Abstract
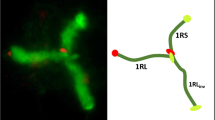
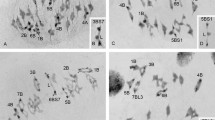
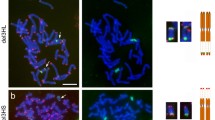
Distal location of chiasmata in chromosome arms is thought to be a consequence of the distal initiation of synapsis. Observations of meiotic behavior of a rye chromosome with an inverted arm show that patterns of chiasma distribution and frequency are also inverted; therefore, the patterns of synapsis and chiasma distribution are independent, and recombination frequency along a chromosome is position-independent and segment-specific. Since cases of random distribution of chiasmata and recombination are known in rye, a genetic mechanism must be present that licenses specific chromosome regions for recombination. Large differences in the metaphase I pairing of the inversion in various combinations of two armed and telocentric chromosomes confirm the major role of the telomere bouquet in early homologue recognition. However, occasional synapsis and chiasmate pairing of the distal regions of normal arms with the proximal regions of the inversion suggest that an alternative mechanism for juxtaposing of homologues must also be present. Synapsis in inversion heterozygotes was mostly complete but in the antiparallel orientation, hence defying homology, but non-homologues never synapsed. Instances of synapsis strictly limited to the chiasma-capable segments of the arm suggest that, in rye, both recombination-dependent and recombination-independent mechanisms for homologue recognition must be present.


Similar content being viewed by others
References
Albini S, Jones GH (1987) Synaptonemal complex spreading in Alium cepa and Alium fistulosum. I. The initiation and sequence of pairing. Chromosoma 95:324–338
Bass HW (2003) Telomere dynamics unique to meiotic prophase: formation and significance of the bouquet. Cell Mol Life Sci 60:2319–2324
Bass HW, Riera-Lizarazu O, Ananiev EV, Bordolo SJ, Rines HW, Phillips RL, Sedat JW, Agard DA, Cande WZ (2000) Evidence for the coincident initiation of homologue pairing and synapsis during the telomere clustering (bouquet) stage of meiotic prophase. J Cell Sci 113:1033–1042
Burnham CR, Stout JT, Weinheimer WH, Knowles RV, Philips RL (1972) Chromosome pairing in maize. Genetics 71:111–126
Carlton PM, Cande WZ (2002) Telomeres act autonomously in maize to organize the meiotic bouquet from a semipolarized chromosome orientation. J Cell Biol 157:231–242
Close TJ, Wanamaker S, Roose ML, Lyon M (2007) HarvEST: an EST database and viewing software. In: Edwards D (ed) Plant bioinformatics: Methods in molecular biology. Humana Press, Totowa, NJ, pp 161–178
Corredor E, Lukaszewski AJ, Pachón P, Allen DC, Naranjo T (2007) Terminal regions of wheat chromosomes select their pairing partners in meiosis. Genetics 177:699–706
Curtis CA, Lukaszewski AJ (1991) Genetic linkage between C-bands and storage protein genes in chromosome 1B of tetraploid wheat. Theor Appl Genet 81:245–252
Dawe RK (1998) Meiotic chromosome organization and segregation in plants. Annu Rev Plant Physiol Plant Mol Biol 49:371–395
De Jong JH, Havekes F, Roca A, Naranjo J (1991) Synapsis and chiasma formation in a ditelo-substituted haploid of rye. Genome 34:109–120
Faris DJ, Haen KM, Gill BS (2000) Saturation mapping of a gene-rich recombination hot spot region in wheat. Genetics 154:823–835
Francki M (2001) Identification of Bilby, a diverged centromeric Ty1-copia retrotransposon family from cereal rye (Secale cereale L.). Genome 44:266–274
Fussel CP (1987) The Rabl orientation: A prelude to synapsis. In: Moens PB (ed) Meiosis. Academic, Orlando, pp 275–299
Gerlach WL, Bedbrook JR (1979) Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res 8:4851–4855
Gerton JL, Hawley RS (2005) Homologous chromosome interactions in meiosis: diversity amidst conservation. Nat Rev 6:477–487
Gill KS, Gill BS, Endo TR, Boyko EV (1996) Identification and high density mapping of gene-rich regions in chromosome group-1 of wheat. Genetics 143:1001–1012
Gillies CB (1974) The nature and extent of synaptonemal complex formation in haploid barley. Chromosoma (Berl.) 48:441–453
Gillies CB (1985) An electron microscopic study of synaptonemal complex formation at zygotene in rye. Chromosoma 92:165–175
Gilles CB, Lukaszewski AJ (1989) Synaptonemal complex formation in rye (Secale cereale) heterozygous for telomeric C-bands. Genome 32:901–907
Harper L, Golubovskaya I, Cande WZ (2004) A bouquet of chromosomes. J Cell Sci 117:4025–4032
Jones GH (1967) The control of chiasma distribution in rye. Chromosoma (Berl.) 22:69–90
Jones GH (1978) Giemsa C-banding of rye meiotic chromosomes and the nature of “terminal” chiasmata. Chromosoma 66:45–57
Jones GH (1987) Chiasmata. In: Moens PB (ed) Meiosis. Academic, Orlando, pp 213–243
Jones LE, Rybka K, Lukaszewski AJ (2002) The effect of a deficiency and a deletion on recombination in chromosome 1BL in wheat. Theor Appl Genet 104:1204–1208
Karp A, Jones RN (1983) Cytogenetics of Lolium perenne. Part. 2. Chiasma distribution in inbred lines. Theor Appl Genet 64:137–145
Khrustaleva LI, Kik C (1998) Cytogenetical studies in the bridge cross Allium cepa x (A. fistulosum x A. roylei). Theor Appl Genet 96:8–14
Künzel G, Korzun L, Meister A (2000) Cytologically integrated physical restriction fragment length polymorphism maps for the barley genome based on translocation breakpoints. Genetics 154:397–412
Loidl J (1990) The initiation of meiotic chromosome pairing: the cytological view. Genome 33:759–778
Lukaszewski AJ (1992) A comparison of physical distribution of recombination in chromosome 1R in diploid rye and in hexaploid triticale. Theor Appl Genet 83:1048–1053
Lukaszewski AJ (1995) Chromatid and chromosome type breakage–fusion–bridge cycles in wheat (Triticum aestivum L.). Genetics 140:1069–108
Lukaszewski AJ (1997a) Further manipulation by centric misdivision of the 1RS.1BL translocation in wheat. Euphytica 94:257–261
Lukaszewski AJ (1997b) The development and meiotic behavior of asymmetrical isochromosomes in wheat. Genetics 145:1155–1160
Lukaszewski AJ (1997c) Construction of midget chromosomes in wheat. Genome 40:566–569
Lukaszewski AJ (2003) Manipulation of recombination in wheat. In: Pogna NE (ed) Proc. 10th Int. Wheat Genet. Symp. Paestum, Italy, pp 73–76
Lukaszewski AJ, Curtis CA (1992a) Recombination pattern and chiasma interference in tetraploid wheat. In: Rajaram S, Saari EE, Hettel GP (eds) Durum wheats: Challenges and opportunities. Wheat special report no. 9. CIMMYT, Mexico, DF, pp 174–177
Lukaszewski AJ, Curtis CA (1992b) Transfer of the Glu-D1 gene from chromosome 1D of breadwheat to chromosome 1R in hexaploid triticale. Plant Breeding 109:203–210
Lukaszewski AJ, Curtis CA (1993) Physical distribution of recombination in B-genome chromosomes of tetraploid wheat. Theor Appl Genet 86:121–127
Lukaszewski AJ, Xu X (1995) Screening large populations of wheat hybrids by C-banding. Cereal Res Comm 23:9–13
Lukaszewski AJ, Rybka K, Korzun V, Malyshev SV, Lapinski B, Whitkus R (2004) Genetic and physical mapping of homoeologous recombination points involving wheat chromosome 2B and rye chromosome 2R. Genome 47:36–45
Maestra B, de Jong JH, Shepherd K, Naranjo T (2002) Chromosome arrangement and behavior of two homologous telosomes at the onset of meiosis in disomic wheat-5RL addition lines with and without the Ph1 locus. Chromosome Res 10:655–667
Maguire MP (1981) A search for the synaptic adjustment phenomenon in maize. Chromosoma 81:717–725
Maguire MP (1986) The pattern of pairing that is effective for crossing over in complex B–A chromosome rearrangements in maize. III. Possible evidence for pairing centers. Chromosoma 94:71–85
Massoudi-Nejad A, Nasuda S, McIntosh RA, Endo TR (2002) Transfer of rye chromosome segments to wheat by gametocidal system. Chromosome Res 10:349–357
Martinez-Perez E, Shaw P, Moore G (2001) The Ph1 locus is needed to ensure specific somatic and meiotic centromere association. Nature 411:204–207
Moses MJ, Poorman PA, Roderick TH, Davidson MT (1982) Synaptonemal complex analysis of mouse chromosome rearrangements. IV. Synapsis and synaptic adjustment in two paracentric inversions. Chromosoma 84:457–474
Pawlowski WP, Cande WZ (2005) Coordinating the events of the meiotic prophase. Trends Cell Biol 15:674–681
Pawlowski WP, Golubovskaya IN, Timofejeva L, Meeley RB, Sheridan WF, Cande WZ (2004) Coordination of meiotic recombination, pairing and synapsis by PHS1. Science 303:89–92
Qi LL, Friebe B, Gill BS (2002) A strategy for enhancing recombination in proximal regions of chromosomes. Chromosome Res 10:645–654
Orellana J, Giraldez R (1981) Metaphase I bond arms and crossing over frequencies in rye. I. Open pollinated varieties. Chromosoma 84:439–449
Rees H, Thompson JB (1956) Genotypic control of chromosome behaviour in rye. III. Chiasma frequency in homozygotes and heterozygotes. Heredity 10:409–424
Rees H, Thompson JB (1958) Genotypic control of chromosome behaviour in rye. V. The distribution pattern of chiasmata between pollen mother cells. Heredity 12:101–111
Salee PJ, Kimber G (1978) An analysis of the pairing of wheat telocentric chromosomes. In: Ramanujan S (ed) Proc. 5th Int. Wheat Genet. Symp. New Dehli, India, pp 408–419
Scherthan H (2007) Telomere attachment and slustering during meiosis. Cell Mol Life Sci 64:117–124
Sybenga J (1965) The quantitative analysis of chromosome pairing and chiasma formation based on the relative frequencies of M I configurations. Introduction: normal diploids. Genetica 36:243–252
Sybenga J (1966a) The quantitative analysis of chromosome pairing and chiasma formation based on the relative frequencies of M I configurations. IV. Interchange heterozygotes. Genetica 37:199–206
Sybenga J (1996b) The zygomere as hypothetical unit of chromosome pairing initiation. Genetica 37:186–198
Yao H, Zhou Q, Li J, Smith H, Yandeau M, Nikolau BJ, Schnable PS (2002) Molecular characterization of meiotic recombination across the 140 kb multigenic a1-sh2 interval of maize. Proc Natl Acad Sci U S A 99:6157–6162
Zickler D (2006) From early homologue recognition to synaptonemal complex formation. Chromosoma 115:158–174
Zwierzykowski Z, Lukaszewski AJ, Naganowska B, Lesniewska A (1999) The pattern of homologous recombination in triploid hybrids of Lolium multiflorum with Festuca pratensis. Genome 42:720–726
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Shaw
Rights and permissions
About this article
Cite this article
Lukaszewski, A.J. Unexpected behavior of an inverted rye chromosome arm in wheat. Chromosoma 117, 569–578 (2008). https://doi.org/10.1007/s00412-008-0174-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-008-0174-4