Abstract
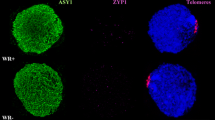
Chromosome pairing in meiosis usually starts in the vicinity of the telomere attachment to the nuclear membrane and congregation of telomeres in the leptotene bouquet is believed responsible for bringing homologue pairs together. In a heterozygote for an inversion of a rye (Secale cereale L.) chromosome arm in wheat, a distal segment of the normal homologue is capable of chiasmate pairing with its counterpart in the inverted arm, located near the centromere. Using 3D imaging confocal microscopy, we observed that some telomeres failed to be incorporated into the bouquet and occupied various positions throughout the entire volume of the nucleus, including the centromere pole. Rye telomeres appeared ca. 21 times more likely to fail to be included in the telomere bouquet than wheat telomeres. The frequency of the out-of-bouquet rye telomere position in leptotene was virtually identical to the frequency of telomeres deviating from Rabl’s orientation in the nuclei of somatic cells, and was similar to the frequency of synapsis of the normal and inverted chromosome arms, but lower than the MI pairing frequency of segments of these two arms normally positioned across the volume of the nucleus. Out-of-position placement of the rye telomeres may be responsible for reduced MI pairing of rye chromosomes in hybrids with wheat and their disproportionate contribution to aneuploidy, but appears responsible for initiating chiasmate pairing of distantly positioned segments of homology in an inversion heterozygote.





Similar content being viewed by others
References
Alleva B, Smolikove S (2017) Moving and stopping: regulation of chromosome movement to promote meiotic chromosome pairing and synapsis. Nucleus 8:613–624
Bass HW, Marshall WF, Sedat JW, Agard DA, Cande WZ (1997) Telomeres cluster de novo before the initiation of synapsis: a three dimensional spatial analysis of telomere positions before and during meiotic prophase. J Cell Biol 137:5–18
Bass HW, Riera-Lizarazu O, Ananiev EV, Bordolo SJ, Rines HW, Phillips RL, Sedat JW, Agard DA, Cande WZ (2000) Evidence for the coincident initiation of homologue pairing and synapsis during the telomere clustering (bouquet) stage of meiotic prophase. J Cell Sci 113:1033–1042
Bass HW (2003) Telomere dynamics unique to meiotic prophase: formation and significance of the bouquet. Cell Mol Life Sci 60:2319–2324
Bauwens S, Van Oostveldt P (1996) Nonradioactive in situ hybridization application manual. 2nd ed., Boehringer Mannheim, Mannheim, Germany p165–171 http://hdl.handle.net/1854/LU-260636
Brakenhoff GJ, van der Voort HTM, van Spornsen EA, Linnemans WAM, Nanninga N (1985) Three dimensional chromatin distribution in neuroblastoma nuclei shown by confocal scanning laser microscopy. Nature 317:748–749
Carlton PM, Cande WZ (2002) Telomeres act autonomously in maize to organize the meiotic bouquet from a semipolarized chromosome orientation. J Cell Biol 157:231–242
Corredor E, Lukaszewski AJ, Pachón P, Allen DC, Naranjo T (2007) Terminal regions of wheat chromosomes select their pairing partners in meiosis. Genetics 177:699–706
Curtis CA, Lukaszewski AJ, Chrzastek M (1991) Metaphase I pairing of deficient chromosomes and genetic mapping of deficiency breakpoints in common wheat. Genome 34:553–560
Dawe RK (1998) Meiotic chromosome organization and segregation in plants. Ann Rev Plant Physiol Plant Mol Biol 49:371–395
Ding X, Xu R, Yu J, Xu T, Zhuang Y, Han M (2007) SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev Cell 12:863–872
Eckardt NA (2008) High-resolution three-dimensional imaging of plant tissues. Plant Cell 20:1423
Francki MG (2001) Identification of Bilby, a diverged centromeric T1-copia retrotransposon family from cereal rye (Secale cereale L). Genome 44:266–274
Fussel CP (1987) The Rabl orientation: a prelude to synapsis. In: Moens PB (ed) Meiosis. Academic Press, Orlando, pp 275–299
Graumann K, Runions J, Evans DE (2010) Characterization of SUN-domain proteins at the higher plant nuclear envelope. Plant J 61:134–144
Howe ES, Murphy SP, Bass HW (2014) Three-dimensional acrylamide fluorescence in situ hybridization for plant cells. In: Pawlowski W, Grelon M, Armstrong S, eds. Dordrecht: plant meiosis. Methods and protocols. Springer International Publishing AG, 53–66
Ito H, Nasuda S, Endo TR (2004) A direct repeat sequence associated with the centromeric retrotransposons in wheat. Genome 47:747–756
Juchimiuk-Kwasniewska J, Brodziak L, Maluszynska J (2011) FISH in analysis of gamma ray-induced micronuclei formation in barley. J Appl Genet 52:23–29
Harper L, Golubovskaya I, Cande WZ (2004) A bouquet of chromosomes. J Cell Sci 117:4025–4032
Linc G, Sepsi A, Molnár-Láng M (2012) A FISH karyotype to study chromosome polymorphisms for the Elytrigia elongata E genome. Cytogenet Genome Res 136:138–144
Lukaszewski AJ (1992) A comparison of physical distribution of recombination in chromosome 1R in diploid rye and in hexaploid triticale. Theor Appl Genet 83:1048–1053
Lukaszewski AJ (1997a) The development and meiotic behavior of asymmetrical isochromosomes in wheat. Genetics 145:1155–1160
Lukaszewski AJ (1997b) Further manipulation by centric misdivision of the 1RS.1BL translocation in wheat. Euphytica 94:257–261
Lukaszewski AJ (2008) Unexpected behavior of an inverted rye chromosome arm in wheat. Chromosoma 117:569–578
Moens PB, Bernei-Moens C, Spyropoulos B (1989) Chromosome core attachment to the meiotic nuclear envelope regulates synapsis in Chloealtis (Orthoptera). Genome 32:601–610
Moore G (2002) Meiosis in allopolyploids—the importance of “Teflon” chromosomes. Trends in Genet 18:456–463
Murphy SP, Bass HW (2012) The maize (Zea mays) desynaptic (dy) mutation defines a pathway for meiotic chromosome segregation, linking nuclear morphology, telomere distribution and synapsis. J Cell Sci 125:3681–3690
Naranjo T (2014) Dynamics of rye telomeres in a wheat background during early meiosis. Cytogenet Genome Res 143:60–68
Naranjo T (2018) Variable patterning of chromatin remodeling, telomere positioning, synapsis, and chiasma formation of individual rye chromosomes in meiosis of wheat-rye additions. Frontiers in Plant Sci 9:880
Naranjo T, Valenzuela N, Perera E (2010) Chiasma frequency is region specific and chromosome conformation-dependent in a rye chromosome added to wheat. Cytogenet Genome Res 129:133–142
Oleszczuk S, Rabiza-Swider J, Zimny J, Lukaszewski AJ (2011) Aneuploidy among androgenic progeny of hexaploid triticale (x Triticosecale Wittmack). Plant Cell Rep 30:575–586
Pawar V, Poulet A, Detourne G, Tatout C, Vanrobays E, Evans DE, Graumann K (2016) A novel family of plant nuclear envelope-associated proteins. J Exp Botany 67:5699–5710
Phillips D, Nibau C, Ramsay L, Waugh R, Jenkins G (2010) Development of a molecular cytogenetic recombination assay for barley. Cytogenet Genome Res 129:154–161
Scherthan H, Weich S, Schwegler H, Härle M, Heyting C, Cremer T (1996) Centromere and telomere movements during early meiotic prophase of mouse and man are associated with the onset of chromosome pairing. J Cell Biol 134:1109–1125
Scherthan H (2001) A bouquet makes ends meet. Nature Rev Mol Cell Biol 2:621–627
Scherthan H (2007) Telomere attachment and clustering during meiosis. Cell Mol Life Sci 64:117–124
Schubert V, Kim YM, Berr A, Fuchs J, Meister A, Marschner S, Schubert I (2007) Random homologous pairing and incomplete sister chromatid alignment are common in angiosperm interphase nuclei. Mol Gen Genomics 278:167–176
Sheehan MJ, Pawlowski WP (2009) Live imaging of rapid chromosome movements in meiotic prophase I in maize. Proc Natl Acad Sci U S A 106:20989–20994
Shimanuki M, Miki F, Ding DQ, Chikashige Y, Hiraoka Y, Horio T, Niwa O (1997) A novel fission yeast gene, kms1C, is required for the formation of meiotic prophase-specific nuclear architecture. Mol Gen Genet 254:238–249
Tiang CL, He Y, Pawlowski WP (2012) Chromosome organization and dynamics during interphase, mitosis, and meiosis in plants. Plant Phys 158:26–34
Tsunewaki K (1964) Genetic studies on a 6x derivative from an 8x triticale. Can J Genet Cytol 6:1–11
Valenzuela NT, Perera E, Naranjo T (2012) Dynamics of rye chromosome 1R regions with high or low crossover frequency in homology search and synapsis development. PLoS One 7:e36385
Varas J, Graumann K, Osman K, Pradillo M, Evans DE, Santos JL, Armstrong SJ (2015) Absence of SUN1 and SUN2 proteins in Arabidopsis thaliana leads to a delay in meiotic progression and defects in synapsis and recombination. Plant J 81:329–346
Zhang P, Li W, Fellers J, Friebe B, Gill BS (2004) BAC-FISH in wheat identifies chromosome landmarks consisting of different types of transposable elements. Chromosoma 112:288–299
Acknowledgements
The authors thank Drs. David Carter (UCR) and Attila Fabian (MTA, ATK) for assistance with the SP8 confocal laser scanning microscope system.
Funding
This study was done with support from the National Institute of Food and Agriculture (NIFA) Project CA-R-BPS-5411-H to AJL and the National Science Foundation Grants (OTKA K108555) and the Hungarian Academy of Sciences (MTA KEP 2018) to GL. KP and DK were partially supported by the Czech Science Foundation (grant award 17-13853S) and together with OS by a grant award LO1204 from the National Program of Sustainability I.
Author information
Authors and Affiliations
Contributions
AJL and GL designed experiments; AJL, EG, and GL performed meiotic experiments; KP, OS, and DK performed mitotic experiments; AJL and GL analyzed data; AJL, GL, and DK wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pernickova, K., Linc, G., Gaal, E. et al. Out-of-position telomeres in meiotic leptotene appear responsible for chiasmate pairing in an inversion heterozygote in wheat (Triticum aestivum L.). Chromosoma 128, 31–39 (2019). https://doi.org/10.1007/s00412-018-0686-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-018-0686-5