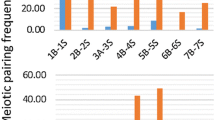
Abstract
Cotton is a model system for studying polyploidization, genomic organization, and genome-size variation because the allotetraploid was formed 1-2 million years ago, which is old enough for sequence divergence but relatively recent to maintain genome stability. In spite of characterizing random genomic sequences in many polyploidy plants, the cytogenetic and sequence data that decipher homoeologous chromosomes are very limited in allopolyploid species. Here, we reported comprehensive analyses of integrated cytogenetic and linkage maps of homoeologous chromosomes 12A and 12D in allotetraploid cotton using fluorescence in situ hybridization and a large number of bacterial artificial chromosomes that were anchored by simple sequence repeat markers in the corresponding linkage maps. Integration of genetic loci into physical localizations showed considerable variation of genome organization, structure, and size between 12A and 12D homoeologous chromosomes. The distal regions of the chromosomes displayed relatively lower levels of structural and size variation than other regions of the chromosomes. The highest level of variation was found in the pericentric regions in the long arms of the two homoeologous chromosomes. The genome-size difference between A and D sub-genomes in allotetraploid cotton was mainly associated with uneven expansion or contraction between different regions of homoeologous chromosomes. As an attempt for studying on the polyploidy homoeologous chromosomes, these results are of general interest to the understanding and future sequencing of complex genomes in plant species.




Similar content being viewed by others
References
Barakat A, Carels N, Bernardi G (1997) The distribution of genes in the genomes of Gramineae. Proc Natl Acad Sci USA 94:6857–6861
Broman KW, Murray JC, Sheffield VC, White RL, Weber JL (1998) Comprehensive human genetic maps: individual and sex-specific variation in recombination. Am J Hum Genet 63:861–869
Chen ZJ (2007) Genetic and epigenetic mechanisms for gene expression and phenotypic variation in plant polyploids. Annu Rev Plant Biol 58:377–406
Chen ZJ, Scheffler BE, Dennis E, Triplett BA, Zhang T, Guo W, Chen X, Stelly DM, Rabinowicz PD, Town CD, Arioli T, Brubaker C, Cantrell RG, Lacape JM, Ulloa M, Chee P, Gingle AR, Haigler CH, Percy R, Saha S, Wilkins T, Wright RJ, Van Deynze A, Zhu Y, Yu S, Abdurakhmonov I, Katageri I, Kumar PA, Mehboob Ur R, Zafar Y, Yu JZ, Kohel RJ, Wendel JF, Paterson AH (2007) Toward sequencing cotton (Gossypium) genomes. Plant Physiol 145:1303–1310
Chen D, Ding Y, Guo W, Zhang T (2009) Molecular mapping of genic male-sterile genes ms 15 , ms 5 and ms 6 in tetraploid cotton. Plant Breed 128:193–198
Collins A, Frezal J, Teague J, Morton NE (1996) A metric map of humans: 23,500 loci in 850 bands. Proc Natl Acad Sci USA 93:14771–14775
Cronn RC, Small RL, Wendel JF (1999) Duplicated genes evolve independently after polyploid formation in cotton. Proc Natl Acad Sci USA 96:14406–14411
Cronn RC, Small RL, Haselkorn T, Wendel JF (2002) Rapid diversification of the cotton genus (Gossypium: Malvaceae) revealed by analysis of sixteen nuclear and chloroplast genes. Am J Bot 89:707–725
De Jong JH, Fransz P, Zabel P (1999) High resolution FISH in plants—techniques and applications. Trends Plant Sci 4:258–263
Dong CG, Ding YZ, Guo WZ, Zhang TZ (2007) Fine mapping of the dominant glandless Gene Gl e2 in Sea island cotton (Gossypium barbadense L.). Chin Sci Bull 52:3105–3109
Fransz P, Armstrong S, Alonso-Blanco C, Fischer TC, Torres-Ruiz RA, Jones G (1998) Cytogenetics for the model system Arabidopsis thaliana. Plant J 13:867–876
Fryxell PA (1992) A revised taxonomic interpretation of Gossypium L. (Malvaceae). Rheedea 2:108–165
Gerstel DU (1953) Chromosome translocations in interspecific hybrids of the genus Gossypium. Evolution 7:234–244
Gill KS, Gill BS, Endo TR (1993) A chromosome region-specific mapping strategy reveals gene-rich telomeric ends in wheat. Chromosoma 102:374–381
Gill KS, Gill BS, Endo TR, Taylor T (1996) Identification and high-density mapping of gene-rich regions in chromosome group 1 of wheat. Genetics 144:1883–1891
Grover CE, Kim H, Wing RA, Paterson AH, Wendel JF (2004) Incongruent patterns of local and global genome size evolution in cotton. Genome Res 14:1474–1482
Grover CE, Kim H, Wing RA, Paterson AH, Wendel JF (2007) Microcolinearity and genome evolution in the AdhA region of diploid and polyploid cotton (Gossypium). Plant J 50:995–1006
Grover CE, Yu Y, Wing RA, Paterson AH, Wendel JF (2008) A phylogenetic analysis of indel dynamics in the cotton genus. Mol Biol Evol 25:1415–1428
Guo W, Cai C, Wang C, Zhao L, Wang L, Zhang T (2008) A preliminary analysis of genome structure and composition in Gossypium hirsutum. BMC Genomics 9:314
Hanson RE, Zwick MS, Choi S, Islam-Faridi MN, McKnight TD, Wing RA, Price HJ, Stelly DM (1995) Fluorescent in situ hybridization of a bacterial artificial chromosome. Genome 38:646–651
Hanson RE, Islam-Faridi MN, Percival EA, Crane CF, Ji Y, McKnight TD, Stelly DM, Price HJ (1996) Distribution of 5S and 18S-28S rDNA loci in a tetraploid cotton (Gossypium hirsutum L.) and its putative diploid ancestors. Chromosoma 105:55–61
Harper LC, Cande WZ (2000) Mapping a new frontier; development of integrated cytogenetic maps in plants. Funct Integr Genomics 1:89–98
Hawkins JS, Kim H, Nason JD, Wing RA, Wendel JF (2006) Differential lineage-specific amplification of transposable elements is responsible for genome size variation in Gossypium. Genome Res 16:1252–1261
Hu Y, Guo WZ, Zhang TZ (2009) Construction of a bacterial artificial chromosome library of TM-1, a standard line for genetics and genomics in Upland cotton. J Integr Plant Biol 51:107–112
Islam-Faridi MN, Childs KL, Klein PE, Hodnett G, Menz MA, Klein RR, Rooney WL, Mullet JE, Stelly DM, Price HJ (2002) A molecular cytogenetic map of sorghum chromosome 1. fluorescence in situ hybridization analysis with mapped bacterial artificial chromosomes. Genetics 161:345–353
Jack Mursal I, Endrizzi JE (1976) A reexamination of the diploidlike meiotic behavior of polyploid cotton. Theor Appl Genet 47:171–178
Ji Y, Zhao X, Paterson AH, Price HJ, Stelly DM (2007) Integrative mapping of Gossypium hirsutum L. by meiotic fluorescent in situ hybridization of a tandemly repetitive sequence (B77). Genetics 176:115–123
Jiang J, Gill BS (2006) Current status and the future of fluorescence in situ hybridization (FISH) in plant genome research. Genome 49:1057–1068
Kapuscinski J (1995) DAPI: a DNA-specific fluorescent probe. Biotech Histochem 70:220–233
Kato A, Vega JM, Han F, Lamb JC, Birchler JA (2005) Advances in plant chromosome identification and cytogenetic techniques. Curr Opin Plant Biol 8:148–154
Kato A, Albert PS, Vega JM, Birchler JA (2006) Sensitive fluorescence in situ hybridization signal detection in maize using directly labeled probes produced by high concentration DNA polymerase nick translation. Biotech Histochem 81:71–78
Kim JS, Klein PE, Klein RR, Price HJ, Mullet JE, Stelly DM (2005) Molecular cytogenetic maps of Sorghum linkage groups 2 and 8. Genetics 169:955–965
Kunzel G, Korzun L, Meister A (2000) Cytologically integrated physical restriction fragment length polymorphism maps for the barley genome based on translocation breakpoints. Genetics 154:397–412
Lakatošová M, Holečková B (2007) Fluorescence in situ hybridisation. Biologia 62:243–250
Liu B, Brubaker CL, Mergeai G, Cronn RC, Wendel JF (2001) Polyploid formation in cotton is not accompanied by rapid genomic changes. Genome 44:321–330
Masterson J (1994) Stomatal size in fossil plants: evidence for polyploidy in majority of angiosperms. Science 264:421–424
Nguyen TB, Giband M, Brottier P, Risterucci AM, Lacape JM (2004) Wide coverage of the tetraploid cotton genome using newly developed microsatellite markers. Theor Appl Genet 109:167–175
Otto SP, Whitton J (2000) Polyploid incidence and evolution. Annu Rev Genet 34:401–437
Rong J, Abbey C, Bowers JE, Brubaker CL, Chang C, Chee PW, Delmonte TA, Ding X, Garza JJ, Marler BS, Park CH, Pierce GJ, Rainey KM, Rastogi VK, Schulze SR, Trolinder NL, Wendel JF, Wilkins TA, Williams-Coplin TD, Wing RA, Wright RJ, Zhao X, Zhu L, Paterson AH (2004) A 3347-locus genetic recombination map of sequence-tagged sites reveals features of genome organization, transmission and evolution of cotton (Gossypium). Genetics 166:389–417
Rong J, Pierce GJ, Waghmare VN, Rogers CJ, Desai A, Chee PW, May OL, Gannaway JR, Wendel JF, Wilkins TA, Paterson AH (2005) Genetic mapping and comparative analysis of seven mutants related to seed fiber development in cotton. Theor Appl Genet 111:1137–1146
Schwarzacher T (2003) DNA, chromosomes, and in situ hybridization. Genome 46:953–962
Seelanan T, Schnabel A, Wendel JF (1997) Congruence and consensus in the cotton tribe. Syst Bot 22:259–290
Tanksley SD, Ganal MW, Prince JP, de Vicente MC, Bonierbale MW, Broun P, Fulton TM, Giovannoni JJ, Grandillo S, Martin GB et al (1992) High density molecular linkage maps of the tomato and potato genomes. Genetics 132:1141–1160
Thuriaux P (1977) Is recombination confined to structural genes on the eukaryotic genome? Nature 268:460–462
Tomkins JP, Peterson DG, Yang TJ, Main D, Wilkins TA, Paterson AH, Wing RA (2001) Development of genomic resources for cotton (Gossypium hirsutum L.): BAC library construction, preliminary STC analysis, and identification of clones associated with fiber development. Mol Breed 8:255–261
Udall JA, Swanson JM, Haller K, Rapp RA, Sparks ME, Hatfield J, Yu Y, Wu Y, Dowd C, Arpat AB, Sickler BA, Wilkins TA, Guo JY, Chen XY, Scheffler J, Taliercio E, Turley R, McFadden H, Payton P, Klueva N, Allen R, Zhang D, Haigler C, Wilkerson C, Suo J, Schulze SR, Pierce ML, Essenberg M, Kim H, Llewellyn DJ, Dennis ES, Kudrna D, Wing R, Paterson AH, Soderlund C, Wendel JF (2006) A global assembly of cotton ESTs. Genome Res 16:441–450
Wang CJ, Harper L, Cande WZ (2006a) High-resolution single-copy gene fluorescence in situ hybridization and its use in the construction of a cytogenetic map of maize chromosome 9. Plant Cell 18:529–544
Wang K, Song X, Han Z, Guo W, Yu JZ, Sun J, Pan J, Kohel RJ, Zhang T (2006b) Complete assignment of the chromosomes of Gossypium hirsutum L. by translocation and fluorescence in situ hybridization mapping. Theor Appl Genet 113:73–80
Wang K, Guo W, Zhang T (2007a) Detection and mapping of homologous and homoeologous segments in homoeologous groups of allotetraploid cotton by BAC-FISH. BMC Genomics 8:178
Wang K, Guo W, Zhang T (2007b) Development of one set of chromosome-specific microsatellite-containing BACs and their physical mapping in Gossypium hirsutum L. Theor Appl Genet 115:675–682
Wang K, Zhang YJ, Guan B, Guo W, Zhang T (2007c) Fluorescence in situ hybridization of bacterial artificial chromosome in cotton. Prog Biochem Biophys 34:1216–1222
Wang K, Guan B, Guo W, Zhou B, Hu Y, Zhu Y, Zhang T (2008) Completely distinguishing individual A-genome chromosomes and their karyotyping analysis by multiple bacterial artificial chromosome-fluorescence in situ hybridization. Genetics 178:1117–1122
Wendel JF (1989) New World tetraploid cottons contain Old World cytoplasm. Proc Natl Acad Sci USA 86:4132–4136
Xu J, Earle ED (1996) High resolution physical mapping of 45S (5.8S, 18S and 25S) rDNA gene loci in the tomato genome using a combination of karyotyping and FISH of pachytene chromosomes. Chromosoma 104:545–550
Yang SS, Cheung F, Lee JJ, Ha M, Wei NE, Sze SH, Stelly DM, Thaxton P, Triplett B, Town CD, Chen ZJ (2006) Accumulation of genome-specific transcripts, transcription factors and phytohormonal regulators during early stages of fiber cell development in allotetraploid cotton. Plant J 47:761–775
Yin J, Guo W, Yang L, Liu L, Zhang T (2006) Physical mapping of the Rf 1 fertility-restoring gene to a 100-kb region in cotton. Theor Appl Genet 112:1318–1325
Zhao XP, Si Y, Hanson RE, Crane CF, Price HJ, Stelly DM, Wendel JF, Paterson AH (1998) Dispersed repetitive DNA has spread to new genomes since polyploid formation in cotton. Genome Res 8:479–492
Acknowledgments
We thank Prof. Xiue Wang of Nanjing Agricultural University and Prof. Weichang Yu of Chinese University of Hong Kong for technical assistance in FISH analysis. This work was supported by the National Natural Science Foundation of China (30700510, 30730067), Natural Science Foundation of Jiangsu Province of China (BK2008333), and State Key Laboratory of Crop Genetics and Germplasm Enhancement (010-60066009, 010-60060305-2). The work in the Z. Jeffrey Chen and David M. Stelly labs was supported by the grant from the US National Science Foundation Plant Genome Research grant (DBI0624077).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ethical standards
The experiments comply with the current laws of China.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Fig. S1
SSR amplification analysis of the monomorphic- and polymorphic-positive BACs of the SSR primer NAU1237. a Figure shows identification of the BAC r081K08 (lane 3) containing the polymorphic locus of NAU1237-155, and t215O23 (lane 4) containing the monomorphic locus NAU1237-140 between TM-1 and Hai7124 using SSR marker NAU1237. Lanes 1 and 2 are TM-1 and Hai7124, respectively. b BAC r043C02 (green signals, arrows), which is derived from chromosome 12A, was FISHed simultaneously (with150 × Cot-1 DNA blocking) with polymorphic BAC r081K08 (red signals, arrowheads); the results reconfirmed that polymorphic BAC r081K08 is located on chromosome 12A. Bar 10 µm. (DOC 482 kb)
Fig. S2
FISH analysis of homoeologous- and centromere-specific BACs on cotton mitotic chromosomes. Homoeologous-specific BACs t283I16 (a), t208C15 (b), and r070L23 (c) produced two pairs of bright signals (arrows and arrowheads) in FISH. These loci were revealed to be localized on chromosomes 12A (arrows, the larger pair of chromosomes) and 12D (arrowheads, the smaller pair of chromosomes) in the subsequently constructed cytogenetic map. Centromere-specific BAC t097G20 (d) was mapped to the centromeric region of all 26 mitotic chromosomes in tetraploid cotton. Further FISHing based on meiotic metaphase chromosomes showed that all signals were localized on the very top of 26 bivalents (e) and proved its centromere-specific nature. Bars 10 µm. (DOC 326 kb)
Fig. S3
FISH analysis of BAC repetitive sequence content. BACs r075A11 (a) and t107F20 (b) were specific for A subgenome chromosomes and all of the chromosomes of mitotic tetraploid cotton cells stained brightly on FISH analysis, indicating a high content of A or AD subgenome-specific repetitive sequences. Arrowheads indicate the original signals of the corresponding BACs. Bars 10 µm. (DOC 167 kb)
Fig. S4
FISH testing five 12D BACs (t215O23, t081H09, t246C12, t107F20, and r010G17) with A-genome diploid cotton, Gossypium arboreum. Five 12D BACs, t215O23, t081H09, t246C12, t107F20, and r010G17 were hybridized to the G. arboreum chromosome. Two 12A BACs t259M16 and t336G12 were used simultaneously in FISH as control. Signals derived from BACs t259M16 (red, arrow) and t336G12 (green, arrow) could be detected but no other signals from five testing BACs could be detected. Bar 10 µm. (DOC 76 kb)
Rights and permissions
About this article
Cite this article
Wang, K., Guo, W., Yang, Z. et al. Structure and size variations between 12A and 12D homoeologous chromosomes based on high-resolution cytogenetic map in allotetraploid cotton. Chromosoma 119, 255–266 (2010). https://doi.org/10.1007/s00412-009-0254-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-009-0254-0