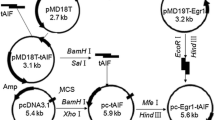
Abstract
Pro-apoptosis in cancer cells has been proposed as a beneficial therapeutic strategy for potentiating the anticancer effects of radiotherapy. TNF-related apoptosis inducing ligand (TRAIL) and Second mitochondria derived activator of caspase (Smac) can induce cell apoptosis. Herein, we designed a conditionally replicating adenoviral co-overexpression vector of TRAIL and Smac regulated by the Egr1 promoter, in which hTERT, E1A-E1B and E1B55K genes were inserted to achieve enhanced tumor targeting characteristics. After breast cancer MDA-MB-231 cells were infected and irradiated, cellular proliferation and colony formation were measured, apoptotic rate was detected by FCM after AnnexinV-FITC/PI staining. To explore the molecular mechanisms of apoptosis, mRNA and protein levels of TRAIL, Smac, Cytochrome c (Cyt c), death receptor 5 (DR5), caspase-8, -9 and -3 were measured by quantitative real-time PCR, ELISA and Western blot, and caspase-3 activity was detected using caspase-3 activity kits. The results showed that TRAIL and/or Smac overexpression enhanced proliferation inhibition and radio-sensitivity through apoptosis. In addition, the combination of IR and overexpression of TRAIL and/or Smac can activate more apoptosis in tumor cells, and the transcriptional levels and protein expressions of Cyt c, DR5, caspase-8, -9 and -3 had similar regularity with apoptotic changes, indicating the molecular mechanisms of TRAIL and Smac involves the mitochondrial pathway. Our findings may have implications for novel radiotherapy plans for breast tumor treatment.





Similar content being viewed by others
References
Abdulkadir SA (2005) Mechanisms of prostate tumorigenesis: roles for transcription factors Nkx3.1 and Egr1. Ann N Y Acad Sci 1059:33–40
Anton M, Gomaa IE, von Lukowicz T, Molls M, Gansbacher B, Würschmidt F (2005) Optimization of radiation controlled gene expression by adenoviral vectors in vitro. Cancer Gene Ther 12:640–646
Ashkenazi A (2008) Targeting the extrinsic apoptosis pathway in cancer. Cytokine Growth Factor Rev 19:325–331
Ashkenazi A, Holland P, Eckhardt SG (2008) Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/tumor necrosis factorerelated apoptosis-inducing ligand (rhApo2L/TRAIL). J Clin Oncol 26:3621–3630
Berk AJ (2005) Recent lessons in gene expression, cell cycle control, and cell biology from adenovirus. Oncogene 24:7673–7685
Cao W, Tian J, Li C, Gao Y, Liu X, Lu J, Wang Y, Wang Z, Svatek RS, Rodriguez R (2017) A novel bladder cancer - specific oncolytic adenovirus by CD46 and its effect combined with cisplatin against cancer cells of CAR negative expression. Virol J 14:149
Choi JW, Lee JS, Kim SW, Yun CO (2012) Evolution of oncolytic adenovirus for cancer treatment. Adv Drug Deliv Rev 64:720–729
Choi JW, Lee YS, Yun CO, Kim SW (2015) Polymeric oncolytic adenovirus for cancer gene therapy. J Control Release 219:181–191
Crompton AM, Kirn DH (2007) From ONYX-015 to armed vaccinia viruses: the education and evolution of oncolytic virus development. Curr Cancer Drug Targets 7:133–139
Fulda S, Vicic D (2012) Targeting IAP proteins for therapeutic intervention in cancer. Nat Rev Drug Discov 11:109–124
Garofalo M, Iovine B, Kuryk L, Capasso C, Hirvinen M, Vitale A, Yliperttula M, Bevilacqua MA, Cerullo V (2016) Oncolytic adenovirus loaded with L-carnosine as novel strategy to enhance the anti-tumor activity. Mol Cancer Ther 15:651–660
Gonzalvez F, Ashkenazi A (2010) New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene 29:4752–4765
Grade M, Wolff HA, Gaedcke J, Ghadimi BM (2012) The molecular basis of chemoradiosensitivity in rectal cancer: implications for personalized therapies. Langenbecks Arch Surg 397:543–555
Hajj C, Goodmn KA (2015) Role of radiotherapy and newer techniques in the treatment of GI cancers. J Clin Oncol 33:1737–1744
Hamacher-Brady A, Choe SC, Krijnse-Locker J, Brady NR (2014) Intramitochondrial recruitment of endolysosomes mediates Smac degradation and constitutes a novel intrinsic apoptosis antagonizing function of XIAP E3 ligase. Cell Death Differ 21:1862–1876
Hemminki O, Parviainen S, Juhila J, Turkki R, Linder N, Lundin J, Kankainen M, Ristimäki A, Koski A, Liikanen I, Oksanen M, Nettelbeck DM, Kairemo K, Partanen K, Joensuu T, Kanerva A, Hemminki A (2015) Immunological data from cancer patients treated with Ad5/3-E2F-Delta24-GMCSF suggests utility for tumor immunotherapy. Oncotarget 6:4467–4481
Hennings J, Hellman P, Ahlström H, Sundin A (2009) Computed tomography, magnetic resonance imaging and 11C-metomidate positron emission tomography for evaluation of adrenal incidentalomas. Eur J Radiol 69:314–323
Huang XL, Wang L, Zhang H, Wang HB, Zhao XP, Qian GX, Hu JF, Ge SF, Fan XQ (2012) Therapeutic efficacy by targeting correction of Notch1-induced aberrants in uveal tumors. PLoS ONE 7:e44301
Kang Z, Sun SY, Cao L (2012) Activating death receptor DR5 as a therapeutic strategy for rhabdomyosarcoma. ISRN Oncol 2012:395952
Kim E, Kim JH, Shin HY, Lee H, Yang JM, Kim J, Sohn JH, Kim H, Yun CO (2003) Ad-mTERT-delta19, a conditional replication-competent adenovirus driven by the human telomerase promoter, selectively replicates in and elicits cytopathic effect in a cancer cell-specific manner. Hum Gene Ther 14:1415–1428
Lanson NA Jr, Friedlander PL, Schwarzenberger P, Kolls JK, Wang G (2003) Replication of an adenoviral vector controlled by the human telomerase reverse transcriptase promoter causes tumor-selective tumor lysis. Cancer Res 63:7936–7941
Li YB, Guo CX, Wang ZC, Dong LH, Guan F, Liu Y, Wang HF, Sun ZW, Gong SL (2013) Radiosensitization of breast cancer cells by TRAIL-endostatin-targeting gene therapy. Neoplasma 60:613–619
Li Y, Li YF, Si CZ, Zhu YH, Jin Y, Zhu TT, Liu MY, Liu GY (2016) CCL21/IL21-armed oncolytic adenovirus enhances antitumor activity against TERT-positive tumor cells. Virus Res 220:172–178
Lumniczky K, Sáfrány G (2006) Cancer gene therapy: combination with radiation therapy and the role of bystander cell killing in the anti-tumor effect. Pathol Oncol Res 12:118–124
Mantwill K, Klein FG, Wang D, Hindupur SV, Ehrenfeld M, Holm PS, Nawroth R (2021) Concepts in oncolytic adenovirus therapy. Int J Mol Sci 22:10522
Mühlethaler-Mottet A, Bourloud KB, Auderset K, Joseph JM, Gross N (2004) Drug-mediated sensitization to TRAIL-induced apoptosis in caspase-8-complemented neuroblastoma cells proceeds via activation of intrinsic and extrinsic pathways and caspase-dependent cleavage of XIAP, Bcl-xL and RIP. Oncogene 23:5415–5425
Mundt AJ, Vijayakumar S, Nemunaitis J, Sandler A, Schwartz H, Hanna N, Peabody T, Senzer N, Chu K, Rasmussen CS, Kessler PD, Rasmussen HS, Warso M, Kufe DW, Gupta TD, Weichselbaum RR (2004) A phase I trial of TNFerade biologic in patients with soft tissue sarcoma in the extremities. Clin Cancer Res 10:5747–5753
Panayotopoulou EG, Müller AK, Börries M, Busch H, Hu G, Lev S (2017) Targeting of apoptotic pathways by SMAC or BH3 mimetics distinctly sensitizes paclitaxel-resistant triple negative breast cancer cells. Oncotarget 8:45088–45104
Shen Q, Brown PH (2005) Transgenic mouse models for the prevention of breast cancer. Mutat Res 576:93–110
Shimizu N, Ohta M, Fujiwara C, Sagara J, Mochizuki N, Oda T, Utiyama H (1992) A gene coding for a zinc finger protein is induced during 12-O-tetradecanoylphorbol-13-acetate-stimulated HL-60 cell differentiation. J Biochem 111:272–277
Wang H, Song X, Zhang H, Zhang J, Shen X, Zhou Y, Fan X, Dai L, Qian G, Hoffman AR, Hu JF, Ge S (2012) Potentiation of tumor radiotherapy by a radiation-inducible oncolytic and oncoapoptotic adenovirus in cervical cancer xenografts. Int J Cancer 130:443–453
Wang J, Li Y, Liu Y, Li Y, Gong S, Fang F, Wang Z (2015) Overexpression of truncated AIF regulated by Egr1 promoter radiation-induced apoptosis on MCF-7 cells. Radiat Environ Biophys 54:413–421
Weichselbaum RR, Hallahan D, Fuks Z, Kufe D (1994) Radiation induction of immediate early genes: effectors of the radiation-stress response. Int J Radiat Oncol Biol Phys 30:229–234
Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA (1995) Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3:673–682
Wu JH, Wang HF, Wang ZC, Xu K, Qi YL, Li JH, Gong SL, Liu Y, Liu Y (2013) Conditionally replicating adenovirus combined with gene-targeted radiotherapy induces apoptosis via TRAIL death receptors in MDA-MB-231 cells. Mol Med Rep 8:299–305
Yoon AR, Hong J, Kim SW, Yun CO (2016) Redirecting adenovirus tropism by genetic, chemical, and mechanical modification of the adenovirus surface for cancer gene therapy. Expert Opin Drug Deliv 13:843–858
Zou W, Liu X, Yue P, Zhou Z, Sporn MB, Lotan R, Khuri FR, Sun S-Y (2004) c-Jun NH 2-terminal kinase-mediated up-regulation of death receptor 5 contributes to induction of apoptosis by the novel synthetic triterpenoid methyl-2-cyano3,12-dioxooleana-1, 9-dien-28-oate in human lung cancer cells. Cancer Res 64:7570–7578
Acknowledgements
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript. This work was supported by grants from the Jilin Health Technology Innovation (2019J029), Science and Technology Developmental Plan of Jilin Province (20180101305JC and 20190201202JC).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, W., Fang, F., Wang, Y. et al. Co-overexpression of TRAIL and Smac sensitizes MDA-MB-231 cells to radiation through apoptosis depending on mitochondrial pathway. Radiat Environ Biophys 61, 37–48 (2022). https://doi.org/10.1007/s00411-021-00961-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-021-00961-3