomannan (u-LAM) test (CFR = 24.5%, 13/53) [39] and patients with DR-TB who were on ART prior to initiating TB treatment (CFR = 21.8%, 119/545) [35]. The factors associated with low CFRs were the same as those noted in studies of DS-TB; with ART initiated during TB treatment associated with a lower CFR of 5.8% (25/429) [47] (Table 3). In the two studies restricted to children, the CFR in those with TBM was 8.9% (11/123) [46], and 6.3% (7/112) [49] among children with HIV, prior to ART initiation (Table 3). Unknown TB treatment outcomes were reported in 10 studies. CFRs were higher when restricted to TB patients with known outcomes (Table 3).
Timing of mortality
Nine studies described the time to death from treatment initiation [17, 28, 31, 33,34,35, 41, 45, 49]. Where time was reported continuously, Kaplan-Meier survival curves were shown over 12 [28] or 24 months [31] and in three studies the median time to death was reported for specific groups [35, 45, 49]. Among 573 ART-naïve children with HIV, treated for TB between 2004 and 2008, 37 children died after a median of 62 days of TB treatment, including seven children who died before ART initiation and 30 who died after ART initiation [49]. Where DR-TB treatment was provided between 2008 and 2009 in different settings, the median time to death was 43 days for those treated at centralised sites compared to 85 days for those treated at decentralised sites [45]. Considering the timing of ART in patients with multidrug-resistant (MDR)-TB treated between 2007 and 2010, the median time to death for those on ART prior to TB treatment was 139 days compared to 321 days for those starting ART after TB treatment [35]. Where time to death from treatment initiation was classified categorically, this varied with deaths reported per month of treatment [41] or at varied time points [17, 33, 34] after starting TB treatment. Survival curves were reported in three studies and time periods varied from 3 months to 3 years [18, 39, 44]. When analysing subgroups, a median survival from diagnosis of 42 days for patients with MDR-TB and 19 days for patient with XDR-TB was reported for patients treated between 2005 and 2006 [18].
Risk factors for TB mortality
We broadly characterised risk factors as demographic, baseline clinical characteristics, TB disease-related, TB treatment-related and HIV-related. However, factors falling into these categories were varied, some being unique to studies focusing on a sub-population (Additional file 1: Tables 4-7).
Demographic factors
Age was referenced in eight studies [17, 28, 33, 36, 41, 44, 45, 49]. The individuals with the highest risk for death were 25–42-year-old adults with XDR-TB, as compared to individuals < 25 years old with XDR-TB (aOR 3.5, 95% CI 1.3–9.6) [28]. Sex was evaluated in eight studies, but results were mixed and not significant [17, 18, 33, 35, 36, 39, 44, 46] (Additional file 1: Table 4).
Clinical factors
Weight was evaluated in three studies. Lower weight at TB diagnosis decreased the odds of survival among TB patients with XDR-TB [28]; being underweight (BMI 16–18.49 kg/m2) or severely underweight (BMI < 16 kg/m2) compared to those with a normal BMI (BMI 18.5 kg/m2–24.9 kg/m2) increased the odds of mortality among adults with DR-TB [35]; and in ART-naive children with HIV being severely underweight (weight-for-age Z-score <− 3) was associated with greater hazard of mortality [49] (Additional file 1: Table 5). A history of previous TB was reported as a risk factor for mortality in four studies [28, 30, 32, 41]. The greatest risk was reported for individuals with XDR-TB who had previously received MDR-TB treatment compared with those that had not (aHR 5.2, 95% CI 1.9–14.1) [32]. In a study restricted to South African miners, one or more previous TB episodes was associated with higher risk of mortality in the first month of treatment (aIRR 2.2, 95% CI 1.5–3.3) and in months 2–6 of treatment (aIRR 1.5, 95% CI 1.2–1.8) [41]. Specific elements of the diagnosis of TB were assessed as risk factors in eight studies [18, 28, 33, 35, 39, 41, 44, 48], six of which reported significant results. The greatest effect was in a group of South African miners treated for TB with microbiological data, where a ‘possible’ TB diagnosis (defined as a negative culture and/or smear) was associated with a greater risk of death within the first month of TB treatment compared to those with a ‘confirmed’ TB diagnosis (defined as a positive culture) (aIRR 6.3, 95%CI 3.2–12.4) [41]. Patients with positive uLAM results compared to those with negative results had a greater risk of death (aHR 4.2, 95% CI 1.5–11.8) [39] and adults with sputum smear-positive DR-TB were at increased risk compared to those with smear-negative disease (HR 3.3, 95% CI 2.1–5.6). Drug resistance was evaluated as a risk factor in four studies [18, 29, 39, 46]. The greatest risk associated with mortality was reported in a study of children with culture confirmed TBM where children with DR-TB had a higher risk for mortality compared to those with DS-TB (aOR 63.9, 95% CI 4.8–843.2) [46]. In a study of TB patients with MDR- and XDR-TB between 2005 and 2006, resistance to more drugs was associated with an increased hazard of mortality [18]. Site of disease was associated with risk of mortality in two studies, both of which reported lower risk in those with extrapulmonary TB when compared to those with pulmonary TB alone [41, 43]. Additionally, in individual studies TB molecular genotypes [36] and the setting of diagnosis (hospital vs ambulatory care) were evaluated [42] (Additional file 1: Table 5). Baseline haemoglobin ≥ 10 g/dL (HR 0.2, 95% CI 0.1–0.6) [43] and culture conversion in patients with XDR-TB, with final follow up sputum as conversion (HR 0.1, 95% CI 0.1–0.3) or reversion (HR 0.2, 95% CI 0.1–0.5) had a protective effect on mortality [17] (Additional file 1: Table 5).
Treatment-related factors
Drugs and regimens used for treating DR-TB were considered in five studies [17, 28, 29, 32, 35]. The use of ethambutol in XDR-TB treatment regimens among PLHIV was associated with an increased risk of mortality (HR 3.1, 95% CI 1.0–9.7) [17]. A significant protective effect was reported for increasing the number of drugs included in XDR-TB regimens (HR 0.6, 95% CI 0.5–0.8) [32] as well as the use of specific drugs (clofazimine and moxifloxacin) (Additional file 1: Table 6).
HIV-related factors
HIV status (independent of ART) was reported as a risk factor in six studies [17, 28,29,30, 33, 46]. The greatest risk of mortality was reported in miners living with HIV not on ART (aIRR 3.6, 95% CI 1.9–6.7 for death in the first month of TB treatment and aIRR 7.8, 95% CI 5.2 to 11.8 2–6 months after starting TB treatment) [41]. In addition, co-infected adults with XDR-TB (aOR 2.9, 95% CI 1.3–6.3 [29]) or MDR-TB (HR 1.9, 95%CI 1.0–3.6) had an increased risk of mortality. For children with TBM, HIV was associated with an increased risk of mortality, but this effect was not significant in the final model when adjusted for drug resistance (aOR 6.2, 95% CI 0.9–41.3) [46]. CD4 count was evaluated as a risk factor in eight studies [18, 30, 35, 38, 40, 42, 44, 49] and found to be associated with mortality in six [18, 30, 38, 40, 42, 44]. Three of these studies analysed CD4 count as a continuous variable and reported increased risk of death as CD4 counts fell. The other three studies considered CD4 count categorically and the greatest effect was reported in adults living with HIV with CD4 < 100 cells/mm3 compared to CD4 > 100 cells/mm3 (aOR 18.0, 95% CI 1.5–210.6) [38]. Two studies reported the effect of HIV viral load [42, 49] with viral suppression (< 5 log copies/ml) associated with decreased risk of mortality in children with HIV (HR 0.4, 95% CI 0.2–0.9) [49]. In adults with XDR-TB, initiating ART was associated with reduced risk of mortality in three studies (HR 0.1, 95% CI 0.0–0.5 [17], aHR 0.3, p value = 0.009 [18], and HR 0.4, 95% CI 0.2–0.8 [32]). Not being on ART was associated with increased risk of death compared to HIV-negative individuals (OR 2.5, 95% CI 1.0 to 6.3) [28]. Timing of ART was evaluated in patients with MDR-TB and initiating ART before initiating TB treatment was associated with increased risk of mortality (OR 1.7, CI 95% 1.0–2.7) [35] (Additional file 1: Table 7).
Discussion
Despite TB being preventable, treatable, and curable, in this systematic review we found that one in four South African patients with a TB diagnosis died. This is higher than the 15% (or one in seven) reported by the WHO for 2018 (in their most recent estimates of TB burden, generated for the Global Tuberculosis Report), because WHO numbers reflect estimates for all incident TB whereas our review is restricted to those on TB treatment, and because the WHO estimate includes deaths prior to TB notification and reflects deaths over a one-year period whereas our review includes some studies with greater periods of follow up and specific populations with higher risk of mortality [2, 50].
In our review, the risk of mortality was highest among patients with DR-TB, where death was observed in more than a third of all patients. In contrast, in those diagnosed with DS-TB, the risk was lower, with death observed in one in eight patients. Risk of mortality was equal for PLHIV and HIV-negative TB patients, with death observed in one in five patients in each group; however, this is because the pooled CFR for the HIV-negative group is based primarily on studies of DR-TB patients.
Individual studies included in this review indicate that HIV remains a major risk factor for TB mortality. This is in line with findings from previous studies as well as a national study evaluating treatment outcomes of all TB patients started on treatment, reporting that patients with HIV on ART had a greater hazard of death compared to patients without HIV, and those not on ART had two-threefold increased risk of mortality compared to patients who were HIV-negative [23, 24, 51]. In our systematic review, the protective effect of ART on TB mortality relates to patients treated between 1995 and 2012, a period when ART was not available for all PLHIV, including TB patients. Earlier work has shown that unknown HIV status is associated with increased risk of mortality compared to a negative status [8, 51], possibly as PLHIV had an unknown status and were not accessing ART. Our study findings are aligned with a meta-analysis reporting that ART during TB treatment reduced mortality by 44–71% [52]. In addition to ART timing, our study emphasizes the important protective role of viral suppression on ART against mortality; the extremely high CFR reported among hospitalised HIV-positive individuals with low CD4 counts < 350 cells/mm3 from suspected bacterial sepsis and disseminated TB, underscores the impact of failure to timeously diagnose and initiate treatment in patients for both TB and HIV, and possibly delayed patient health seeking behavior. In South Africa, the response to treating HIV and TB has shifted considerably with all PLHIV eligible for ART as of 2016 [53]. This increased access to ART is likely to reduce mortality in TB patients, but studies in our review did not cover this period.
Previous TB treatment was reported as a risk factor in two studies we reviewed [28, 32]. Studies of routine data have also shown significant associations between previous treatment history and increased TB mortality [8, 23, 24, 51], suggesting this may relate to the proportion of undiagnosed DR-TB in patients with previous TB treatment [8], a higher likelihood of extensive lung damage, and previous TB episode-related co-morbidities [51]. Drug regimens administered to TB patients may impact the risk of mortality, but these effects are unclear. As all DR-TB treatment outcomes were reported for 2015 or earlier, our review did not include the use of bedaquiline, delamanid, or newer shorter regimens for DR-TB but indicated protective effects for moxifloxacin, clofazimine, and the number of drugs included in XDR-TB treatment regimens [32]. These results have been confirmed in patients with HIV and DR-TB, treated with at least one WHO DR-TB Group A drug (moxifloxacin, levofloxacin, bedaquiline, or linezolid), who were at a significantly lower risk of death compared to those not treated with a group A drug [54]. Ethambutol was reported to have a threefold increased risk of mortality in the small sample of XDR patients with HIV [17]; however, this is likely reflective of a period where patients had limited treatment options rather than the effect of the drug.
Multiple studies reported advancing age increasing the risk of mortality in adults [28, 33, 41, 44]; especially for those over 50 years. This is comparable to studies conducted on smaller segments of the South African population [8, 51] as well as the study of the National TB treatment register for DS-TB [24] and indicates that older patients may have differing treatment needs. This is also reflective of changing trends in the profile of people dying from TB in South Africa, with mortality decreasing in those under 50 years, but increasing in older patients [55]. Although mixed results for sex and mortality [8, 24, 51, 56] are reported, results from the analyses of the National TB treatment register and mortality registrations indicate that men are more likely to die from TB than women [24]. While this may reflect biological differences, it is in part attributed to women’s higher participation in HIV-related services [24, 55], better adherence to TB treatment and lower risk of LTFU [51].
A strength of this systematic review is that we included studies with moderate to large samples of TB patients that were reasonably representative of target study populations; that used medical records and other objective measures to establish exposure and outcomes; and sampled comparison groups from comparable sources. Multiple factors compromised the quality of the evidence, including the lack of prospective studies and RCTs, and the reliance on statistical procedures rather than sampling or design strategies to control for potential confounders. This increased the potential for unknown systematic bias. Inconsistency in risk factors and comparator variables made it difficult to assess the quality for each of the outcomes. Variances could not be pooled and estimated across studies, and we were unable to perform a meta-analysis of risk factors for TB mortality. A further limitation is that collectively the studies in this review do not accurately represent the population of people with TB in South Africa. In this review, the proportion of patients with DR-TB or living with HIV was much higher than estimated for South Africa and the proportion of children was under-represented. In our review 41% of TB patients included were miners in South Africa, but we had insufficient data to evaluate additional risk factors for mortality in this population such as silicosis. This review did not provide sufficient data to consider co-morbidities and final causes of death, nor was there sufficient data relating to sociostructural determinants of health such as those related to socioeconomic status, healthcare system access and quality and behavioural factors (e.g. cigarette smoking). Finally, this study evaluated studies published between 2010 and 2018 and included data on patients treated between 1995 and 2015. We acknowledge that this does not include earlier studies which may inform the results, nor does it include studies which address the impact of COVID-19 on TB mortality.
Conclusions
Introducing standardised variables and minimum reporting requirements for TB cohorts would support future comparative work. We recommend at a minimum including sex; age with WHO age bands as a minimum; weight, including BMI; HIV status including HIV unknown, negative, and positive with an indication of ART regimen and timing; immune suppression with CD4 counts; diagnostic tests used with DST; and the place or level of care where the diagnosis was made. Published articles should include explicit statements about follow-up and the duration of follow-up and should include all WHO TB treatment outcome categories with descriptors of those who are LTFU. While sex was not identified as a definitive risk factor for TB mortality, specific interventions which target improvements in case finding and retention in care should focus on the differing needs of males and females. Based on existing evidence it is important to further examine the impact of factors such as pregnancy, diabetes mellitus, heart disease, chronic lung disease, and malignancy, which are associated with increased TB mortality [6, 57,58,59,60]. Further, we note the limited data on TB mortality and risk factors for mortality in children and adolescents indicating the importance of further studies of these groups. Additional studies on how sociostructural determinants of health impact TB mortality outcomes are also needed. Finally, given the likely impact of COVID-19 on TB and TB mortality, we recommend that an additional review be conducted to examine this.
Availability of data and materials
The data generated or analysed during this study are included in this published article and its supplementary information files.
Abbreviations
- ART:
-
Antiretroviral therapy
- CFR:
-
Case fatality ratio
- CI:
-
Confidence interval
- DR:
-
Drug-resistant
- DS:
-
Drug-susceptible
- DST:
-
Drug-susceptible test
- HR:
-
Hazard ratio
- IRR:
-
Incident rate ratio
- LTFU:
-
loss to follow-up
- MDR:
-
Multidrug-resistant
- NOS:
-
Newcastle-Ottawa scale
- OR:
-
Odds ratio
- PLHIV:
-
People living with HIV
- RCT:
-
Randomised control trial
- RR:
-
Relative risk
- TB:
-
Tuberculosis
- TBM:
-
Tuberculosis meningitis
- u-LAM:
-
Urinary lipoarabinomannan
- WHO:
-
World Health Organization
- XDR:
-
Extensively drug-resistant
References
World Health Organization. Definitions and reporting framework for tuberculosis – 2013 revision (Updated December 2014 and January 2020). Geneva, Switzerland: WHO Press, 2020. Report No.: 978 92 4 150534 5.
World Health Organization. Global Tuberculosis Report 2022. Geneva: Switzerland; 2022.
World Health Organization. The End TB Strategy. Geneva: Switzerland; 2015.
World Health Organization. Global Tuberculosis Report 2020. Geneva: Switzerland; 2020.
Statistics South Africa. Mortality and causes of death in South Africa: Findings from death notification, 2018. Pretoria, South Africa., 2021.
Waitt CJ, Squire SB. A systematic review of risk factors for death in adults during and after tuberculosis treatment. Int J Tuberc Lung Dis. 2011;15(7):871–85.
Osman M, Seddon JA, Dunbar R, Draper HR, Lombard C, Beyers N. The complex relationship between human immunodeficiency virus infection and death in adults being treated for tuberculosis in Cape Town. South Africa BMC Public Health. 2015;15:556.
Heunis JC, Kigozi NG, Chikobvu P, Botha S, van Rensburg HD. Risk factors for mortality in TB patients: a 10-year electronic record review in a South African province. BMC Public Health. 2017;17(1):38.
Ershova JV, Podewils LJ, Bronner LE, Stockwell HG, Dlamini SS, Mametja LD. Evaluation of adherence to national treatment guidelines among tuberculosis patients in three provinces of South Africa. S Afr Med J. 2014;104(5):362–8.
Mabunda TE, Ramalivhana NJ, Dambisya YM. Mortality associated with tuberculosis/HIV co-infection among patients on TB treatment in the Limpopo province. South Africa Afr Health Sci. 2014;14(4):849–54.
Gandhi NR, Shah NS, Andrews JR, et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 2010;181(1):80–6.
Schnippel K, Shearer K, Evans D, Berhanu R, Dlamini S, Ndjeka N. Predictors of mortality and treatment success during treatment for rifampicin-resistant tuberculosis within the South African National TB Programme, 2009 to 2011: a cohort analysis of the national case register. Int J Infect Dis. 2015;39:89–94.
MacPherson P, Houben RM, Glynn JR, Corbett EL, Kranzer K. Pre-treatment loss to follow-up in tuberculosis patients in low- and lower-middle-income countries and high-burden countries: a systematic review and meta-analysis. Bull World Health Organ. 2014;92(2):126–38.
Omar T, Variava E, Moroe E, et al. Undiagnosed TB in adults dying at home from natural causes in a high TB burden setting: a post-mortem study. Int J Tuberc Lung Dis. 2015;19(11):1320–5.
Tiemensma M, Burger EH. Sudden and unexpected deaths in an adult population, Cape Town, South Africa, 2001–2005. S Afr Med J. 2012;102(2):90–4.
Shean KP, Willcox PA, Siwendu SN, et al. Treatment outcome and follow-up of multidrug-resistant tuberculosis patients, West Coast/Winelands, South Africa, 1992–2002. Int J Tuberc Lung Dis. 2008;12(10):1182–9.
Pietersen E, Ignatius E, Streicher EM, et al. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. Lancet. 2014;383(9924):1230–9.
Gandhi NR, Andrews JR, Brust JC, et al. Risk factors for mortality among MDR- and XDR-TB patients in a high HIV prevalence setting. Int J Tuberc Lung Dis. 2012;16(1):90–7.
Baker MA, Harries AD, Jeon CY, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9(81):81.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339: b2700.
Swartz MK. PRISMA 2020: an update. Journal of Pediatric Health Care. 2021;35(4):351.
Osman M, du Preez K, Seddon JA, et al. Mortality in South African children and adolescents routinely treated for tuberculosis. Pediatrics. 2021;147(4):e2020032490.
Osman M, van Schalkwyk C, Naidoo P, et al. Mortality during tuberculosis treatment in South Africa using an 8-year analysis of the national tuberculosis treatment register. Scientific Reports. 2021;11(1):15894.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Wells G, Shea B, O'Connell D, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2000.
Sterne JA, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.
Kvasnovsky CL, Cegielski JP, Erasmus R, Siwisa NO, Thomas K, der Walt ML. Extensively drug-resistant TB in Eastern Cape, South Africa: high mortality in HIV-negative and HIV-positive patients. J Acquir Immune Defic Syndr. 2011;57(2):146–52.
Pietersen E, Peter J, Streicher E, et al. High frequency of resistance, lack of clinical benefit, and poor outcomes in capreomycin treated South African patients with extensively drug-resistant tuberculosis. PLoS One. 2015;10(4): e0123655.
Marais S, Pepper DJ, Schutz C, Wilkinson RJ, Meintjes G. Presentation and outcome of tuberculous meningitis in a high HIV prevalence setting. PLoS One. 2011;6(5): e20077.
O’Donnell MR, Padayatchi N, Kvasnovsky C, Werner L, Master I, Horsburgh CR Jr. Treatment outcomes for extensively drug-resistant tuberculosis and HIV co-infection. Emerg Infect Dis. 2013;19(3):416–24.
Dheda K, Shean K, Zumla A, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet. 2010;375(9728):1798–807.
Olaleye AO, Beke AK. Survival of smear-positive multidrug resistant tuberculosis patients in Witbank, South Africa: a retrospective cohort study. Infect Dis. 2016;48(6):422–7.
Janssen S, Schutz C, Ward A, et al. Mortality in severe human immunodeficiency virus-tuberculosis associates with innate immune activation and dysfunction of monocytes. Clin Infect Dis. 2017;65(1):73–82.
Umanah T, Ncayiyana J, Padanilam X, Nyasulu PS. Treatment outcomes in multidrug resistant tuberculosis-human immunodeficiency virus Co-infected patients on anti-retroviral therapy at Sizwe Tropical Disease Hospital Johannesburg. South Africa BMC Infect Dis. 2015;15:478.
Marais E, Mlambo CK, Lewis JJ, et al. Treatment outcomes of multidrug-resistant tuberculosis patients in Gauteng. South Africa Infection. 2014;42(2):405–13.
Brust JC, Gandhi NR, Carrara H, Osburn G, Padayatchi N. High treatment failure and default rates for patients with multidrug-resistant tuberculosis in KwaZulu-Natal, South Africa, 2000–2003. Int J Tuberc Lung Dis. 2010;14(4):413–9.
Pepper DJ, Marais S, Wilkinson RJ, Bhaijee F, De Azevedo V, Meintjes G. Barriers to initiation of antiretrovirals during antituberculosis therapy in Africa. PLoS One. 2011;6(5): e19484.
Lawn SD, Kerkhoff AD, Burton R, et al. Diagnostic accuracy, incremental yield and prognostic value of Determine TB-LAM for routine diagnostic testing for tuberculosis in HIV-infected patients requiring acute hospital admission in South Africa: a prospective cohort. BMC Med. 2017;15(1):67.
Griesel R, Stewart A, van der Plas H, Sikhondze W, Mendelson M, Maartens G. Prognostic indicators in the World Health Organization’s algorithm for seriously ill HIV-infected inpatients with suspected tuberculosis. AIDS Res Ther. 2018;15(1):5.
Field N, Lim MS, Murray J, Dowdeswell RJ, Glynn JR, Sonnenberg P. Timing, rates, and causes of death in a large South African tuberculosis programme. BMC Infect Dis. 2014;14:3858.
Kerkhoff AD, Meintjes G, Burton R, Vogt M, Wood R, Lawn SD. Relationship between blood concentrations of hepcidin and anemia severity, mycobacterial burden, and mortality among patients with HIV-associated tuberculosis. J Infect Dis. 2016;213(1):61–70.
Kendon MA, Knight S, Ross A, Giddy J. Timing of antiretroviral therapy initiation in adults with HIV-associated tuberculosis: outcomes of therapy in an urban hospital in KwaZulu-Natal. S Afr Med J. 2012;102(12):931–5.
Brust JCM, Shah NS, Mlisana K, et al. Improved survival and cure rates with concurrent treatment for multidrug-resistant tuberculosis-human immunodeficiency virus coinfection in South Africa. Clin Infect Dis. 2018;66(8):1246–53.
Loveday M, Wallengren K, Voce A, et al. Comparing early treatment outcomes of MDR-TB in decentralised and centralised settings in KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis. 2012;16(2):209–15.
Seddon JA, Visser DH, Bartens M, et al. Impact of drug resistance on clinical outcome in children with tuberculous meningitis. Pediatr Infect Dis J. 2012;31(7):711–6.
Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362(8):697–706.
Churchyard GJ, Fielding K, Roux S, et al. Twelve-monthly versus six-monthly radiological screening for active case-finding of tuberculosis: a randomised controlled trial. Thorax. 2010;66(2):134–9.
Yotebieng M, Van Rie A, Moultrie H, et al. Effect on mortality and virological response of delaying antiretroviral therapy initiation in children receiving tuberculosis treatment. AIDS. 2010;24(9):1341–9.
World Health Organization. Global Tuberculosis Programme, Global Tuberculosis Report, Data, CSV Files.2022 Available from: https://www.who.int/teams/global-tuberculosis-programme/data#csv_files [Accessed 03 Jan 2023].
Berry KM, Rodriguez CA, Berhanu RH, et al. Treatment outcomes among children, adolescents, and adults on treatment for tuberculosis in two metropolitan municipalities in Gauteng Province, South Africa. BMC Public Health. 2019;19(1):973.
Odone A, Amadasi S, White RG, Cohen T, Grant AD, Houben RM. The impact of antiretroviral therapy on mortality in HIV positive people during tuberculosis treatment: a systematic review and meta-analysis. PLoS One. 2014;9(11): e112017.
Osman M, du Preez K, Naidoo P, et al. Key changes in the public health response to TB and HIV in South Africa. Int J Tuberc Lung Dis. 2020;24(8):857–9.
Bisson GP, Bastos M, Campbell JR, et al. Mortality in adults with multidrug-resistant tuberculosis and HIV by antiretroviral therapy and tuberculosis drug use: an individual patient data meta-analysis. The Lancet. 2020;396(10248):402–11.
Loveday M, Mzobe YN, Pillay Y, Barron P. Figures of the dead: a decade of tuberculosis mortality registrations in South Africa. S Afr Med J. 2019;109(10):728–32.
Abdullahi OA, Ngari MM, Sanga D, Katana G, Willetts A. Mortality during treatment for tuberculosis; a review of surveillance data in a rural county in Kenya. PLoS One. 2019;14(7): e0219191.
de Almeida CPB, Ziegelmann PK, Couban R, Wang L, Busse JW, Silva DR. Predictors of in-hospital mortality among patients with pulmonary tuberculosis: a systematic review and meta-analysis. Sci Rep. 2018;8(1):7230.
Huangfu P, Ugarte-Gil C, Golub J, Pearson F, Critchley J. The effects of diabetes on tuberculosis treatment outcomes: an updated systematic review and meta-analysis. Int J Tuberc Lung Dis. 2019;23(7):783–96.
Grange J, Adhikari M, Ahmed Y, et al. Tuberculosis in association with HIV/AIDS emerges as a major nonobstetric cause of maternal mortality in Sub-Saharan Africa. Int J Gynaecol Obstet. 2010;108(3):181–3.
Bekker A, Schaaf HS, Draper HR, Kriel M, Hesseling AC. Tuberculosis disease during pregnancy and treatment outcomes in HIV-infected and uninfected women at a referral hospital in Cape Town. PLoS One. 2016;11(11): e0164249.
Acknowledgements
Not applicable.
Funding
This study was funded by the South African TB Think Tank through a consultancy to the Desmond Tutu TB Centre through funding from the Bill and Melinda Gates Foundation (Investment ID OPP1173131). This was made possible through funding by the South African Medical Research Council (SA MRC) through its Division of Research Capacity Development under the Bongani Mayosi National Health Scholars Program from funding received from the Public Health Enhancement Fund/South African National Department of Health to MO. The contents of any publications from any studies during this degree are solely the responsibility of the authors and do not necessarily represent the official views of the SA MRC or the funders.
KDP is supported by the Fogarty International Center of the National Institutes of Health under Award Number K43TW011006. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ACH is financially supported by the South African National Research Foundation through a South African Research Chairs Initiative (SARChI), and KDP received grant-holder linked student support. The financial assistance of the NRF towards this research is hereby acknowledged. Opinions expressed, and conclusions arrived at, are those of the authors and are not necessarily to be attributed to the NRF.
JAS is supported by a Clinician Scientist Fellowship jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement (MR/R007942/1).
MM vd Z is supported by a career development grant from the EDCTP2 program supported by the European Union (grant number TMA2019SFP-2836 TB lung-FACT2) and by the Fogarty
International Center of the NIH (award number K43TW011028). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ELV is supported by a Spanish Pediatrics Association (AEP) fellowship and a Ramon Areces Foundation fellowship.
MMC is jointly funded by the UK Medical Research Council (MRC) and the UK Foreign, Commonwealth & Development Office (FCDO) under the MRC/FCDO Concordat agreement and is a Senior Fellow of the EDCTP2 programme supported by the European Union.
Author information
Authors and Affiliations
Contributions
MO, TJN, RD, ACH, KN, JAS, ELV, and FMM contributed to the protocol and study design. All authors contributed to article reviews and data extraction. TJN, MMC, GH, JAS, ELV, MvdZ, and MO produced the first draft of the manuscript. TJN, MMC, JAS, and MO revised the manuscript based on reviewer feedback. All authors reviewed the manuscript and contributed to the final draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary material. Description of data: Complete list of outcomes and variables for which data were sought; PRISMA 2020 checklist. Supplementary Table 1. Newcastle-Ottawa quality assessment scale for cohort studies, South Africa, 2010-2018 (n=21). Supplementary Table 2. Cochrane risk of bias tool for randomised trials, South Africa, 2010-2018 (n=2). Supplementary Table 3. Newcastle-Ottawa quality assessment scale for case control studies, South Africa, 2010-2018 (n=1). Supplementary Table 4. Demographic risk factors for TB mortality. Supplementary Table 5. Clinical risk factors for TB mortality, South Africa, 2010-2018. Supplementary Table 6. Tuberculosis treatment-related risk factors for TB mortality, South Africa, 2010-2018. Supplementary Table 7. HIV and antiretroviral therapy related risk factors for TB mortality, South Africa, 2010-2018.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nicholson, T.J., Hoddinott, G., Seddon, J.A. et al. A systematic review of risk factors for mortality among tuberculosis patients in South Africa. Syst Rev 12, 23 (2023). https://doi.org/10.1186/s13643-023-02175-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-023-02175-8