Abstract
Purpose
Crimean-Congo hemorrhagic fever (CCHF) is a viral tick-borne illness. Although its etiopathogenesis is not clearly understood, it is known to be a Nairovirus. We aimed to examine the viral effects of intense systemic inflammation and vascular damage on the pulmonary vascular beds and lung tissues.
Methods
A total of 45 patients who were diagnosed with CCHF were considered for this retrospective study. In this patient group, those whose lungs had been visualized via thoracic computer tomography (CT) were entered into the study. Diameters of the pulmonary trunk, main pulmonary arteries, atria, and ventricles were measured. Study group measurements were compared with the control group, which included patients with normal thoracic CT.
Results
Overall, 90 patients were enrolled in the study, with 45 patients in the study group and 45 in the control group. In the study group, the man-to-woman balance was 3/2. The average age in the study group was 54.07 ± 17.91 years. In comparing the average diameters of pulmonary arteries in the study and control groups, the study group’s average pulmonary artery diameter was significantly larger than the control group (p < 0.001).
Conclusions
The increase in diameters of the pulmonary trunks and main pulmonary arteries due to CCHF was first shown in this current study. Moreover, due to our findings, it should be noted that with the rise in pulmonary artery diameter in CCHF, pulmonary hypertension can appear acutely, and this condition can be significantly alter clinical course and follow-up of the viral illness.
Similar content being viewed by others
Introduction
Crimean-Congo hemorrhagic fever (CCHF) is a viral tick-borne illness. It is especially prevalent in late spring and summer months and is endemic in many geographic regions such as Africa and the Balkans. The virus is a member of the genus Nairovirus of the Bunyavirus family and can lead to life-threatening bleeding [1]. Although its etiopathogenesis is not clearly understood, the virus is transmitted via tick bite [2] and creates an intense systemic inflammatory response. Nonspecific symptoms like fever, weakness, and myalgia can appear during the acute period. In later stages of the illness, the virus can cause life-threatening clinical conditions, including shock and spontaneous severe bleeding caused by decreased platelet levels. There has been an increase in the number of cases worldwide and in Turkey over the past few years [1, 2]. Surprisingly, studies investigating the pulmonary system in CCHF are rather rare. Little is known about the pathophysiological changes that result from systemic inflammation and vascular damage caused by the virus, or its clinical effects on pulmonary vascular beds and lung tissues [1–4]. In our study, we examined the consequences of this systemic inflammation on the pulmonary artery, apart from changes induced by direct and indirect vascular endothelial damage.
Materials and Methods
A total of 90 patients who were diagnosed with CCHF and referred to the Emergency Service and Infectious Diseases Department between May 2010 and September 2015 were included for consideration in this retrospective study. The diagnosis of CCHF was made with genome detection of the virus through either an enzyme-linked immunosorbent assay or polymerase chain reaction after processing blood samples in a virology lab. In this patient group, those whose lungs were examined via thoracic computed tomography (CT) were entered into the study. Patients with blood diseases, malignancies, acute or chronic lung and heart diseases, medicines that may cause pulmonary hypertension, mediastinal pathology, or history of thoracic radiotherapy or surgery in addition to CCHF were excluded from the study. Patient’s medical information was gained from their files. The ethics committee provided approval for the study.
In addition to demographics including age, sex, occupation, and location, tick exposure, clinical diseases, patient medical history, and thoracic CT diameter measurements of the pulmonary trunk, the main pulmonary arteries, atria, and ventricles were measured. Measurements were performed on the widest diameter based on the axial images in the mediastinal window in a thoracic CT taken at the level of the pulmonary artery bifurcation, and vertical to the long axis. Two radiologists with at least 5 years experience performed measurements by doing at least two measurements at different times. The average of four measurements was used for statistical analysis. The study group was compared with the control group, which included patients who underwent thoracic CT due to solitary pulmonary nodule follow-up. The control patients demonstrated similar demographics and were without additional diseases.
Statistical Analysis
Quantitative data are expressed as the mean ± SD. Independent sample t-tests were used to compare the continuous data between groups. Categorical variables are presented as a count and percentage. A p value of <0.05 was considered significant. Analyses were performed using SPSS 19 (IBM SPSS Statistics 19, SPSS Inc., IBM Co., Somers, NY).
Results
Overall, 90 patients were enrolled in the study, with 45 patients in the study group and 45 in the control group. In the study group, the man-to-woman balance was 3/2, while in the control group the man/woman balance was 26/19. In terms of sex, both groups were similar.
According to the age distribution, the average age in the study group was 54.07 ± 17.91 years, while the average age in the control group was 53.56 ± 10.96 years. In both groups, the average age was similar and consistent. When the age distribution was examined with regard to sex, the average ages were also similar. The average age of women in the study group was 52.33 ± 14.92 years, while the average age of men in the study group was 55.22 ± 19.84 years. Similarly, the average age of women in the control group was 52.32 ± 8.58 years, while it was 54.46 ± 12.5 years for men. The average ages of patients in both groups showed a homogenous distribution independent of sex.
In comparing the average diameters of pulmonary arteries in the study and control groups, the study group’s average pulmonary artery diameter was significantly larger than the control group (p < 0.001) (Figs. 1, 2, 3). Measurements of the average diameters of the pulmonary trunks and the left and right main pulmonary arteries from thoracic CT are summarized in Table 1. In addition, in comparing the measurements of both groups, a statistically significant difference was not detected, except for the average left ventricle diameter (p > 0.05). The diameters of the left atrium, right atrium, left ventricle, and right ventricle measured from thoracic CT are summarized in Table 1.
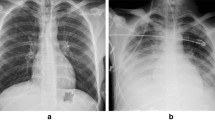
The same patient described in Fig. 1 was examined for the enlargements of the pulmonary trunk and right and left main pulmonary arteries, in addition to diameter measurements, via enhanced thoracic CT
Alveolar and ground glass opacities are seen in the parenchymal window of the same patient described in Fig. 1 on thoracic CT
Furthermore, 16 patients were examined by echocardiography during their hospital admission or after their hospitalization. The 16 patients’ average systolic pulmonary artery pressure was 44.69 ± 14.66 mmHg (range between 30 and 90 mmHg), which is higher than normal. Measurements of cardiac and pulmonary artery dimensions in both groups were compared based on sex. There were no statistically significant differences between any parameters apart from the right ventricle diameter. Moreover, in the study group, the average right ventricle diameter was 51.56 ± 15.92 mm in women, which was significantly wider than in men (42.07 ± 9.89 mm) (p < 0.05) (Table 2).
A total of 17 patients (37.7 %) out of 45 died in the study group. Moreover, 7 out of 16 patients who had a high sPAP in echocardiography died. There was no significant relationship between the average diameters of the cardiac and pulmonary artery dimensions and survival rates (Table 3).
Discussion
In CCHF, the clinical course of disease begins with the viral entry into the body through the skin, mucosa, or inhalation. The virus is met by the body’s monocytes, macrophages, and dendritic cells; then, if the virus is not sufficiently eliminated by the host’s defense cells, it is replicated [5–7]. The virus first spreads to regional lymph nodes, and then to the other organ systems, through vascular pathways. The virus damages the liver, spleen, and endothelial cells due to its direct cytotoxic effect. This evokes cytokine and chemokine release from defense cells with non-cytopathic effects, and can lead to endothelial damage as well as an intense inflammatory response. When factors and mechanisms related to this pathogenesis are considered generally, the prognosis of the disease corresponds to viral burden, the sufficiency of the host’s immune system, and the inflammatory response [8–12]. There are similar diseases caused by hantavirus, which belongs to the Bunyavirus family. These other diseases include viral hemorrhagic fevers, and are similar in both pathophysiological mechanisms and in clinical characteristics. Hantavirus pulmonary syndrome (HPS) is one such disease, which utilizes similar clinical and pathophysiological mechanisms compared with the lung symptoms of CCHF. These two viruses cause similar immune responses and systemic inflammation in the host [13, 14]. Furthermore, when examining lung symptoms of HPS caused by both CCHF and the hantavirus, the affinities of these two members of the Bunyavirus family for lung tissue are better understood.
The effects of CCHF on the lung have not been clearly delineated, and there is limited information concerning these effects. When pathophysiological mechanisms at the molecular level are taken into consideration, this process, caused by numerous inflammatory factors, is associated with increased endothelial damage and vascular permeability. In turn, these conditions are closely related to clinical conditions like pleural effusion, alveolar hemorrhage, acute respiratory distress syndrome (ARDS), and shock occurring in the lungs of patients with CCHF [15, 16]. For this reason, it can be stated that the CCHF virus, which causes an intense systemic inflammatory response, leads to an intense inflammatory response in vascular and defensive cells of the lungs in a similar fashion. Thus, changes happening due to this intense inflammatory response and endothelial damage in pulmonary vascular beds, especially in pulmonary arteries, have become significant in revealing the etiopathogenesis related to the lungs in CCHF. As indicated in our study, a significant rise in the diameter of the pulmonary artery of CCHF patients can be the evidence of this intense inflammatory response and endothelial damage. Furthermore, 16 patients with CCHF were evaluated with echocardiography, and a high sPAP was detected in all of them; the average sPAP value was 44.69 ± 14.66 mmHg. In addition, the lowest sPAP value was 30 mmHg, while the highest was 90 mmHg. In the echocardiography of one patient in the study group, which was performed during the patient’s hospitalization, the sPAP and other cardiac symptoms were detected at normal levels. However, the sPAP was 40 mmHg higher than normal in the echocardiography done on the 10th day of hospitalization. In the follow-up examinations, there was clinical recovery and the sPAP value decreased to normal levels.
Engin et al. evaluated 52 patients with CCHF by echocardiography. As a result, when they separated the patients into two groups consisting of severe and mild forms based on their clinical symptoms, the average sPAP value measured by echocardiography in both groups was high (48 and 36 mmHg, respectively). When the patients were grouped as fatal and nonfatal and compared, the sPAP was 50 mmHg in the fatal group and 39 mmHg in the nonfatal group. In that study, the comparisons among all groups were statistically significant (p < 0.05) [17]. These results suggest that pulmonary hypertension found in echocardiographic examinations is based on coagulation deficits, vascular endothelial damage, and an inflammatory response that occurs with CCHF’s pathophysiology. In our study, the increased diameter of the pulmonary trunks and main pulmonary arteries in patients with CCHF might also be the result of pulmonary hypertension occurring during the clinical and pathophysiological processes of the disease.
Our choice to conduct a retrospective study is our first limitation; however, patients’ medical information and radiological images had been properly recorded, and there were no difficulties in retrieving their data. Another limitation is that echocardiography could not be performed on all patients. The reason for this was that some patients’ general condition was not suitable for an echocardiographic evaluation. In addition, an optimal measurement method is necessary to conduct the follow-up of the patients with echocardiography during their hospitalization and clinical follow-up period, and perform an evaluation of cardiac and pulmonary artery dimensions with thoracic CT in a parallel manner.
In conclusion, pathophysiological changes in CCHF have not yet been clearly elucidated. However, in many studies conducted at the molecular level, there are an intense systemic inflammation and endothelial damage at the basis of the disease. The difference in the severity of clinical findings among patients is related to the viral burden, the presence of intense inflammation, endothelial damage, and the response provided by the immune system of the host. Nevertheless, there is still little known about the effect of CCHF on the lungs. In a few studies cited here, clinical consequences like pleural effusion, alveolar hemorrhage, ARDS, and shock were mentioned, and the fact that the diameters of pulmonary trunks and main pulmonary arteries increase due to CCHF was first shown in the current study. Moreover, due to our findings, it should be noted that with the rise in pulmonary artery diameter in CCHF, pulmonary hypertension can appear over an acute period, and this condition can be significant over the clinical course and follow-up of the disease.
References
Leblebicioglu H, Ozaras R, Irmak H, Sencan I (2016) Crimean-congo hemorrhagic fever in turkey: current status and future challenges. Antiviral Res 126:21–34
Leblebicioglu H, Sunbul M, Bodur H, Ozaras R, Network CCHFR (2016) Discharge criteria for Crimean-Congo haemorrhagic fever in endemic areas. J Infect 72(4):500–501
Ergönül O (2006) Crimean-Congo haemorrhagic fever. Lancet Infect Dis 6:203–214
Swanepoel R, Gill DE, Shepherd AJ, Leman PA, Mynhardt JH, Harvey S (1989) The clinical pathology of Crimean-Congo haemorrhagic fever. Clin Infect Dis 11:794–800
Ergonul O (2012) Crimean-Congo hemorrhagic fever virus: new outbreaks, new discoveries. Curr Opin Virol. 2:215–220
Whitehouse CA (2004) Crimean-Congo hemorrhagic fever. Antivir Res 64:145–160
Pshenichnaya NY, Nenadskaya SA (2015) Probable Crimean-Congo hemorrhagicfever virus transmission occurred after aerosol-generatingmedical procedures in Russia: nosocomial cluster. Int J Infect Dis 33:120–122
Connolly-Andersen AM, Moll G, Andersson C, Akerström S, Karlberg H, Douagi I et al (2011) Crimean-Congo hemorrhagic fever virus activates endothelial cells. J Virol 85:7766–7774
Burt FJ, Swanepoel R, Shieh WJ, Smith JF, Leman PA, Greer PW et al (1997) Immunohistochemical and in situ localization of Crimean- Congo hemorrhagic fever (CCHF) virus in human tissues and implications for CCHF pathogenesis. Arch Pathol Lab Med 121:839–846
Rodrigues R, Paranhos-Baccalà G, Vernet G, Peyrefitte CN (2012) Crimean-Congo hemorrhagic fever virus-infected hepatocytes induceER-stress and apoptosis crosstalk. PLoS One 7:e29712
Fraisier C, Rodrigues R, Hai VV, Belghazi M, Bourdon S, Paranhos-Baccala G et al (2014) Hepatocyte pathway alterations in response to in vitro Crimean Congo hemorrhagic fever virus infection. Virus Res 179:187–203
Bente DA, Alimonti JB, ShiehWJ Camus G, Ströher U, Zaki S et al (2010) Pathogenesis and immune response of Crimean-Congo hemorrhagic fever virus in a STAT-1 knockout mouse model. J Virol 84:11089–11100
Saggioro FP, Rossi MA, Duarte MI, Martin CC, Alves VA, Moreli ML et al (2007) Hantavirus infection induces a typical myocarditis that may be responsible for myocardial depression and shock in hantaviruspulmonary syndrome. J Infect Dis 195:1541–1549
Schnittler HJ, Feldman H (2003) Viral hemorahagic fever—a vascular disease? Thromb Haemost 89:967–972
Sannikova IV, Pacechnikov VD, Maleev VV (2007) Respiratory lesions in Crimean-Congo haemorrhagic fever. Ter Arkh 79:20–23
Papa A, Tsergouli K, Çağlayık DY, Bino S, Como N, Uyar Y, Korukluoglu G (2016) Cytokines as biomarkers of Crimean-Congo hemorrhagic fever. J Med Virol 88(1):21–27
Engin A, Yilmaz MB, Elaldi N, Erdem A, Yalta K, Tandogan I, Kaya S, Bakır M, Dokmetas I (2009) Crimean-Congo hemorrhagic fever: does it involve the heart? Int J Infect Dis 13(3):369–373
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Aktaş, T., Aktaş, F., Özmen, Z. et al. Does Crimean-Congo Hemorrhagic Fever Cause a Vasculitic Reaction with Pulmonary Artery Enlargement and Acute Pulmonary Hypertension?. Lung 194, 807–812 (2016). https://doi.org/10.1007/s00408-016-9913-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-016-9913-0